Ex vivo evaluation of 4 different viscoelastic assays for detecting moderate to severe coagulopathy during liver transplantation
Kenichi A. Tanaka is currently involved in a clinical study supported by TEM Innovations (Munich, Germany). Ezeldeen Abuelkasem, Michael A. Mazzeffi, Shu Yang Lu, Raymond M. Planinsic, and Tetsuro Sakai have no conflict of interest to report. TEM Innovations was not involved in the study design, data analysis, or manuscript preparation.
Financial support for this work was provided solely by the University of Pittsburgh Medical Center and the Department of Anesthesiology (Pittsburgh, PA). TEM Innovations (Munich, Germany) provided 2 ROTEM devices for this study at the University of Pittsburgh Medical Center (Pittsburgh, PA).
Abstract
Prolonged prothrombin time (PT) and its ratio are routinely used for the assessment of candidates for liver transplantation (LT), but intraoperative coagulation management of transfusion is hindered by its long turnaround time. Abnormal reaction time (R time) on thromboelastography (TEG) or clotting time (CT) of rotational thromboelastometry (ROTEM) are presumably an alternative, but there is a paucity of clinical data on abnormal R time/CT values compared to PT during LT. After receiving institutional review board approval and informed consent, we obtained blood samples from 36 LT patients for international normalized ratio (INR), factor (F) X level, and viscoelastic tests (EXTEM/INTEM and kaolin/rapid TEG) at baseline and 30 minutes after graft reperfusion. Receiver operating characteristic (ROC) curves were calculated for INR > 1.5 and viscoelastic R time/CT thresholds to assess the ability to diagnose FX deficiency at the moderate (<50%) or severe (<35%) level. The FX deficiency data were calculated using cutoff values of INR (>1.5) and abnormal R time/CT for TEG and ROTEM. Tissue factor (TF)–activated INR and EXTEM-CT performed well in diagnosing FX below 50%, but rapid TEG with combined TF and kaolin activators failed. Improved performance of INTEM-CT in diagnosing FX below 35% underlies multifactorial deficiency involving both intrinsic and common pathways. In conclusion, the differences among different viscoelastic tests and clinical situations should be carefully considered when they are used to guide transfusion during LT.
Abbreviations
-
- AUC
-
- area under the curve
-
- CT
-
- clotting time
-
- F
-
- factor
-
- HCT
-
- hematocrit
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- MA
-
- maximum amplitude
-
- MCF
-
- maximum clot firmness
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NPV
-
- negative predictive value
-
- PCC
-
- prothrombin complex concentrate
-
- PPV
-
- positive predictive value
-
- PRBC
-
- packed red blood cell
-
- PT
-
- prothrombin time
-
- ROC
-
- receiver operating characteristic
-
- ROTEM
-
- rotational thromboelastometry
-
- R time
-
- reaction time
-
- TEG
-
- thromboelastography
-
- TF
-
- tissue factor
Coagulation abnormalities are frequently observed in patients with end-stage liver disease, which affects the synthesis and clearance of procoagulant and anticoagulant proteins. Hemostatic function may be maintained because low procoagulant levels are counterbalanced by reduced anticoagulant proteins.1-3 However, preexisting deficiencies of coagulation factors can be exacerbated by hemorrhage and hemodilution due to fluid replacement in liver transplantation (LT), and plasma transfusion is often necessary to maintain hemostatic function.4-6 For the evaluation of coagulopathy in LT, prothrombin time (PT) and the international normalized ratio (INR) are most commonly used because of the high incidences of PT-sensitive factor (F) deficiencies (FII, FV, FVII, and FX). In general, PT/INR measurement in plasma takes 30-90 minutes,7, 8 which potentially hinders prompt assessment of coagulopathy and the need for plasma replacement. Thromboelastography (TEG; Haemonetics, Niles, IL) and rotational thromboelastometry (ROTEM; TEM Innovations, Munich, Germany) are viscoelastic coagulation monitors, in which a whole blood sample is used to assess the onset and quality of clot formation.8-11 A prolonged onset of clot formation on TEG (reaction time; R time) or ROTEM (clotting time; CT) has been used to guide plasma transfusion,12-15 and this parameter can be obtained in a short time (10-15 minutes).16 The reference ranges of R time and CT are different for 4 types of coagulation activators used in TEG and ROTEM.11 For example, rapid TEG reagent contains tissue factor (TF), phospholipids, and kaolin, activating both extrinsic and intrinsic pathways of coagulation,17 whereas EXTEM reagent only contains TF and phospholipids for extrinsic pathway activation. It was thus hypothesized that TEG and ROTEM assays have different performance characteristics in identifying PT-sensitive factor deficiencies during LT.
Patients and Methods
This study was conducted under local institutional review board approval at the University of Pittsburgh (No. PRO12120173). After written, informed consent, blood samples were obtained from 36 adult patients undergoing LT (May 23, 2013 to April 1, 2014). The anesthetic and surgical managements were previously described in detail.18 LTs using grafts from deceased donors and living donors were included in the study. The piggyback technique was used for the graft implantation. During this study period, percutaneous venovenous bypass was only used for living donor LTs. The intraoperative cell salvage was routinely used, except in the recipients with hepatic malignancy. Packed red blood cells (PRBCs) were administered to maintain hematocrit (HCT) above 26%. The decision to transfuse hemostatic products was based on the visual assessment of microvascular bleeding along with kaolin TEG.
Blood samples for viscoelastic and standard hematological tests were simultaneously drawn from an existing arterial catheter at baseline and at 30 minutes after the reperfusion of the graft liver.
Kaolin and rapid TEG tests were performed using native whole blood (350 µL) according to the manufacturer's instructions. Both tests are activated with kaolin, but TF and phospholipids are additionally contained in the rapid TEG reagent.17 EXTEM and INTEM tests were performed on ROTEM using citrated whole blood (300 µL) mixed with CaCl2 and a coagulation activator. The latter consists of 20 µL of TF and phospholipids for EXTEM, or ellagic acid for INTEM. The onset of clotting (seconds), R time (seconds) for TEG and CT (seconds) for ROTEM, and clot strength (mm), maximum amplitude (MA) for TEG, and maximum clot firmness (MCF) for ROTEM were obtained from each run. All measurements were performed by a designated group of 6 technicians who had completed the standard in-house training.
- FX is involved in the final step of thrombin generation with a cofactor FV as the prothrombinase (FXa-FVa) complex;
- FII, FV, and FX are similarly decreased to the range of 15%-35% in the LT affected by hemodilution and blood loss.19
In the 1-stage clotting assay for FX level, the plasma that is only deficient for FX is added to the patient's plasma sample (vol/vol 1:1). The mixing of FX-deficient plasma corrects severely depleted factors other than FX in the patient's plasma, and thus the degree of correction of PT after mixing can be attributed to the patient's residual FX activity. A false-positive prolongation of INR and TEG/ROTEM CT can occur in severe fibrinogen deficiency.
It is clinically important to distinguish defects in thrombin generation from fibrinogen deficiency because plasma transfusion is reserved for the former, and cryoprecipitate or fibrinogen concentrate is preferably used in the latter.20 Therefore, FX deficiency was used as a surrogate of clinical indication for plasma, and diagnostic ability of INR, TEG-R time, and ROTEM-CT to detect moderate (<50%) or severe (<35%) FX deficiency was determined using the upper cutoff value of each test: INR > 1.5, kaolin TEG-R time > 480 seconds, rapid TEG-R time > 110 seconds, EXTEM-CT > 80 seconds, and INTEM-CT > 240 seconds.11, 21
Statistical Analyses
Data were descriptively summarized as the median and interquartile range (IQR; 25%-75%). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of INR, TEG-R time, and ROTEM-CT were calculated along with the receiver operating characteristic (ROC) curves to predict low FX activity (<50% or 35%) as measured by the plasma FX assay.19, 21
Results
The demographic data of patients are presented in Table 1. Total fluid volume (median) was 6 L including crystalloid and 5% albumin. Transfusion of PRBCs, plasma, platelets, and cryoprecipitate was administered in 83.3%, 58.3%, 16.6%, and 11.1% of 36 patients from the incision to the time of reperfusion. There were no statistical differences in HCT and platelet count values between baseline and reperfusion (Table 2). However, median INR was elevated from 1.9 to 3.4 from baseline to reperfusion. Fibrinogen and FX levels were decreased by 33.6% and 39.3%, respectively from baseline to reperfusion (Table 2).
| Characteristic | Value |
|---|---|
| Age, years | 55.5 (50.3-60.0) |
| Sex male, % | 69.4 |
| Weight, kg | 83.5 (68.3-97.8) |
| Surgical data | |
| MELD score | 18.5 (14.0-34.8) |
| Redo transplant | 11.1% |
| Venovenous bypass use | 19.4% |
| LT duration, minutes | 470 (319-690) |
| Fluid usage, mL | |
| Crystalloids | 3500 (2800-5230) |
| Colloids | 2500 (1500-3500) |
| Salvaged blood | 725 (0-1312) |
| Blood product usage, units | |
| PRBCs | 6 (3-9) |
| Plasma | 4 (2.5-6) |
| Apheresis platelet | 1 (0-2) |
| Cryoprecipitate | 1 (0-1) |
- NOTE: Data shown as median (IQR) unless otherwise indicated.
| Normal Reference Range | Baseline | Reperfusion | P Value | |
|---|---|---|---|---|
| Laboratory results | ||||
| HCT, % | Male, 38-48.8; female, 34.1-43.3 | 30.5 (25.3-35.0) | 29.0 (27.0-32.0) | 0.71 |
| Platelet count, × 103/µL | 156-369 | 54.0 (38.3-75.0) | 54.5 (46.0-81.3) | 0.41 |
| INR | 0.8-1.2 | 1.9 (1.5-2.6) | 3.4 (2.5-5.0) | <0.001 |
| Fibrinogen, mg/dL | 205-508 | 151 (118-236) | 96.0 (63.0-134) | <0.001 |
| FX, % | 70-150 | 48.5 (39.0-74.0) | 28.0 (16.3-36.8) | <0.001 |
| TEG/ROTEM results | ||||
| Coagulation time, seconds | ||||
| Kaolin R time | 180-480 | 489 (386-608) | 534 (441-635) | 0.20 |
| Rapid R time | 78-110 | 48.0 (36.0-72.0) | 57.0 (30.0-84.0) | 0.47 |
| EXTEM-CT | 35-80 | 79.5 (68.3-107) | 127 (99.3-177) | <0.001 |
| INTEM-CT | 100-240 | 189 (172-216) | 271 (243-326) | <0.001 |
| Clot amplitude, mm | ||||
| Kaolin MA | 51-69 | 50.1 (41.8-58.9) | 41.1 (33.3-49.0) | 0.002 |
| Rapid MA | 54-72 | 50.0 (36.9-60.4) | 38.1 (30.7-44.9) | 0.011 |
| EXTEM MCF | 53-72 | 42.5 (37.0-52.0) | 37.0 (30.0-43.5) | 0.015 |
| INTEM MCF | 53-72 | 44.5 (40.0-49.8) | 37.5 (29.8-45.0) | 0.008 |
- NOTE: Data shown as median (IQR) for each test.
The R time values on kaolin and rapid TEG were not significantly prolonged after reperfusion, but EXTEM-CT and INTEM-CT values after reperfusion were both prolonged by approximately 1.5-fold from the baseline (Table 2). The scatterplots of INR, kaolin TEG, EXTEM, and INTEM demonstrate the similar patterns for INR, EXTEM-CT, and INTEM-CT in which more samples were widely distributed in the abnormal range after reperfusion (Fig. 1). The majority of samples were distributed close to the upper normal limit at baseline and reperfusion for kaolin TEG-R time (Fig. 1). All TEG/ROTEM tests showed decreases in MA or MCF values in parallel with lower fibrinogen after the reperfusion (Table 2).
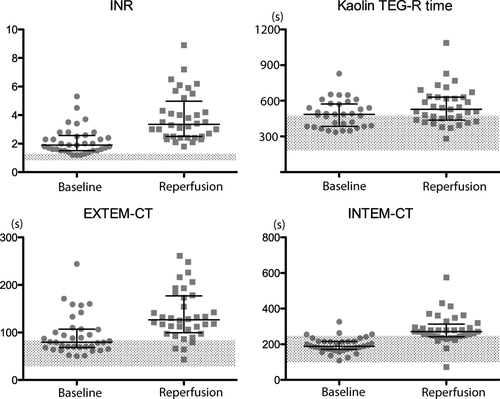
Data scatters for INR, kaolin TEG-R time, EXTEM-CT, and INTEM-CT. The bars indicate the median (long bar) and IQRs (short bars). Shaded areas indicate the normal reference ranges. The median values after the reperfusion showed statistically significant prolongations from the baseline (cf, Table 2).
For the detection of FX below 50%, the sensitivity was best for INR at 98.1%, followed by EXTEM-CT, kaolin TEG-R time, and INTEM-CT at 85.2%, 61.1%, and 55.6%, respectively (Table 3). The sensitivity of rapid TEG-R time was far lower than other tests. The area under the curve (AUC) values of the ROC curves were 0.941 for INR, 0.865 for EXTEM-CT, and 0.798 for INTEM-CT (Fig. 3). The AUCs of kaolin and rapid TEG-R time were below 0.620.
| FX Level (%) | ||
|---|---|---|
| <50% | <35% | |
| INR > 1.5 | ||
| Sensitivity | 98.1 | 100 |
| Specificity | 50.0 | 24.4 |
| PPV | 85.5 | 50.0 |
| NPV | 90.0 | 100 |
| Kaolin TEG-R time > 480 seconds | ||
| Sensitivity | 61.1 | 67.7 |
| Specificity | 50.0 | 68.3 |
| PPV | 78.6 | 50.0 |
| NPV | 30.0 | 66.6 |
| Rapid TEG-R time > 110 seconds | ||
| Sensitivity | 11.1 | 16.1 |
| Specificity | 88.9 | 95.1 |
| PPV | 75.0 | 75.0 |
| NPV | 25.0 | 60.9 |
| EXTEM CT > 80 seconds | ||
| Sensitivity | 85.2 | 90.3 |
| Specificity | 83.3 | 48.8 |
| PPV | 95.8 | 58.3 |
| NPV | 62.5 | 83.3 |
| INTEM CT > 240 seconds | ||
| Sensitivity | 55.6 | 74.2 |
| Specificity | 83.3 | 75.6 |
| PPV | 90.9 | 69.7 |
| NPV | 38.5 | 79.5 |
For the detection of FX below 35%, the sensitivity remained excellent for INR and EXTEM-CT at 100% and 90.3%, respectively, but higher false-positive rates reduced the specificity (Table 3). ROC analysis showed the AUC of 0.893 for INR, and 0.783 for EXTEM-CT. The sensitivity and specificity of INTEM-CT for FX < 35% were approximately 75%, and the AUC of 0.818 was comparable to those of INR and EXTEM-CT. The AUCs of kaolin and rapid TEG-R time remained below 0.664.
Discussion
In our present study, 4 types of viscoelastic tests showed distinctive characteristics in diagnosing moderate (FX < 50%) or severe (FX < 35%) coagulopathy during LT (Table 2; Fig. 1). EXTEM-CT had the highest sensitivity and specificity next to INR for moderate coagulopathy by the ROC analysis (Fig. 2). Severe coagulopathy represented with FX below 35% was most frequently detected by INR and EXTEM-CT (sensitivity, 90%-100%), but false-positive rates increased (Table 3). Importantly, the deficiency of FV, a crucial cofactor of FX, has been observed in severe postreperfusion coagulopathy in LT.12, 19 FV deficiency can partly explain improved sensitivities of INTEM-CT, and to a lesser extent for kaolin and rapid TEG-R time (Table 3). Low prothrombinase formation (FXa-FVa) affects both extrinsic and intrinsic pathways, but this defect is detected earlier using a TF-triggered assay (PT) compared to a contact-activated test in trauma-induced coagulopathy.22 Indeed, Nascimento et al.21 recently showed that kaolin TEG-R time is inferior to INR in detecting PT-sensitive factor deficits due to trauma-induced coagulopathy. In their study, INR of >1.5 and kaolin TEG-R time over 480 seconds demonstrated a sensitivity of 67% and 33%, respectively, for vitamin K–dependent factor levels < 50%. Similarly, Dunham et al.23 reported that kaolin and rapid TEG-R time values were in normal range in 45.5% and 40.9% of warfarin-anticoagulated plasma samples (mean INR, 2.8). In a recent large prospective randomized trial of the ratio-based plasma transfusion in 2 groups of major trauma patients (1:1:1 or 1:1:2 ratio for plasma/platelets/PRBCs; n = 680), abnormal kaolin TEG-R time over 480 seconds was reported in only 4.3% on admission, whereas INR ratio above 1.5 was observed at a higher rate (26%-27%).24 These findings collectively support our present findings that contact-activated viscoelastic tests are less sensitive than TF-activated tests in moderate coagulopathy during LT. There are also differences among different contact activators because the sensitivity differed between INTEM-CT and kaolin TEG-R time in severe FX deficiency (Table 2; Fig. 2).
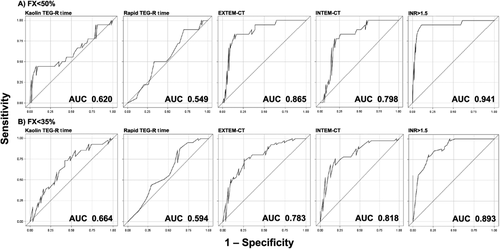
ROC curves for different viscoelastic clotting cutoffs for the detection of FX activity (A) below 50% or (B) below 35%. INR > 1.5, kaolin TEG-R time > 480 seconds, rapid TEG-R time > 110 seconds, EXTEM-CT > 80 seconds, and INTEM-CT > 240 seconds were used as cutoff values.
Early fluid resuscitation is typically implemented to replace surgical blood loss, and large amounts of crystalloid and albumin were administered in our cases (Table 1). It is possible that ongoing hemodilution hindered the increase of coagulation factors despite plasma transfusion. On the contrary, other investigators hypothesized that aggressive fluid resuscitation could paradoxically increase bleeding by increasing central venous pressure and microvascular bleeding. Massicotte et al.25 recently reported their restrictive transfusion (hemoglobin trigger for PRBCs, 5.8 g/dL) including intraoperative phlebotomy and cell salvage during LT. Their 500 patients (median Model for End-Stage Liver Disease [MELD], 23) received the mean (standard deviation) of 0.5 (1.3), 0.2 (1.1), and 0.1 (1.0) units for PRBCs, plasma, and platelets, respectively; 79.6% of their transplant patients were managed without transfusion. There are multiple reasons that may explain these differences in transfusion. First, Massicotte et al.25 used the lower threshold for PRBC transfusion (HCT, 17%-18%), whereas we transfused to maintain HCT above 26%. Second, the starting platelet count was much lower in our patients than in theirs (54 × 103/L versus 93 × 103/L). Some of our patients could have been at a higher risk of bleeding due to thrombocytopenia, although our median MELD score was lower than their median value (18.5 versus 23). Lastly, surgical approaches may be quite different between 2 centers; for example, 19.4% of our patients received a venovenous bypass, which is associated with higher requirements for PRBCs, plasma, and cell salvaged units.18 Taken together, severe coagulopathy after reperfusion was documented in our coagulation tests, and high transfusion requirements were due to multifactorial causes including coagulopathy, transfusion practice, surgical, and anesthetic protocols.
Clinical implementation of cutoff values for EXTEM-CT and INTEM-CT had been previously demonstrated20 during LT. Among their 266 patients, 85.3% were without plasma transfusion, and 71.4% were done without platelet transfusion. Their primary hemostatic therapy was fibrinogen, which was given in 57.5% (n = 153) of patients at the mean dose of 6.3 g. Prothrombin complex concentrate (PCC) was administered in 34.9% (n = 93) of bleeding patients when EXTEM-CT exceeded 80 seconds. Plasma transfusion was given (15-20 mL/kg) when bleeding persisted after PCC and the INTEM-CT exceeded 240 seconds. The authors speculated that plasma was required to correct severe coagulopathy due to FV and/or FXI, but the incidence was less frequent (14.7%; n = 39). Our ex vivo data on the differential use of EXTEM and INTEM are corroborated by their practical experience in using PCC for moderate coagulopathy, and subsequently transfusing plasma for severe coagulopathy. This use of PCC is considered off-label for our institution, but vitamin K–dependent factor replacement with PCC is known to avoid volume overload.26 Low transfusion rates in the study by Kirchner et al.20 may be partly explained by the defined transfusion protocol under viscoelastic testing, and avoidance of high venous pressure by low-volume hemostatic resuscitation. Further clinical studies of PCC are warranted to investigate the efficacy and safety of PCC in this setting.
PT/INR is the most commonly used test in diagnosing coagulopathy associated with liver disease. However, its major disadvantage is a long turnaround time (30-90 minutes),7, 8 and a rapid point-of-care PT (<5 minutes) suffers from a larger bias at higher INR ratios over 1.5, and HCT below 30%.8 Viscoelastic testing with a reasonable turnaround time (15-25 minutes) can overcome some of the limitations of conventional coagulation testing.16, 27
Several important limitations of our study need to be discussed. First, kaolin TEG was the only test used for transfusion management, and thus transfusion volumes were not influenced by any other viscoelastic test. Although a direct clinical comparison of TEG and ROTEM in transfusion outcomes would be ideal, such a trial needs a better understanding of different cutoff values in a specific target population. Our present data provides important preliminary data for future clinical trials. Second, the use of native whole blood for TEGs per our study protocol might have affected their sensitivity to FX levels. However, the aforementioned 2 studies failed to demonstrate a high sensitivity in blood collected in 3.2% sodium citrate.21, 23 Third, the sample size of our study was small (n = 36), and the operating characteristics of TEG and ROTEM in our transplant patients cannot be inferred to other clinical settings without caution.
In conclusion, the present study demonstrates that 4 types of viscoelastic tests perform distinctively in the diagnosis of moderate or severe coagulopathy based on FX deficiency during LT. TF-activated INR and EXTEM-CT performed well in diagnosing FX below 50%, but rapid TEG with combined TF and kaolin activators failed. Improved performance of INTEM-CT (and to a lesser extent, kaolin TEG-R) in diagnosing FX below 35% appears to underlie multifactorial deficiency involving both intrinsic and common pathways.20 Clinical effectiveness of TEG and ROTEM in LT depends on not only the optimal cutoff value(s), but also on the patient demographic as well as anesthetic and surgical practice.4, 18, 20, 25