Presentation, diagnosis, and management of early hepatic venous outflow complications in whole cadaveric liver transplant
Potential conflict of interest: Nothing to report.
Abstract
Early hepatic venous outflow obstruction (HVOO) can be a devastating complication leading to graft loss after liver transplantation (LT). A retrospective study on 777 adult LT recipients over a 5-year period (August 2007 to August 2012) was undertaken to determine the incidence of early HVOO presenting within 3 months of transplant, its clinical features and management, and potential technical risk factors related to the implanting technique. Cases of early HVOO were screened for by identifying recipients with problematic ascites within 3 months of transplant. Definitive diagnosis for HVOO was based on a wedge pressure of >12 mm Hg. Considering only whole livers, the incidence of early problematic ascites was 3% (20/695) of which more than one-third (35%, 7/20) were then confirmed to have HVOO. Overall, the incidence of early HVOO was 1% (7/695). Two hepatic veins (HVs) with extension piggybacks (PBs; n = 423) were the dominant implanting technique in the time period of study rather than the 3 HV PB (n = 182) and caval replacement techniques (n = 82). Considering the implantation technique, all cases of HVOO occurred after 2 HVs when extension PBs had been used with an incidence of 1.7% (7/423). Institutionally, early HVOO was mainly managed surgically by either cavoplasty within a month of transplant (n = 4) or retransplant (n = 1), and the remainder (n = 2) were medically managed with diuretics. In conclusion, early HVOO is rare, and there is no evidence from this study that a given implantation technique is at a higher risk of developing HVOO (2 HV with extension versus 3 HV and caval replacement; P = 0.11). However, early revisional surgery for HVOO can preserve graft function with retransplantation being reserved for when surgical cavoplasty or radiological stenting is technically not possible. Liver Transpl 21:914-921, 2015. © 2015 AASLD.
Abbreviations
-
- AIH
-
- autoimmune hepatitis
-
- ALF
-
- acute liver failure
-
- CLD
-
- chronic liver disease
-
- CT
-
- computed tomography
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after cardiac death
-
- FHV
-
- free hepatic vein
-
- GRWR
-
- graft-to-recipient weight ratio
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HV
-
- hepatic vein
-
- HVOO
-
- hepatic venous outflow obstruction
-
- ICB
-
- intracerebral bleed
-
- IVC
-
- infrahepatic cava
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NT
-
- neurotrauma
-
- PB
-
- piggyback
-
- PSC
-
- primary sclerosing cholangitis
-
- RA
-
- right atrial
-
- SAH
-
- subarachnoid hemorrhage
-
- TPCS
-
- temporary portocaval shunt
-
- VBDS
-
- vanishing bile duct syndrome
-
- VV
-
- veno-veno
Conventional liver transplantation (LT) was first described with caval replacement.1 Drawbacks to this approach of implantation were the reduction in venous return, splanchnic bed congestion and low renal flow, which were addressed with the use of venovenous bypass. To preserve the retrohepatic cava and counter these problems, the piggyback (PB) LT was developed, where the donor suprahepatic cava was anastomosed to the recipient common orifice of 2 hepatic veins (HV), either the left and middle HV or right and middle HV.2, 3 The PB technique was further refined by the development of the 3 HV PB.4, 5 Fundamental to the advantages gained from PB implantation are the temporary portocaval shunt (TPCS)6 and partial side clamping of the cava that minimize recipient hemodynamic disturbances and bowel congestion.7 Additional modifications to the classical PB LT implanting anastomosis were side-to-side,6 end-to-side cavocavostomy8 with additional consideration for the outflow anastomosis being required for living-related, split, reduced, and auxiliary grafts.9 In all implanting techniques, there are reported problems with hepatic venous outflow obstruction (HVOO)9-12 with the incidence increasing up to 15% in partial grafts.9, 13
This article describes a 5-year single-institute experience of early HV outflow complications in adult cadaveric whole-graft recipients focusing on its presentation, diagnosis, technical risk factors, and management.
PATIENTS AND METHODS
Patient Group: Identifying, Diagnosing, and Definition of Early HVOO
A retrospective analysis of 777 consecutive LTs over a 5-year period (August 2007 to August 2012) at a single institution was undertaken. Pediatric recipients (<16 years), living-related, auxiliary, and reduced/split livers were excluded from the analysis. Clinical data were extracted from prospectively maintained transplant and theatre databases, then correlated with electronically documented clinical outcome (iSOFT Clinical Manager Client, version 1.4.579 Computer Sciences Corporation, USA) and supplemented where need be with review of traditional article clinical notes. The study was performed with institutional ethical approval and within the remit of the ethical guidelines of the 1975 Declaration of Helsinki.
Recipients with potential early outflow problems were identified as recipients with problematic ascites, requiring diuretics (spironolactone and/or frusemide) in the first 3 months after LT, as recorded on discharge summaries, clinic follow-up letters, or identified from their listing diagnosis for retransplant. Investigations for recipients with problematic ascites typically included ascitic fluid analysis for albumin, amylase, triglycerides, bacterial culture, cell count, and cytology. Screening ultrasound was performed for vascular anatomy patency and flow direction, supplemented with liver biopsy if required. A computed tomography (CT) scan was then used for visualization of the vascular implanting anatomy and graft appearance. If preceding investigations remained negative and outflow remained a diagnostic concern, then venography and pressure studies were performed.14-17 At the time of venography, transjugular liver biopsy was often performed if there was no recent biopsy. Definitive diagnosis of HVOO was made if the wedged HV pressure was greater than the sinusoidal pressure, ie, >12 mm Hg.18
Surgical Techniques
Donation after brain death (DBD) and donation after cardiac death (DCD) livers were procured using standard techniques. Only controlled DCD livers were used (category 3 and 4) according to Maastricht classification19 and a modified Casavilla's super-rapid technique was employed, with emphasis on rapid donor hepatectomy.20, 21 Institutionally, the maximum accepted warm ischemia time (from oxygen saturations of 70% or systolic pressure of 50 mm Hg to aortic cannulation) was 30 minutes. The preservation solution was University of Wisconsin in all cases.
Implanting techniques used during the period of study included both caval replacement and PB. In PB implantation, 3 HV, 2 HV with extension, side-to-side, and cavostomy/triangulation variants were used. In PB implantation, a TPCS was typically fashioned.3 Institutionally, veno-veno (VV) bypass is no longer routine but was used on a selective basis where the recipient was not tolerant of a trial cross clamp as reflected by mean arterial pressures of less than 60 mm Hg or if there was excessive bleeding during hepatectomy. In cases of domino transplant, institutional practice was to put the amyloid donor on VV bypass to facilitate hepatectomy en bloc with cava. The amyloid liver would then be implanted using the PB technique, and the amyloid donor would have caval implantation.
Operative cavoplasty for HVOO involved Pringle of the hilus, clamping of the suprahepatic and infrahepatic cava. The caval anastomosis was then taken down, and the common orifice of the recipient HVs and donor cava were refashioned to facilitate triangulation of the anastomosis that was sutured with 4/0 Prolene. VV bypass facilities were available if needed.
Statistical Analysis
Results are reported as mean ± standard deviations or percentages. Continuous variables were compared using the Wilcoxon rank sum test and categorical variables were entered into contingency tables to be evaluated by the Pearson chi-square and Fisher's exact test. Statistical significance was set at P < 0.05. Analysis was performed using SPSS, version 17 (IBM, Armonk, NY).
RESULTS
Transplant Activity
Over the 5-year period of study 777 adult transplants were done, of which 25 were living related, 49 right lobes, 2 left lobes, and 6 auxiliary. The remainder (n = 695) received whole cadaveric livers of which DCD made up 24%, DBD made up 75%, and domino made up 1% of grafts used (see Table 1). Considering only recipients of whole livers, the average age was 55 ± 2 years with an average Model for End-Stage Liver Disease (MELD) score of 14 ± 2. Retransplantation made up 10% (n = 69/695) of transplant activity. Chronic liver disease (CLD; 85%, n = 592/695) was the main indication for transplant and acute liver failure (ALF; 15%, n = 103/695) accounted for the remainder (see Table 1). In CLD, the leading reasons for transplant were alcohol-related liver disease (23%, n = 136/592), hepatitis C virus (HCV; 17%, n = 101/592), primary sclerosing cholangitis (PSC; 8%, n = 50/592), primary biliary cirrhosis (8%, n = 47/592), and hepatocellular carcinoma (HCC; 22%, n = 131/592).
| Venous Implantation Technique | |||
|---|---|---|---|
| Recipient and Liver Graft Characteristics | Caval Replacement, n = 82 | 3 HV PB, n = 182 | 2 HV With Extension PB, n = 423 |
| Recipient age, years | 44.9 ± 14.9 | 51.4 ± 21.1 | 50.2 ± 13.5 |
| MELD | 14 ± 6.3 | 14.7 ± 5.9 | 15.3 ± 6.7 |
| CLD/ALF | 73/9 | 156/26 | 355/68 |
| Redo LT | 20 | 11 | 38 |
| DBD/DCD/Domino | 68/14/0 | 132/46/4 | 312/105/6 |
- NOTE: The total number of whole cadaveric LTs presented n = 687, excluded from summary are PB variants of triangulation (0.5%, n = 3) and side to side (0.8%, n = 5) that are used only sporadically. Continuous data were presented as mean ±standard deviation. Implantation techniques summarized caval replacement, 3 HV PB, and 2 HV with extension PB. For each implantation technique, a breakdown of number of cases was done for ALF, CLD, redo LT, and MELD score. Also for each implantation technique, a summary was shown of donor livers numbers from domino recipient, DBD, and DCD.
Implantation Techniques
Table 1 summarizes the main implanting techniques used in the time period of study. PB was the dominant approach for implantation in 88% of cases (n = 613/695) over caval replacement (12%, n = 82/695), and of the PB variant with 2 HVs with extensions was favored (69%, n = 423/613), followed by 3 HVs (29.7%, n = 182/613) with triangulation (0.5%, n = 3/613), and side to side (0.8%, n = 5/613). VV bypass was used in 8% (n = 55/695) of transplants over the time period studied, typically being used in the retransplants (31%, n = 17/55), acute Budd-Chiari (15%, n = 8/55), and familial amyloid polyneuropathy (18%, n = 10/55). In ALF (n = 103), a PB approach was preferred (91%, 94/103) with caval replacement being used only occasionally (9%, 9/103). ALF recipients, where caval replacement was the favored implanting technique, was in the setting of acute Budd-Chiari (n = 8) or an early retransplant for HVOO (n = 1).
Early Ascites After Transplant
Of the 695 recipients who received a whole liver, 3% (n = 20/695) were identified with symptomatic ascites needing medical management and investigation in the first 3 months after transplant. The majority were still inpatients, 75% (n = 15/20), presenting with either ongoing high drain losses (n = 8) and/or abdominal distension (n = 11), and 1 patient was associated with a pleural effusion. The readmissions with symptomatic ascites, 25% (n = 5/20), typically occurred at a month after transplant with the presenting complaint of abdominal distension. After further investigation (CT, pressure studies, and biopsy), just more than one-third (35%) of these early cases of ascites were confirmed to have HVOO (n = 7). The remaining diagnoses of early ascites after transplant were initial poor graft function (n = 3), chylous fluid loss (n = 2), no clear diagnosis (n = 4), veno-occlusive disease (n = 2), pancreatitis (n = 1), and portal vein stenosis (n = 1). These non-HVOO early cases of ascites were managed medically, and the portal vein stenosis was stented by interventional radiology.
Characteristics and Management of Early HVOO
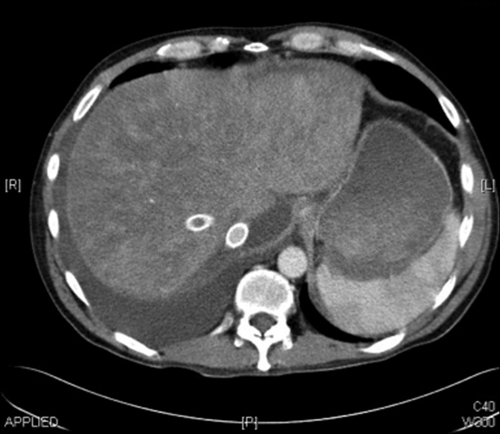
Seven cases of early HVOO were identified; these cases were dispersed throughout the 5-year period of study (Table 2 summarizes recipient and donor clinical characteristics). Overall, the incidence of early HVOO was 1% (7/695) with diagnosis being confirmed at 35 ± 23 days after transplant based on CT, pressure studies, and biopsy. Of these, graft function was normal in 1 with the remaining cases having mild cholestasis at the time of diagnosis. All had preserved renal function before commencement of diuretics (estimated glomerular filtration rate > 60). Typical CT findings for the diagnosis of HVOO were a heterogeneous liver with nonvisualization of HVs and normal inflow vasculature (see Fig. 1). The average wedge pressure was 22 ± 10.5 mm Hg (see Fig. 2 for illustrative case) and characteristic histology findings were perivenular cell drop out and hemorrhagic congestion (see Fig. 3).
| Patient Number | Recipient Age, Years | Sex | Disease | Recipient Weight, kg | Graft Type | Donor Cause of Death | Graft Weight, kg | Explant Weight, kg | GRWR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | Male | Glycogen storage disease | 49 | DBD | SAH | 1.29 | 4.925 | 2.6 |
| 2 | 72 | Female | HCV HCC | 60 | DCD | SAH | 1.41 | 1.48 | 2.4 |
| 3 | 22 | Male | AIH | 60 | DCD | NT | 1.69 | 2.56 | 2.8 |
| 4 | 43 | Male | VBDS | 63 | DBD | ICB | 0.79 | 2.33 | 1.25 |
| 5 | 50 | Male | HCV HCC | 77 | DBD | ICB | 1.94 | 1.61 | 2.5 |
| 6 | 39 | Male | PSC | 81 | DBD | SAH | 1.33 | 2.29 | 1.6 |
| 7 | 38 | Female | PSC | 55 | DBD | ICB | 1.25 | Not done | 2.2 |
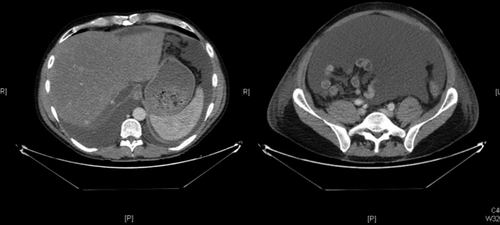
Postcontrast biphasic CT scan of abdomen demonstrating heterogenous enhancement of graft parenchyma, consistent with venous congestion. The major HVs are not visualized, and a large volume of ascites is present.
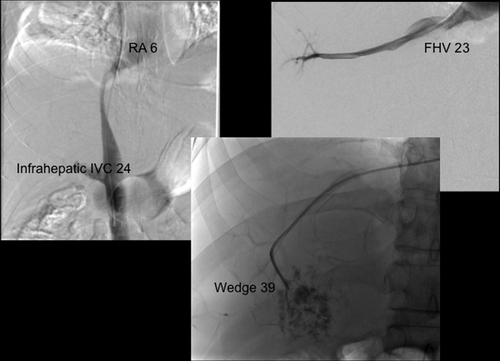
The cavogram demonstrates a displacement and extrinsic compression of the retrohepatic cava. The RA pressure is 6 mm Hg, the IVC pressure is 23 mm Hg, FHV pressure is 23 mm Hg, and wedge pressure is 39 mm Hg. Pressure measurements are consistent with HV outflow, with the level of obstruction being at the HV anastomosis with associated extrinsic compression of the retrohepatic cava.
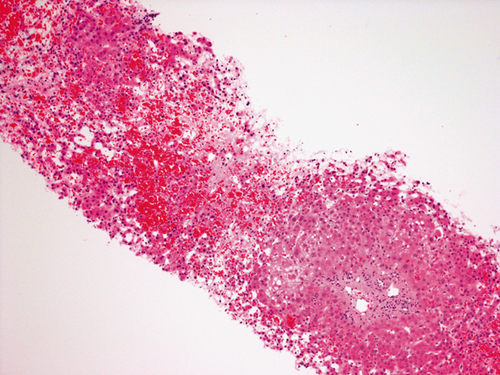
Hematoxylin-eosin stain of formalin fixed allograft core needle biopsy. Demonstrating characteristic features of venous outflow of centrilobular hepatocyte drop out, centrilobular ectasia of sinusoids, and centrilobular patchy acute hemorrhage.
Considering implantation technique, the cases of early HVOO that were identified occurred when 2 HVs with extension PB had been used, giving an incidence of 1.6% (7/423) for that technique. Statistically, with the present study design and numbers for each implanting technique, it cannot be demonstrated that 2 HVs with extension gives a higher risk of early HVOO (P = 0.114). In just under one-third of HVOO cases (29%, n = 2/7) medical management with diuretics was successful with the remainder requiring surgical management (71%, n = 5/7). Operative cavoplasty was successful in 80% of cases (n = 4/5). The HVOO cases that were successfully managed by cavoplasty were all inpatients and revision was within a month of transplant (see Table 3 for summary of HVOO clinical, diagnostic findings, and management strategy).
| Patient Numbers | LT Year | HVOO Presentation | Diagnosis Time, Days | Location | Ultrasound | CT | Liver Biopsy | Transjugular Wedge HV Pressure, Mm Hg | Management |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2008 | Ascites, drain losses | 30 | Inpatient | Ascites | HVs not visualized, congested liver, ascites | Yes | 33a | Cavoplasty; Operative findings: congested liver, twisting at HV anastomoses |
| 2 | 2008 | Pleural effusion | 18 | Inpatient | Ascites | HVs not visualized, congested liver, ascites | Yes | 23 | Cavoplasty; Operative findings: congested liver, stenoses at HV anastomoses |
| 3 | 2010 | Ascites | 16 | Inpatient | Ascites | HVs not visualized, congested liver, ascites | Yes | 12 | Cavoplasty; Operative findings: congested liver, stenoses, and twisting of HV anastomoses |
| 4 | 2011 | Ascites | 25 | Inpatient | Ascites | HVs not visualized, congested liver, ascites | Yes | Not done | Cavoplasty; Operative findings: congested liver, stenoses, at HV anastomoses |
| 5 | 2011 | Ascites | 82 | Outpatient | Ascites | HVs not visualized, congested liver, ascites | Yes | 39 | Re-LT; Operative findings: congested liver, stenoses at HV anastomoses, with a fibrous flap lined by endothelium |
| 6 | 2012 | Ascites | 46 | Outpatient | Ascites | Ascites, narrowing at anastomoses | Yes | 15 | Medical/Diuretics; Spironolactone 150 mg once daily |
| 7 | 2012 | Ascites | 29 | Outpatient | Ascites | Ascites, narrowing at anastomoses | Yes | 20 | Medical/Diuretics; frusemide 20 mg once daily, spironolactone 25 mg once daily |
- NOTE: Patient numbers correlate with those in Table 2.
- a Intraoperative splenic pulp pressure.
The 1 patient for whom cavoplasty was unsuccessful was readmitted 6 weeks after transplant presenting with tense ascites and diuretic-related renal dysfunction. The indication for transplant was HCC on a background of HCV. There was no underlying procoagulant disorder identified in the recipient and the cause of death in the donor was an intracerebral bleed. Figures 1 and 2 show the CT and pressure studies that supported the diagnosis of HVOO. A right lobe transjugular biopsy at the time of the pressure studies also corroborated the radiological and clinical diagnosis of HVOO. Histologically the findings were of centrilobular patchy acute hemorrhage with centrilobular necrosis. There was no associated thrombosis, rejection, or steatosis (see Fig. 3). Exploratory laparotomy for cavoplasty was decided upon, but at the time of the operation, the finding of severe portal hypertension combined with a large, congested liver led to the procedure being abandoned. Interventional radiology then deployed stents in the cava and HV in an attempt to relieve HVOO (see Fig. 4). After 3 days, the stents thrombosed despite anticoagulation (see Fig. 5). The patient was relisted and successfully retransplanted a week later. The implanting technique was caval replacement on VV bypass.
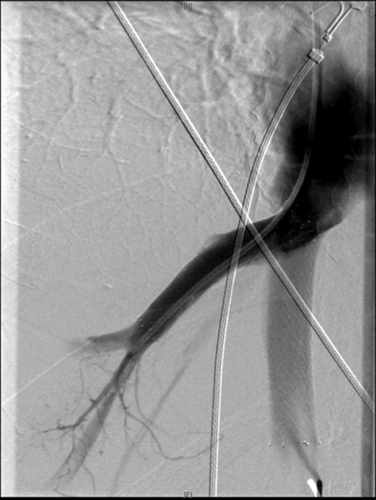
Fluoroscopic-guided stent insertion. The right HV was catheterized, and a 10-mm by 6-cm stent was placed via a transjugular route. A 14-mm by 8-cm stent was deployed in the retrohepatic cava via the right femoral vein.
CT demonstrating a patent caval stent and an occluded HV stent (no contrast within). There is associated heterogeneous enhancement of the liver.
DISCUSSION
PB transplant evolved to counter the physiological problems of caval replacement, but concerns remain that the advantages gained are negated by a higher incidence of HVOO. In the literature, the reported incidence of HVOO is < 2% for caval replacement,9, 10 3% to 4% for PB,11, 12 with the incidence increasing up to 5% to 15% in pediatric recipients and partial grafts.13 Considering the PB variants, the incidence of HVOO ranges from 2.4% to 6.4% for standard PB and 1.8% to 5.5% for side to side, with advocates of the 3 HV technique reporting 1%.11, 12, 22 In the present report, the overall incidence of early HVOO was low at 1%, making it a rare complication, classically, presenting at a month after transplant with progressive ascites and minimal derangement in graft biochemistry.
In previous published series, there are typically 4 phases of HVOO described: (1) immediate graft congestion on reperfusion, requiring the anastomosis to be refashioned or the right diaphragm to be plicated to adjust for the mismatch between a small graft and hepatic bed11, 14; (2) acute Budd-Chiari presenting with graft dysfunction and ascites within 48 hours generally requiring retransplant as the HVs have thrombosed; (3) ascites within 3 months that typically is managed with diuretics; and (4) chronic through a combination of intimal hyperplasia/fibrosis that is increasingly managed by interventional radiology before retransplant is considered.11, 22
In the present study, there are a number of limitations. First, it is retrospective, not randomized and driven by institutional preference. Additionally, the early cases of HVOO for this study were identified by the presenting complaint of problematic ascites, which will have missed cases of HVOO that were identified intraoperatively with congestion on reperfusion and immediately amended surgically, either by re-fashioning the anastomosis or plicating the diaphragm. The other subset of early HVOO that would not of been captured in the present study design is the case of transient ascites that reflects a compromise to right HV outflow, which occurs in the 3 HV PB; in that case, the liver remodels to produce right lobe atrophy.
When medical management for HVOO fails, there are a number of endovascular options in the form of angioplasty or stents. However, most experience on stenting for HVOO is from partial rather than whole grafts,13, 23 and there is a reluctance to use this strategy early or in the presence of thrombosis because of the potential risk of anastomotic rupture.22, 24 Another consideration is that stents can migrate, and if deployment is not carefully planned, they may compromise retransplant or cavoplasty.13, 14 A number of different surgical strategies have been used to resolve HVOO such as termino-terminal rescue cavocavostomy,25 renosplenic shunt,26 cavoatrial shunt27 and side-to-side stapled cavocavostomy.28 Ultimately, if HVOO rescue strategies are unsuccessful, retransplant is then required.
In conclusion, early HVOO can be a graft-losing complication that highlights the technical vulnerabilities of the alternative implanting techniques. This study illustrates that early HVOO is rare, and there is no evidence from this study, as it is not sufficiently powered, that a given implantation technique is more vulnerable to developing early HVOO. However, early revisional surgery for HVOO can preserve graft function with retransplantation being reserved for cases where surgical cavoplasty or radiological stenting is technically not possible.