Longterm outcomes of auxiliary partial orthotopic liver transplantation in preadolescent children with fulminant hepatic failure
The authors received financial support from National Institutes of Health Grant 5T32HL007854-19, the John Jones Surgical Society Research Fellowship, the Louis V. Gerstner Foundation, the Columbia Department of Surgery, the Irving Institute Foundation Grant, and the Kenneth A. Forde Surgical Research Award.
Potential conflict of interest: Nothing to report.
Abstract
By preserving part of the native liver, auxiliary partial orthotopic liver transplantation (APOLT) provides the advantage of potential immunosuppression (ISP) withdrawal if the native liver recovers but has had limited acceptance, especially in the United States, due to technical complications and low rates of native liver regeneration. No previous study has evaluated APOLT specifically for preadolescent children with fulminant hepatic failure (FHF). This population might benefit especially based on greater capacity for liver regeneration. Data from 13 preadolescent children who underwent APOLT were compared to 13 matched controls who underwent orthotopic liver transplantation (OLT) for FHF from 1996 to 2013. There were no significant differences in patient demographics or survival between the 2 groups. However, all surviving OLT recipients (10/13) remain on ISP, while all but 1 surviving APOLT recipient (12/13) showed native liver regeneration, and the first 10 recipients (76.9%) are currently off ISP with 2 additional patients currently weaning. In our experience, APOLT produced excellent survival and high rates of native liver regeneration in preadolescent children with FHF. This represents the largest series to date to report such outcomes. Liberating these children from lifelong ISP without the downside of increased surgical morbidity makes APOLT an attractive alternative. In conclusion, we therefore propose that, with the availability of technical expertise and with the technical modifications above, APOLT for FHF should be strongly considered for preteenage children with FHF. Liver Transplantation 22 485-494 2016 AASLD
Abbreviations
-
- APOLT
-
- auxiliary partial orthotopic liver transplantation
-
- CT
-
- computed tomography
-
- FHF
-
- fulminant hepatic failure
-
- HAT
-
- hepatic artery thrombosis
-
- HIDA
-
- hepatobiliary iminodiacetic acid
-
- INR
-
- international normalized ratio
-
- ISP
-
- immunosuppression
-
- IVC
-
- inferior vena cava
-
- OLT
-
- orthotopic liver transplantation
Auxiliary partial orthotopic liver transplantation (APOLT) refers to a transplant that replaces part of the liver with an orthotopically placed allograft. Thus, the recipient gains a partial liver allograft while retaining part of the native liver. This procedure was first reported by Bismuth's group in Villejuif, France in 1985.1 It has been used for treating metabolic disorders,2, 3 cirrhosis,4 viral hepatitis,5 and small-for-size syndrome.6 In 1991, Gubernatis et al.7 from Hanover, Germany reported the first use of APOLT to treat fulminant hepatic failure (FHF),8 which affects approximately 50 preadolescent children per year.9 Since then, several case reports or case series have been published regarding APOLT for FHF, including by our group.5, 10-17 One of the greatest advantages of APOLT is the opportunity to withdraw immunosuppression (ISP) entirely in the event of native liver regeneration. There have been several reports of this ideal outcome: Rosenthal et al.11 (1/1 withdrawn); Azoulay et al.18 (2/12); Jaeck et al.12 (6/15); Pereira et al.19 (2/7); Kobayashi et al.20 (1/5); Kobayashi et al.5 (1/1); and Faraj et al.17 (11/20).
Despite these possible beneficial outcomes, use of APOLT for FHF has been met with reluctance due to the technical complexity of the surgery, its relatively high morbidity, especially vascular thrombosis, biliary leaks, and strictures, and lack of improved outcomes.18 In fact, reluctance is so great that we are currently the only group in the United States performing APOLT to our knowledge. However, 4 relatively small case series of APOLT for FHF give renewed consideration to the use of APOLT specifically in children younger than 10 years of age due to significantly better survival and liver regeneration rates (see Table 1 for summary of outcomes based on age).16, 17, 21, 22
| Number of APOLT Recipients | Survival Rate, % | ISP Weaning, % | |||||
|---|---|---|---|---|---|---|---|
| Study | Total Recipients | Recipients <10 Years of Age | Overall Survival | Recipients <10 Years of Age | Overall Rate | Recipients ≥10 Years of Age | Recipients <10 Years of Age |
| Sudan et al. (1997)21 | 7 | 4 | 57.1 | 75.0 | 85.7 | 66.7 | 100 |
| Kato et al. (2006)16 | 6 | 6 | 100 | 100 | 16.7 | – | 16.7a |
| Faraj et al. (2010)17 | 20 | 8 | 85.0 | 100 | 55.0 | 25.0 | 75.0b |
| Quaglia et al. (2008)22 | 40 | 6 | 87.5 | 100 | 50.0 | 47.1 | 83.3c |
- a There was some degree of regeneration in all patients at time of publication, and all 6 subsequently survived and were weaned off ISP.
- b In the cohort of patients younger than 10 years old, 6/8 were weaned from ISP at time of publication, but 8/8 (100%) had native liver regeneration.
- c If those being actively weaned are included, these percentages are 62.5% (all patients), 58.8% (cohort ≥10 years old), and 100% (cohort <10 years old).
On the basis of these encouraging preliminary data that APOLT might be especially beneficial for preadolescent children with FHF, we continued to offer APOLT to these patients. We herein present our longterm outcomes of 13 children aged <10 years who underwent APOLT for FHF. Results are compared to 13 age-matched children who received whole liver allografts for FHF at the same centers.
Patients and Methods
Patient Selection and Follow-up
The 2-center retrospective chart review was approved by the Columbia University institutional review board (protocol IRB-AAAM8706). The centers were University of Miami/Jackson Memorial Hospital and New York Presbyterian–Columbia University Medical Center. Our APOLT cohort consisted of all children younger than 10 years of age who underwent APOLT for FHF (n = 13) at the participating centers, 6 of whom were included in a previous report. Because there is no other adequate age-matched control group available for comparison in the literature and data in national registries is heterogeneous and center-specific, we chose an age-matched group of 13 children with FHF who underwent conventional orthotopic liver transplantation (OLT) at the same institutions. The control population includes all children in this age group who received OLT for FHF at either institution from 1996 to 2013. Therefore, the APOLT and OLT groups represent the entirety of our experience transplanting preteenaged children for FHF during this time period. Both groups were well matched for demographics and pretransplant state of health (see Tables 2 and 3). The only significant difference between groups is that all APOLT surgeries except 1 were performed at later time points. There were no specific selection criteria to determine which patients received OLT versus APOLT. Instead, all patients starting in 2005 were offered both APOLT and OLT without regard to the etiology of the FHF. The parents were informed in detail about the risks and benefits of each, which include increased hepatic artery thrombosis (HAT), biliary complications, neurologic sequelae, and retransplantation for APOLT and increased rejection and inability to wean ISP for OLT. The parents ultimately chose between the 2 approaches. All chose APOLT. Exclusion criteria, which none of the patients met for APOLT, were suspicion of cerebral edema, multiorgan failure, and hemodynamic instability. Surgeries were performed at the University of Miami between 1996 and 2009 and at New York Presbyterian–Columbia University Medical Center between 2003 and 2013. Of the APOLT surgeries, 9 took place in Miami and 4 at New York Presbyterian–Columbia University Medical Center. All but 2 OLT surgeries were performed in Miami. Except for the 1996 case, all other APOLT cases were performed after 2005, because APOLT became routinely offered at that point. Liver regeneration was assessed using computed tomography (CT) to measure volumetric changes, ultrasound to measure blood inflow and outflow, nuclear medicine scan (technetium-99m or hepatobiliary iminodiacetic acid [HIDA]) to assess hepatocyte activity, and biopsy to assess histology.
| Patient | Transplant Date | Etiology | Patient Age at Transplant | Donor Age, Years | ISP (Current) | Complications |
|---|---|---|---|---|---|---|
| 1 | August 1, 1996 | Hepatitis A | 6 years, 6 months | 52 | Off | Bile leak |
| 2 | April 17, 2005 | Idiopathic | 4 years, 2 months | 17 | Off | Bowel obstruction; acute rejection episode |
| 3 | September 14, 2005 | Autoimmune Hepatitis | 1 years, 0 months | 4 | Off | Acute rejection episode |
| 4 | October 31, 2005 | Idiopathic | 8 years, 2 months | 40 | Off | HAT requiring retransplantation; acute rejection episode |
| 5 | November 11, 2005 | Idiopathic | 1 years, 6 months | 10 | Off | Acute rejection episode |
| 6 | March 6, 2006 | Idiopathic | 8 months | 19 | Off | Biliary strictures; abdominal abscess |
| 7 | August 7, 2007 | Idiopathic | 2 years, 1 month | 32 | Off | Bowel obstruction |
| 8 | July 1, 2009 | Idiopathic | 6 years, 7 months | 59 | Off | None |
| 9 | October 22, 2009 | Autoimmune hepatitis | 1 years, 9 months | 33 | Azothioprine (note: treatment for autoimmune hepatitis, not rejection) | Autoimmune hepatitis |
| 10 | November 26, 2010 | Drug toxicity (Azithromycin) versus Autoimmune hepatitis | 7 years, 6 months | 4 | Mycophenolate mofetil (note: treatment for autoimmune hepatitis, not rejection) | Abscess; autoimmune hepatitis |
| 11 | August 6, 2012 | Idiopathic | 3 months | 13 | Tacrolimus; mycophenolate mofetil | Abscess; fungal peritonitis; voriconazole overdose; idiopathic; thrombocytopenic purpura; respiratory failure |
| 12 | November 16, 2012 | Idiopathic | 2 years, 1 month | 2 | Weaning | CM viremia |
| 13 | January 30, 2013 | Idiopathic | 3 years, 3 months | 15 | Weaning | Anastomotic bleed |
| Patient | Transplant Date | Etiology | Patient Age at Transplant | Donor Age, Years | Complications |
|---|---|---|---|---|---|
| 1 | January 23, 1997 | Idiopathic | 2 years, 4 months | 45 | Bile leak; bowel perforation; primary graft nonfunction requiring retransplantation; sepsis → death |
| 2 | August 21, 1997 | Idiopathic | 7 years, 0 months | 15 | None |
| 3 | March 30, 1998 | Idiopathic | 5 years, 1 month | 10 | Aplastic anemia; sepsis → death |
| 4 | July 25, 1998 | Idiopathic | 7 years, 2 months | 8 | Acute rejection episode |
| 5 | August 24, 1998 | Idiopathic | 5 years, 0 months | 50 | None |
| 6 | April 14, 1999 | Idiopathic | 2 years, 8 months | 21 | Acute rejection episode |
| 7 | January 23, 2000 | Idiopathic | 10 months | 0.13 | Esophageal perforation; HAT requiring retransplantation; diabetic ketoacidosis → death |
| 8 | March 13, 2003 | Idiopathic | 7 years, 0 months | 11 | Acute rejection episode |
| 9 | April 21, 2003 | Idiopathic | 3 years, 1 month | 8 | Bile leak; fungal peritonitis; pneumonia; gastrointestinal bleed; acute rejection episode |
| 10 | October 9, 2003 | Idiopathic | 6 years, 6 months | 47 | Inflammatory bowel disease |
| 11 | February 20, 2004 | Idiopathic | 2 years, 4 months | 2 | Acute rejection episode; chylothorax |
| 12 | July 4, 2004 | Idiopathic | 5 years, 10 months | 37 | None |
| 13 | September 9, 2006 | Idiopathic | 8 years, 7 months | 5 | Wound infection |
Technical Approach to APOLT Surgery
We first remove the recipient's left liver lobe using the standard technique, preserving the middle hepatic vein. We perform an extensive dissection around the left suprahepatic inferior vena cava (IVC), ligating all vessels including the left phrenic veins. The purpose of this is to create additional space to elongate the left hepatic orifice, ensuring that outflow is as wide as possible.
On the back table, the structures of the hepatic hilum are dissected in the usual manner for OLT. Left lateral segments or left lobes are prepared in the standard manner for split-liver grafts. The middle hepatic vein is preserved for left lobes but not left lateral segments.
Using the extra space created in the dissection of the suprahepatic IVC, the recipient's left hepatic vein orifice is extended onto the left cranial aspect of the IVC and anastomosed to the donor hepatic vein to create a large outflow tract (Fig. 1).
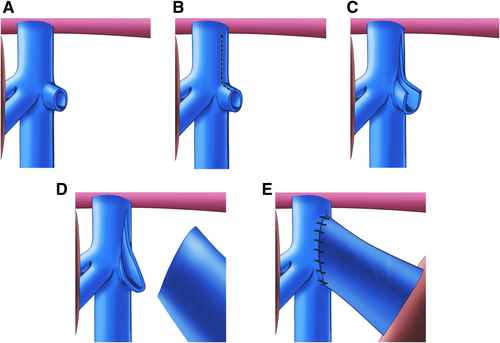
The native left portal vein is resected at its takeoff from the portal bifurcation, and its orifice is enlarged proximally. The donor portal vein is then anastomosed to the enlarged native left portal vein orifice with or without an interpositional iliac vein graft (Fig. 2).

A portion of the donor carotid or iliac artery is anastomosed to the infrarenal aorta and used as an arterial conduit between the aorta and the donor celiac artery. The purpose of this conduit is to avoid anastomosis to the small-caliber native left hepatic artery. It might also prevent a steal from the allograft to the native liver, which can occur once the native liver has recovered if the allograft is instead connected to the left hepatic artery.
The bile duct is anastomosed to the small intestine in an end-to-side fashion over an internal stent after the creation of a Roux-en-Y.
Grafts
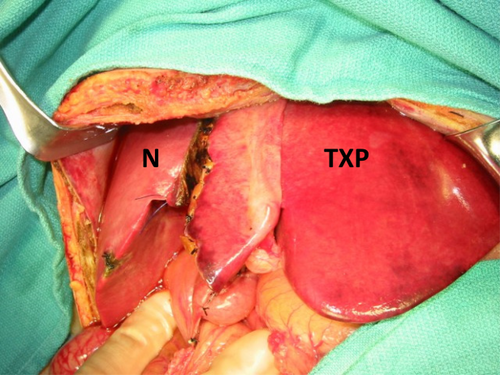
All APOLT grafts were either left lateral segments or left lobes (Fig. 3).
Postoperative Management
The ISP regimen was equivalent for both cohorts for at least the first 6 months per center protocol. This 6-month period was chosen for the APOLT group because avoiding rejection of the life-supporting graft was the most important goal during this time period. After that, decisions regarding weaning were made based on evidence of native liver regeneration. Specifically, if the graft had no evidence of rejection based on laboratory values, we evaluated the native liver function with technetium-99m sulfur colloid scan (hepatobiliary scan) or HIDA to evaluate native liver function, CT to evaluate size and appearance of the native liver, and ultrasound to evaluate blood flow to the native liver. Imaging was repeated approximately every 3-6 months until the native graft appeared normal, at which point biopsy of both livers was performed for confirmation. The criteria for beginning ISP withdrawal were good liver function with normal anatomy, blood flow, and histology. Steroids were weaned first, followed by mycophenolate mofetil then tacrolimus. Tacrolimus was decreased by approximately 20% at each follow-up visit as long as transaminase levels remained below 100 ng/dL. Above this level, weaning was suspended and biopsy of both livers was performed to confirm rejection of the graft and rule out disease of the native liver. If the native liver was confirmed to be normal, weaning proceeded more quickly as enzyme elevations could be attributed to rejection.
Statistics
Mean values of continuous data were compared using Student t tests or nonparametrics as appropriate. Categorical variables were compared using the chi-square test. Survival analysis (log-rank test) was performed using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA).
Results
Patient Demographics
Patient demographics are summarized in Tables 2 and 3. For the APOLT versus OLT groups, the average age was 3.5 ± 2.8 versus 4.9 ± 2.4 years; sex was 84.6% male versus 53.8% male; donor age was 23.1 ± 18.6 versus 19.9 ± 18.2 years; mean pretransplant total bilirubin was 19.8 versus 23.1 mg/dL; and mean pretransplant international normalized ratio was 3.9 versus 4.5. There were no statistically significant differences in these values between groups (see Table 4 for comparison of groups). There was similarly no significant difference in average encephalopathy level for the APOLT versus OLT groups (2.8 versus 3.4; P = 0.16). No patients in either group had failure of any other organ system. The etiology of FHF was idiopathic for all recipients of OLT. For recipients of APOLT, etiology of FHF was idiopathic (n = 9), definitive acute autoimmune hepatitis (n = 2), acute autoimmune hepatitis versus drug toxicity from azithromycin (n = 1), and hepatitis A (n = 1). Of the OLT group, 5 (38.5%) received partial liver grafts and 8 (61.5%) received whole organs. Donor-to-recipient weight ratio was 1.7 ± 0.7 versus 0.8 ± 0.5 for the APOLT and OLT groups, respectively.
| APOLT (n = 13) | OLT (n = 13) | P Value | |
|---|---|---|---|
| Age at transplantation, mean ± SD, year | 3.5 ± 2.8 | 4.9 ± 2.4 | 0.19 |
| Donor age, mean ± SD, year | 23.1 ± 18.6 | 19.9 ± 18.2 | 0.67 |
| Recipient sex, male, n (%) | 11 (84.6) | 7 (53.8) | 0.20 |
| Pretransplantation weight, mean, pounds | 39.3 | 39.1 | 0.98 |
| Pretransplantation total bilirubin, mean, mg/dL | 19.8 | 23.1 | 0.15 |
| Pretransplantation INR | 3.9 | 4.5 | 0.51 |
| Time from admission to transplantation, mean ± SD, days | 3.5 ± 2.4 | 6.2 ± 5.5 | 0.14 |
| Time from transplantation to discharge, median ± SD, days | 15 ± 19.2 | 17 ± 38.9 | 0.42 |
| Overall survival, n (%) | 13 (100.0) | 10 (76.9) | 0.22 |
| Graft failure, n (%) | 1 (7.7) | 2 (15.4) | 0.99 |
| Retransplantation, n (%) | 1 (7.7) | 2 (15.4) | 0.99 |
Clinical Course and Complications
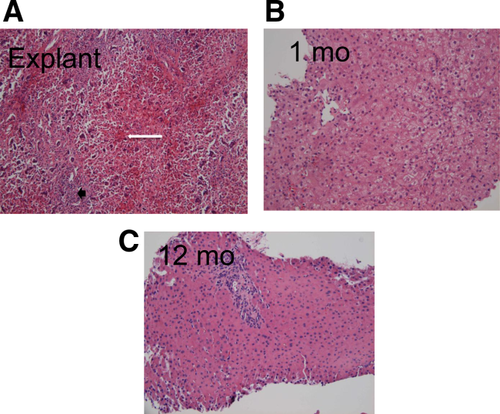
Clinical course and outcomes for both groups are compared in Table 4. Complications are summarized in Tables 2 and 3. At time of transplantation, native liver histology of all specimens showed submassive to massive necrosis without fibrosis or regenerative nodules (Fig. 4). No patients received either steroids or treatment specific for autoimmune hepatitis before transplantation, even if this etiology was suspected. Initial ISP for all patients was tacrolimus and steroids ± mycophenolate mofetil. Steroids were weaned at 3-6 months after transplantation. Average follow-up in the APOLT versus OLT group was 6.3 years (range, 1.2-17.3 years) versus 10.1 years (range, 0.1-16.3 years; P = 0.12). Median length of follow-up of surviving patients was 6.3 years in the APOLT group versus 10.8 years in the OLT group. There were 2 graft failures in the OLT group and 1 in the APOLT group. The 1 graft failure in the APOLT group resulted from HAT, and the patient is alive and well after retransplantation, keeping the native liver in place (redo APOLT). Both of the graft failures in the OLT group led to patient death, 1 after retransplantation for primary graft nonfunction and 1 after retransplantation for HAT. Overall, there were 3 patient deaths in the OLT group and none in the APOLT group, representing a nonsignificant trend toward improved survival after APOLT compared to OLT (P = 0.08 for log-rank test of survival curves). The deaths were due to sepsis (n = 2) and diabetic ketoacidosis (n = 1). The 1-, 3-, and 5-year survival in the OLT group was 84.6%, 76.9%, and 76.9%. In contrast, of all APOLT patients whose transplant date made them eligible to reach these time points, all remain alive. One-year graft survival was more similar between the groups: 92.3% in the APOLT group versus 84.6% in the OLT group (P > 0.99). Of the 2 failed OLT grafts, 1 was a partial liver graft and 1 was a whole organ.
For the OLT group, complications included acute rejection episodes (n = 4), aplastic anemia (n = 2), bile leak (n = 2), bowel perforation (n = 2), HAT (n = 1), gastrointestinal bleed (n = 1), fungal peritonitis (n = 1), pneumonia (n = 1), pleural effusions (n = 1), primary graft nonfunction (n = 1), esophageal perforation (n = 1), and chylothorax (n = 1). For the APOLT group, incidence of acute rejection episodes before ISP withdrawal was 30.8% (4/13). Other postoperative complications included abscess (n = 3), recurrent autoimmune hepatitis (n = 2), HAT (n = 1), bile leak (n = 1), biliary stricture (n = 1), bowel obstruction (n = 1), anastomotic bleed (n = 1), fungal peritonitis (n = 1), and cytomegalovirus viremia (n = 1).
Native Liver Regeneration and ISP Weaning
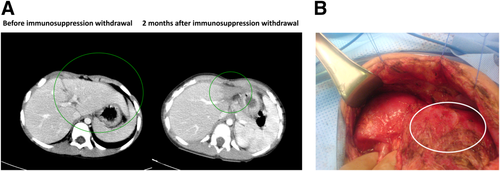
Twelve of 13 (92.3%) native livers have regenerated after APOLT. The first 10 (76.9%) APOLT recipients are currently off ISP with fully regenerated native livers, and ISP is being weaned in the remaining 2 whose livers have regenerated. Of those weaned, the median time from transplantation to discontinuation was 1010 ± 642 days. Histology was used to confirm native liver recovery before instituting ISP withdrawal (Fig. 4). Average time to native liver recovery based on histology was 406.2 ± 352.8 days. Noninvasive imaging also confirmed normal native liver perfusion and function in the patients who were weaned. In contrast, normal perfusion and radiotracer uptake were never seen in the 1 native liver that did not regenerate. Although none of these tests prove that the native liver is functional, this was demonstrated ipso facto by the ability of the native liver to function after the graft atrophies in the absence of ISP. After ISP withdrawal, the allograft liver became completely atrophic in 7 patients, after which no further intervention was required, and was removed in 2 other patients in whom the atrophic graft became infected (Fig. 5).
Discussion
In our experience with APOLT for young children with FHF, which is the largest reported cohort to date, we have demonstrated excellent outcomes. Neither the overall survival nor graft survival rates differed significantly between the APOLT and OLT groups. However, most of the children who received APOLT were later able to be weaned entirely from ISP when their native livers recovered, thus sparing them a lifetime of immunosuppressive therapy and its associated risks of infection and posttransplant lymphoproliferative disease. These results confirm the findings in previous case series, which had smaller cohorts of children younger than 10 years old and which did not aim to evaluate this specific population. In 1997, Sudan et al.21 reported on 7 patients (3 teenagers and 4 children of <10 years old) after APOLT for FHF. Although overall survival was 57%, 3 of 4 patients younger than 10 years old survived with native liver regeneration allowing complete withdrawal from ISP. In 2006, our group presented and published our preliminary data of early outcomes of 6 patients of APOLT for FHF with 100% survival and evidence of some degree of native liver regeneration in all patients and total weaning of ISP in 1 patient.16 More recently, the King's College group published their outcomes in children with FHF in 2008 and 2010.17, 22 They included 20 children (12 teenagers and 8 children of <10 years old) and showed 85% longterm patient survival with significant rates of native liver regeneration and 11/20 patients completely weaned from ISP. Notably, however, all children younger than 10 years old survived and showed native liver regeneration.
We emphasize that, even though almost all patients could be weaned from ISP due to native liver regeneration, lack of liver recovery and ISP withdrawal is not a failure of this approach. Instead, it simply represents the current standard of care (ie, transplantation followed by lifelong ISP). We acknowledge that 1 limitation of this analysis is that all OLT cases were performed in the years before the APOLT cases, and the temporal distribution might bias the results if more recent cases have better outcomes.
We believe the biggest reason for our successful outcomes is our patient selection. As described above, APOLT has not been shown to have better outcomes than OLT in most previous studies; however, best results seem to occur in younger patients.17 Young children may benefit most from APOLT for FHF for 3 reasons. First, they have greater potential for native liver regeneration.23 Second, they would most benefit from being spared lifelong ISP because of their presumed long projected life span.24 Third, it is likely that the smaller size of children younger than 10 years old is another reason for the greater success of APOLT in these patients, as they can tolerate a left lobe, which makes the procedure less technically demanding.
Our excellent outcomes might also be attributed to our development of several technical modifications to the APOLT procedure. First is the extensive dissection of the left aspect of the suprahepatic IVC, allowing the hepatic vein anastomosis to be performed in a way that maximizes the outflow tract. Second is the creation of an extended native portal vein orifice for anastomosis and use of an aortic conduit for maximizing inflow. The portal vein modification also decreases the risk of thrombosis, which was shown to occur more frequently in APOLT in 1 study.25 Maximizing portal inflow to the graft is important because the native liver often outcompetes the graft for portal flow, sometimes leading to failure.14, 26-28 In FHF, however, the opposite is true because of the increased presinusoidal pressure resulting from necrosis, further favoring the graft initially.29 Over time, the regenerated native liver draws more flow, favoring its survival and causing atrophy of the graft that is no longer needed. This makes APOLT particularly suited to the treatment of FHF because portal flow is naturally diverted toward the liver that needs it most at any given time.
One potential drawback of using APOLT for young children with FHF is the risk of disease recurrence in the native liver. This occurred in 2 APOLT recipients, who are now managed with either low-dose mycophenolate mofetil or azathioprine. However, this low-level ISP is less toxic than the higher dose of calcineurin inhibitors they would have required indefinitely after OLT. Another potential drawback is that our technical modifications could delay regeneration of the native graft. The comparatively larger left portal vein may cause a steal of portal blood flow that would limit inflow to the native graft, thus inhibiting repair and regeneration. This was not apparent in most cases but might have been the case with patients 9 (delayed native liver recovery) and 11 (failed native liver recovery), who had the most extreme size discrepancy. We believe that this risk is worthwhile because the most important purpose of liver transplantation in FHF is to provide adequate initial graft function. However, very small children might be less favorable candidates for APOLT.
The use of APOLT has not yet gained momentum in the United States outside of our group, despite these potential benefits in children with FHF, although it is still performed in Europe. Perhaps this is because of the technical challenges and potential morbidity of the APOLT operation. It may also be due to the somewhat less successful outcomes in adult patients with FHF. Even in young children, APOLT might still not be ideal for all patients. Although all of our recipients were hemodynamically stable before transplantation, surgeons should use caution in choosing APOLT for extremely ill patients because they may not tolerate the more complex surgery. However, we have demonstrated that APOLT can be performed with acceptable morbidity and excellent outcomes with our technical modifications, especially in preteenage children. We therefore propose that, with the availability of technical expertise and with the technical modifications above, APOLT for FHF should be considered for preteenage children with FHF, especially by other American centers that have thus far shown little interest in the procedure. Moreover, we believe that either a prospective study or randomized controlled study with a larger cohort is indicated to confirm our findings. Finally, increased performance of APOLT will allow evaluation of longterm health outcomes between APOLT recipients off ISP and traditional OLT recipients, which requires a large cohort who have survived 10-20 years after their transplants, and which we hypothesize will further validate the use of APOLT.
Acknowledgements
The authors thank medical illustrator Peter Kuempel, M.A.M.S., of the COACH Surgery program/CollectedMed at Columbia University for Figs. 1 and 2. All illustrations were used with permission from CollectedMed.