Local allograft irradiation as an adjunct for treating severe resistant rejection after liver transplantation in adults
Potential conflict of interest: Nothing to report.
Abstract
Acute rejection after liver transplantation occurs in one-third of all recipients and can be managed with conventional rejection therapy in the majority of cases. In rare instances, patients with severe acute rejection may be refractory to or have contraindications for conventional therapies. This case series evaluates the role of local allograft irradiation (LAI) as an adjunct for patients with rejection that is refractory to or contraindicated for conventional therapies. Additionally, the literature on the use of radiation therapy for reversing rejection in solid organ transplantation is reviewed. Five patients underwent 9 LAI treatments: 2 had refractory rejection, and 1 each had a malignancy, a concurrent life-threatening infection, and serum sickness with antibody therapy. Conventional rejection therapies included steroids, calcineurin inhibitors, and antithymocyte globulin. LAI consisted of 3 cycles of 1.5 Gy directed toward the liver allograft. Two of the 5 patients remained alive with excellent graft function. Six of the 9 treatments were successful in rescuing the liver allograft (reversing the rejection episode). Treatment success was associated with lower pretreatment serum bilirubin levels and higher pretreatment alanine aminotransferase levels. Compared with patients with immunosuppression-responsive severe acute rejection, those requiring LAI trended toward a later onset of first rejection. In conclusion, local irradiation of liver allografts can be a useful adjunct in patients for whom conventional options have been exhausted or cannot be used. The ability of LAI to reverse allograft dysfunction and promote patient survival appears to be greatest before the onset of severe cholestatic injury. Liver Transpl 21:47-56, 2015. © 2014 AASLD.
Abbreviations
-
- ACR
-
- acute cellular rejection
-
- AIH
-
- autoimmune hepatitis
-
- ALT
-
- alanine aminotransferase
-
- ATG
-
- antithymocyte globulin
-
- HCV
-
- hepatitis C virus
-
- LAI
-
- local allograft irradiation
-
- LFT
-
- liver function test
-
- OLT
-
- orthotopic liver transplantation
-
- PTLD
-
- posttransplant lymphoproliferative disorder
-
- R-CHOP
-
- rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
-
- RILD
-
- radiation-induced liver disease
-
- SD
-
- standard deviation
-
- WBC
-
- white blood cell
One-third of orthotopic liver transplantation (OLT) recipients experience acute rejection within the first 2 years after transplantation.1 Fortunately, graft survival among those experiencing acute rejection remains relatively unaffected, with survival at 5 and 10 years approaching 75.0% and 64.2%, respectively.1 Before the advent of modern immunosuppression, roughly 10% of liver allografts were lost to acute rejection, with median patient survival of 131 months.2 Currently, most acute cellular rejection (ACR) episodes after liver transplantation are resolved with steroids.3 In recipients with steroid-resistant rejection, conventional immunosuppression options for treating ACR of the liver include an escalation of maintenance immunosuppression, the addition of anti-interleukin receptor antibodies such as basiliximab and daclizumab, and depleting antibody therapies such as antithymocyte globulin (ATG).4, 5 Infrequently, severe acute rejection may pose an existential threat to liver allografts and to recipient survival. Such rare circumstances include allograft rejection episodes that are refractory to conventional rejection therapies, rejection episodes that cannot be treated by immunosuppression escalation because of concurrent life-threatening infections and malignancies, and recipient intolerance of antirejection medications. In these situations, progression to fulminant liver failure or chronic allograft rejection may eventually lead to patient mortality.
Before the successful introduction of calcineurin inhibitors and antibody treatments such as OKT3, ATG, and basiliximab, external-beam total lymphoid irradiation therapy was used for induction and maintenance immunosuppression.6, 7 Because of its dose-dependent toxicity, it was abandoned as an immunosuppressive strategy in solid organ transplantation in favor of the newer immunosuppressive agents with fewer adverse effect profiles. However, external-beam radiation therapy has been sporadically employed for refractory rejection treatment in solid organ transplantation, including kidney transplantation8-12 and pediatric heart transplantation.13 The only report of allograft radiation in liver transplantation is for pediatric liver recipients with refractory rejection.14 In that series of 14 pediatric liver recipients, complete reversal of rejection was achieved in 6 children, and partial reversal of rejection was achieved in 2 children; this allowed a bridge to transplantation. To the authors' knowledge, there are no case series to date reporting the use of external-beam irradiation to the liver allograft to treat severe rejection after liver transplantation in adult recipients.
This report details the first single-center case series with local liver allograft irradiation to reverse severe rejection among adult liver transplant recipients who had exhausted conventional antirejection treatment options. The literature regarding the use of irradiation for reversing rejection in solid organ transplantation is also reviewed.
PATIENTS AND METHODS
A retrospective data analysis of adult deceased donor OLT performed at our center between 2002 and 2012 was performed. Liver transplants involving pediatric donors, pediatric recipients, and living donors were excluded from the analysis. Institutional review board approval was obtained for this data analysis.
Immunosuppression Protocols
All recipients received 1 g of methylprednisolone intravenously and 1.5 g of mycophenolate mofetil orally in the preoperative area before transplantation. After deceased donor liver transplantation, maintenance immunosuppression consisted of tacrolimus or cyclosporine, mycophenolate mofetil, and tapering doses of prednisone. Steroids were tapered over a period of 3 months, whereas mycophenolate mofetil was continued at 1 g twice per day [as guided by the white blood cell (WBC) count]. Calcineurin inhibitors were introduced on posttransplant day 3 (with goal serum trough levels of 250-300 ng/mL for cyclosporine and 8-12 ng/mL for tacrolimus during the first 3 months). Patients with a diagnosis of autoimmune hepatitis (AIH) were administered 3 doses of ATG (1.5 mg/kg) on posttransplant days 0, 1, and 2.
Diagnosis of Allograft Rejection
Patients were followed in the clinic every week for the first month, every 2 weeks for the second and third months, every month up to 1 year, and subsequently every 3 to 6 months at the discretion of the treating transplant hepatologist. Patients were evaluated for rejection in the case of laboratory liver function test (LFT) abnormalities or clinical manifestations of liver allograft dysfunction. Rejection was confirmed through transjugular liver biopsies performed by experienced interventional radiologists. Dedicated liver transplant pathologists in consultation with the hepatologist analyzed the liver biopsy specimens. ACR was identified and graded by the presence and extent of portal inflammation, bile duct inflammation, and venous endothelial inflammation in conjunction with the Banff schema.15 ACR was graded as mild (Banff score = 1-3), moderate (Banff score = 4 or 5), or severe (Banff score = 6-9). Biopsies were also examined for features consistent with chronic rejection, active hepatitis C virus (HCV), and AIH.
Conventional Treatment for Acute Allograft Rejection
All biopsy-proved mild and moderate rejection episodes were treated with 250 mg of intravenous methylprednisolone for 3 days, which was followed by tapering doses. Severe and steroid-resistant rejection was additionally treated with rabbit ATG (Thymoglobulin; Genzyme Corp., Cambridge, MA) at a dose of 1.5 mg/kg/day for 5 to 7 days on the basis of the patient's biochemical response. Patients underwent a follow-up biopsy approximately 2 weeks after the completion of their rejection treatment to assess their response to therapy.
Indications for Local Allograft Irradiation (LAI)
Patients with ongoing rejection-related liver allograft dysfunction were referred for LAI after exhaustion of the aforementioned conventional therapeutic options. Indications for LAI included (1) severe rejection refractory to ATG, (2) intolerance of ATG, (3) concurrent life-threatening infections precluding immunosuppression escalation, and (4) a concurrent malignancy precluding immunosuppression escalation.
Protocol for LAI
LAI was delivered with 3-dimensional conformal external-beam radiation on an outpatient basis. The radiation target volumes included the whole liver allograft plus a 2-cm margin. Opposed anteroposterior-posteroanterior (AP-PA) photon beams were used with 3 consecutive daily doses of 1.5 Gy for a total dose of 4.5 Gy. Maintenance immunosuppression was continued during LAI treatment. Post-LAI biopsies were performed as dictated by each patient's clinical status and LFTs. In general, post-LAI transjugular biopsies were performed 1 to 3 months after LAI.
Analysis
The outcomes of patients undergoing LAI were compared with those of a control cohort of patients with severe steroid-resistant rejection who did not require LAI. LAI was considered successful if there was a reversal of rejection or patient survival for at least 1 month after treatment. Liver function was analyzed by comparisons of alanine aminotransferase (ALT) and total bilirubin levels between the groups with the Mann-Whitney U test. Changes in ALT and total bilirubin before and after treatment were compared with the Wilcoxon rank test for paired samples. Other continuous variables were compared with the Mann-Whitney U test, and categorical variables were compared with Fisher's exact test. All statistical analyses were performed in JMP 11.0 (SAS Institute, Cary, NC) with a statistical significance level of P < 0.05.
RESULTS
Between 2002 and 2012, 558 patients underwent OLT at our transplant center and fit the inclusion criteria. There were 64 episodes of severe ACR (defined as a Banff score > 6) in 54 patients. Five patients required 9 LAI treatments (Table 1).
| Patient, Age (Years), Sex | OLT Indication | Conventional Treatments | LAI Indication | Time to LAI After OLT (Months) | Pre-LAI Liver Biopsy | Post-LAI Liver Biopsy | ALT Before/After LAI (U/L) | Total Bilirubin Before/After LAI (mg/dL) | Recipient Status |
|---|---|---|---|---|---|---|---|---|---|
| 1, 50, M | HCV | Pegylated interferon and ribavirin, steroids, ATG | Refractory, HCV | 78 | ACR (Banff 5/9), bile duct destruction, cholestasis | ACR resolution, chronic ductopenic rejection, severe cholestasis; 1 month after LAI | 233/187 | 10.5/9.9 | Deceased (4 months after LAI; 82 months after OLT) |
| 2, 61, M | HCV, alcohol | Antibiotics, ATG | Nocardia brain abscess | 8 | ACR (Banff 8/9), canalicular plugging, cholestasis, bile lake formation | — | 171/131 | 21.3/22 | Deceased (10 days after LAI; 9 months after OLT) |
| 3, 29, M | AIH | Steroids, ATG | ATG serum sickness | 58 | ACR (Banff 6/9), portal fibrosis with early bridging fibrosis | — | 584/263 | 0.7/1.4 | |
| 61 | Recurrent AIH and ACR (Banff 6/9), portal fibrosis; 2 months after first LAI | — | 263/137 | 1.4/1.8 | |||||
| 65 | Nonbridging fibrosis, canalicular cholestasis, early chronic rejection; 4 months after second LAI | — | 163/398 | 14.7/24.3 | Deceased (21 months after first LAI; 79 months after OLT) | ||||
| 4, 36, M | AIH | Steroids, ATG, azathioprine | Refractory, recurrent AIH | 94 | Portal and lobular hepatitis with mild fibrosis | — | 281/444 | 1.2/0.8 | |
| 97 | — | 444/556 | 0.8/0.9 | ||||||
| 102 | ACR resolution (Banff 0/9); 2 months after LAI | 490/119 | 1.6/0.6 | Alive | |||||
| 5, 34, F | AIH | Steroids, R-CHOP | PTLD | 16 | ACR (Banff 7/9) | ACR resolution (Banff 0/9); 3 months after LAI | 1713/838 | 0.3/1.0 | Alive |
Patient Profiles
Patient 1
Patient 1 underwent liver transplantation at the age of 50 years for HCV cirrhosis. Seventy-two months after transplantation, elevations in LFTs were detected, and severe ACR was confirmed by liver biopsy with findings of portal, bile duct, and venous endothelial inflammation (Banff score = 8/9). Six months before, he had undergone treatment with pegylated interferon and ribavirin for recurrence of HCV viremia. Despite treatment with steroids and ATG, there was no improvement in the liver allograft dysfunction, with interval biopsies demonstrating continued ACR with typical findings of acute bile duct destruction, endothelial swelling with neutrophilic infiltrates, and portal necrosis (Banff score = 5/9). There was a pathologic presence of piecemeal necrosis of periportal hepatocytes presumed to be due to both acute rejection and recurrent HCV infection. Additionally, there was new evidence of bile duct destruction and cholestasis. Additionally, the HCV DNA level at that time was 7.43 log. A course of plasmapheresis was attempted, with no improvement in his condition. Having exhausted all available conventional options, the patient underwent a course of LAI. Repeat liver biopsy performed 18 days after the completion of LAI revealed an absence of both acute rejection and HCV with no portal, bile duct, or endothelial inflammation (Banff score = 0/9). However, the biopsy displayed chronic ductopenic rejection of the graft with severe canalicular cholestasis. His total WBC count was 4800/µL before LAI and stayed relatively stable, with counts of 3900/µL 7 days after LAI, 9200/µL 14 days after LAI, and 10,000/µL 50 days after LAI. Clinically, the cholestasis continued to worsen, and he died 4 months after LAI.
Patient 2
Patient 2 was a 61-year-old man who underwent liver transplantation for alcoholic and HCV cirrhosis. Six months after transplantation, he was diagnosed with a Nocardia brain abscess. While completing the antimicrobial course of treatment, he was rehospitalized for new-onset seizures, elevations in LFTs, and progressive multiorgan dysfunction. Liver biopsy revealed severe acute rejection (Banff score = 8/9) with canalicular plugging, cholestasis, and bile lake formation without evidence of HCV recurrence. There was no clinical response to a 3-day course of ATG. Because of the risk of Nocardia reactivation with increased systemic immunosuppression or additional ATG, a course of LAI was administered. Before LAI, his WBC count was 5000/µL. His WBC count decreased to 3300/µL 2 days after LAI and increased to 9600/µL 5 days after LAI. He did not respond to LAI and died 10 days after LAI.
Patient 3
Patient 3 underwent liver transplantation for AIH liver failure at the age of 29 years. In the first 4 years after transplantation, he experienced several episodes of acute rejection. Liver biopsies indicated that these rejection episodes also displayed components of AIH. ATG was required for 1 rejection episode, and the remaining rejection episodes were steroid-responsive. Fifty-eight months after transplantation, he developed serum sickness with ATG during the treatment of his second steroid-resistant severe ACR episode. LAI was used at that time with complete resolution of ACR. His pre-LAI WBC count was 3900/µL and remained stable at 3300/µL 18 days after LAI. Fourteen months after that episode of rejection, a recurrence of severe ACR (Banff 6/9) was treated with a second course of LAI. The second LAI treatment resulted in complete clinical improvement and partial improvement in LFTs. The WBC count before the second LAI was 4500/µL and remained stable at 3800/µL 20 days after LAI. Liver biopsy performed 4 months later revealed progression to chronic rejection with additional findings of cholestasis and possible recurrence of AIH. The patient was not eligible for relisting and retransplantation because of a documented history of medication noncompliance. As a result, the patient underwent a third course of LAI for residual LFT abnormalities and clinical liver dysfunction. His WBC remained unchanged at this time, with 5600/µL before LAI and 5300/µL 30 days after LAI. Despite treatment, he had progressive cholestasis and died 1.5 months after the third LAI treatment.
Patient 4
Patient 4 was a 36-year-old man who underwent liver transplantation for AIH. Eighty-four months after transplantation, he developed severe ACR, with a biopsy sample displaying a mixed inflammatory infiltrate compatible with recurrent AIH and ACR. The rejection was refractory to conventional treatment, which included steroids and ATG for rejection and azathioprine for AIH. After the first LAI treatment, there was clinical improvement but stable elevations in LFTs. A second treatment was administered 3 months later, with complete clinical improvement but persistent stable elevations in LFTs. The pre-LAI WBC before the second treatment was 10,000/µL, with no significant change 40 days after LAI at 9100/µL. A third treatment was subsequently administered 4 months later for LFT elevations and a new increase in total bilirubin to 1.6 mg/dL. The WBC count before this treatment was 9800/µL, and it remained stable at 11,800/µL 60 days after LAI. This treatment resulted in steady decreases in LFTs and resolution of the bilirubinemia. He continued to have a functioning liver allograft more than 10 years after his transplant.
Patient 5
Patient 5 was a 34-year-old woman who underwent liver transplantation for AIH. She developed posttransplant lymphoproliferative disorder (PTLD) 18 months after transplantation with bilateral pulmonary and mediastinal involvement. PTLD was treated with immunosuppression minimization and chemotherapy [rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)]. During the fourth cycle of chemotherapy, she developed severe acute rejection. Her calcineurin inhibitor dose was increased, and ATG was avoided because of her continued PTLD treatment. She underwent 1 cycle of LAI. As a result of her concurrent chemotherapy, her WBC counts were low at 1300/µL before LAI and ranged from 400 to 4200/µL after LAI. Follow-up liver biopsy performed 11 months after LAI demonstrated complete resolution of rejection with no evidence of radiation-related injury. At the time of this writing, she was in remission of PTLD with continued graft function 5 years after liver transplantation and 3.5 years after LAI.
Recipient Outcomes
There were no significant differences in recipient age, sex, race, or liver disease etiology between those patients who received LAI and the control cohort of patients with ATG-responsive severe acute rejection (Table 2). The etiology of liver failure in 2 of the 5 patients undergoing LAI was HCV cirrhosis, and the other 3 patients had AIH-related liver failure. The LAI group trended toward having a later onset of the first rejection episode after liver transplantation in comparison with the controls (32.8 ± 37.8 versus 8.5 ± 11.6 months, P = 0.11).
| LAI (n = 5) | Controls (n = 53) | P Value | |
|---|---|---|---|
| Transplant age, years [mean (SD)] | 42.0 (13.2) | 46.3 (12.4) | 0.46 |
| Males [n (%)] | 4 (80.0) | 30 (56.6) | 0.39 |
| African Americans [n (%)] | 2 (40.0) | 15 (28.3) | 0.20 |
| Etiology [n (%)] | 0.08 | ||
| HCV | 2 (40) | 8 (15) | |
| AIH | 3 (60) | 3 (6) | |
| Hepatocellular carcinoma | 0 | 11 (21) | |
| Acute hepatic necrosis | 0 | 7 (13) | |
| Alcoholic | 0 | 8 (15) | |
| Metabolic | 0 | 4 (8) | |
| Primary sclerosing cholangitis | 0 | 7 (13) | |
| Other | 0 | 5 (9) | |
| Time to first rejection, months [mean (SD)] | 32.8 (37.8) | 8.5 (11.6) | 0.11 |
| Number of rejections [mean (SD)] | 1.6 (0.9) | 1.2 (0.5) | 0.08 |
| Infection rate [n (%)] | 4 (80.0) | 25 (47.2) | 0.22 |
| Patient survival [% (95% CI)] | 0.42 | ||
| 1 year | 80.0 (30.9-97.3) | 86.6 (74.4-93.5) | |
| 5 year | 80.0 (30.9-97.4) | 80.6 (67.5-89.2) | |
| 10 year | 26.7 (3.6-77.7) | 46.6 (27.7-66.6) |
In the LAI cohort, the 2 deaths occurred 10 days (patient 2) and 4 months (patient 1) after a single unsuccessful LAI treatment. In patient 3, who underwent 3 LAI treatments, death occurred 21 months after the first LAI treatment (Table 1). Two of the 3 deaths in the LAI cohort occurred more than 5 years after transplantation, whereas 1 death occurred within 1 year after transplantation. Compared with the patients who were alive after LAI treatment, the deceased patients trended toward having higher preradiation bilirubin levels (18.5 ± 8.3 versus 1.0 ± 0.9 mg/dL, P = 0.08) and last measured bilirubin levels (27.2 ± 4.0 versus 0.5 ± 0.2 mg/dL, P = 0.08).
Among the 9 LAI treatments, 6 were successful in treating rejection, and 3 were unsuccessful. Success was defined as biopsy-proved reversal of rejection or clinical improvement with patient survival for at least 1 month after treatment. LAI treatment success was significantly associated with lower pretreatment bilirubin levels (1.0 ± 0.5 versus 17.4 ± 8.6 mg/dL, P = 0.02) and lower posttreatment bilirubin levels (1.1 ± 0.4 versus 21.5 ± 10.5 mg/dL, P = 0.02; Fig. 1A). LAI treatment success was also significantly associated with higher serum ALT levels before treatment (629.2 ± 545.1 versus 140.0 ± 111.8, P = 0.02) but with no difference in ALT after treatment (Fig. 1B). There were no significant differences in a paired analysis of total bilirubin and ALT before and after LAI on the basis of treatment success.
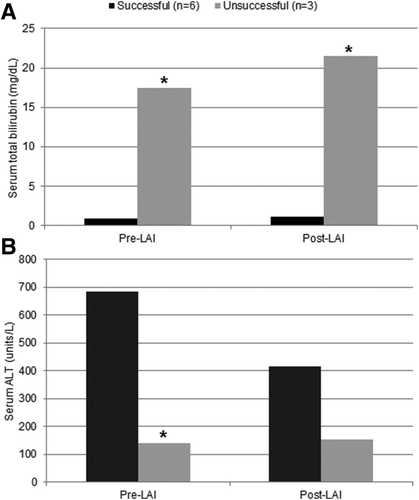
Comparison of successful and unsuccessful LAI treatments: (A) total bilirubin and (B) ALT before and after LAI treatment (*P < 0.05).
DISCUSSION
ACR, a previously devastating complication after liver transplantation, is today treatable as a result of early detection and advances in immunosuppression.16-18 However, in liver transplant recipients who do not respond to conventional therapy, acute rejection remains a graft-threatening complication. To the best of the authors' knowledge, this is the first reported case series describing the use of LAI to treat severe refractory rejection in adult liver transplant recipients. This report illustrates that for recipients with severe acute rejection that is refractory to or contraindicated for conventional antirejection therapy, LAI can be an effective adjunct to salvage the allograft and prolong survival in those without evidence of cholestatic disease.
The indications for the use of LAI for treating rejection are not clearly defined in the literature. We used LAI as a salvage therapy to prevent rapid progression of rejection leading to liver failure. This aggressive approach was especially required for patients with concurrent infections or malignancy because they were otherwise ineligible for relisting and retransplantation. In our series, indications for LAI included (1) rejection refractory to conventional therapies (2/5), (2) contraindications to immunosuppression escalation from a Nocardia brain abscess (1/5), (3) ATG serum sickness (1/5), and (4) PTLD (1/5).
Some studies have evaluated allograft irradiation for the treatment of acute rejection in solid organ transplantation (Table 3). Successful reversal of acute rejection with LAI of the kidney allograft was first reported in 1973 by Fidler et al.8 for 4 kidney transplant patients with life-threatening infections (pneumonias, septic pyarthroses, and osteomyelitis). In that series, all 4 patients had complete reversal of rejection with no cases of graft or recipient loss. In 1981, a subsequent trial of radiation therapy randomized 62 kidney transplant recipients to steroids with or without LAI and found that the LAI group had increased recurrent rejection and lower graft survival (22% versus 54%).12 Notably, in that series, irradiation was used as a first-line therapy for rejection. Other single-center reports of kidney allograft irradiation in the era before calcineurin inhibitors displayed mixed results.9, 10 More recently, in 2000, Chen et al.19 evaluated the role of kidney allograft irradiation in 53 kidney transplant recipients with rejection refractory to OKT3, ATG (ATGAM), and calcineurin inhibitors. Graft survival from the initiation of radiation therapy was 83% at 1 month and 36% at 5 years, with an improved response to radiation noted in female patients, those with 1 or 2 human leukocyte antigen DR matches, those with creatinine levels less than 3.0 mg/dL, and those with a maximum panel reactive antibody less than 70%. For pediatric heart transplant recipients, Asano et al.13 reported the use of total lymphoid irradiation with acute rejection that was refractory to steroids, methotrexate, and ATG. In that series, 5 of the 11 children were alive for 39.9 ± 18.2 months after irradiation, 4 children died 4.1 ± 3.6 months after irradiation, and 1 child underwent retransplantation 59 months after irradiation.
| Study | Year Published | Organ | Study Type | Patient Population (n) | Irradiation Indication | Irradiation Technique | Outcome |
|---|---|---|---|---|---|---|---|
| Fidler et al.8 | 1973 | Adult kidney transplant | Observational | 4 | Life-threatening infections | Local graft irradiation; cumulative dose 3-9 Gy | 100% complete reversal of rejection |
| Pilepich et al.12 | 1983 | Adult kidney transplant | Randomized control trial | 62 (pulse steroids alone versus pulse steroids + irradiation) | All rejection episodes | Local graft irradiation; cumulative dose 5.25 Gy | Higher rates of recurrent rejection and lower graft survival with irradiation |
| Jagetia et al.9 | 1996 | Adult kidney transplant | Observational | 6 | Refractory rejection to steroids, ATGAM, OKT3, others | Local graft irradiation, cumulative dose 4.5-6 Gy | Complete reversal in 1/6 patients, partial reversal in 3/6 patients |
| Noyes et al.10 | 1996 | Adult kidney transplant | Observational | 72 | Refractory rejection to steroids, OKT3 | Local graft irradiation, cumulative dose 8 Gy | 83% responded with decreased creatinine; 60% with continued graft survival to median 16 months follow-up |
| Chen et al.19 | 2000 | Adult kidney transplant | Observational | 53 | Refractory rejection to steroids, OKT3, ATGAM | Local graft irradiation, cumulative dose 6 Gy | 83% 1 month graft survival; 36% 5-year graft survival |
| Asano et al.13 | 2002 | Pediatric heart | Observational | 11 | Refractory rejection to steroids, methotrexate, ATG | Total lymphoid irradiation; cumulative dose 6-8.4 Gy | 5 patients alive; 6 patients died |
| Stephenne et al.14 | 2005 | Pediatric liver | Observational | 14 | Refractory rejection to steroids, OKT3, ATG | LAI; cumulative dose 4.5 Gy | Complete reversal in 6 patients; partial reversal in 4 patients; death in 4 patients |
In contrast to kidney allografts and heart allografts, liver allografts have a high regenerative capacity after immunologic and radiation-induced injury. The sole report of irradiation for rejection in liver transplantation is a pediatric liver transplant series by Stephenne et al.14 (Table 3). Between 1986 and 1990, 14 pediatric recipients with acute rejection refractory to steroids, OKT3, and ATG underwent LAI. The children received a cumulative radiation dose of 4.5 Gy over 3 days, and treatment success was defined by continued graft function and no requirement for further rejection treatment. The same dose of LAI was used in our study. The main etiologies of liver disease in that cohort were biliary atresia and metabolic diseases. One child had cryptogenic cirrhosis. Four of the 14 children underwent irradiation within 15 days after transplantation, 5 children underwent irradiation 15 to 30 days after transplantation, and the remaining 5 children underwent irradiation 30 to 45 days after transplantation. Irradiation resulted in complete rejection reversal in 5 of the 14 children, partial reversal in 4 children, and death in 4 children. The causes of death were adenovirus infection, AIH recurrence, and perioperative complications after retransplantation. Overall, 3 of the 14 children (21.4%) eventually required retransplantation. At 15 years after transplantation, 7 of the 14 children remained alive. After irradiation, 1 child developed an Epstein-Barr virus–related malignancy, and 2 children developed adenovirus hepatitis; however, the incidences of these adverse events were not significantly different from those of a control cohort. Additionally, there was no increase in fibrosis on long-term biopsy in comparison with controls and no radiation-specific histologic findings. To our knowledge, there are no reports of liver irradiation for treating rejection in adult liver transplantation. The only case report of LAI in adult liver transplantation describes the use of 24 Gy for the successful treatment of a focal posttransplant lymphoma in the absence of rejection.20
In our present series of LAI treatment for rejection in adult liver transplantation, 2 of the 5 patients recovered from acute rejection after LAI and remained alive with excellent graft function. Nine treatments were administered to 5 patients, with 6 of the treatments resulting in resolution of rejection episodes. In the 3 patients for whom LAI treatment failed, high serum bilirubin levels were observed at the time of therapy initiation. In addition, the pre-LAI liver biopsy samples for these recipients demonstrated evidence of progression to chronic cholestatic rejection. Another common feature in patients who failed to respond to LAI was the presence of late acute rejection. Late acute rejection by definition occurs 1 to 6 months after transplantation. It is clinically less responsive to immunosuppression, has increased progression to irreversible ductopenic rejection, and pathologically demonstrates a less characteristic pattern of ACR.21-23 This was observed in patient 3, in whom the first 2 noncholestatic acute rejection episodes responded to LAI, but the later onset third cholestatic rejection episode did not respond to LAI.
Radiation toxicity is a concern with any form of radiotherapy. In the liver, radiation-induced liver disease (RILD) manifests as a clinical syndrome consisting of anicteric hepatomegaly, ascites, and transaminitis. RILD typically has an onset 2 to 4 months after radiation therapy and is pathologically notable for centrilobular venous obstruction.24 Patient 3 in our series developed cholestasis approximately 2 months after his second allograft irradiation. However, he did not have any histologic evidence of RILD, which includes a loss of hepatocytes around the central vein of lobules and evidence of trapped erythrocytes.25 Normally, whole liver irradiation has been associated with the development of RILD at doses higher than 30 Gy in a dose-dependent fashion.26-28 In our protocol, 3 daily fractions of 1.5 Gy were directed at the allograft for a total of 4.5 Gy of external-beam radiation. Even with the multiple treatments, patient 3 received a total radiation dose of only 13.5 Gy, which is well below the described threshold for developing RILD. Furthermore, the liver biopsy samples obtained after LAI did not show signs of RILD in any of the patients. Patient 4 was successfully treated with LAI but did not have follow-up biopsies to evaluate for RILD because of the complete clinical response. Patient 5 had a follow-up liver biopsy performed 11 months after LAI with no evidence of radiation-related injury.
The precise mechanisms by which allograft irradiation reverses acute rejection are yet to be defined. Radiotherapy may decrease the antigenicity of the graft and induce donor-specific tolerance by destroying antigen carrier cells and passenger lymphocytes traveling from the graft to the host.14, 20 Total lymphoid irradiation, which has been used in refractory heart and kidney allograft rejection with mixed results, is believed to suppress T cells, block interleukin-2 pathways, and promote donor-specific tolerance.13, 29-31 In animal models, lymphoid radiation has also been proposed to function through apoptosis of graft-infiltrating host leukocytes and immunoregulation.32 These putative mechanisms of lymphocyte modulation and destruction suggest that LAI may be particularly effective in liver transplant recipients with AIH and PTLD because both AIH and PTLD are mediated primarily by lymphocytes. In contrast, HCV hepatitis is primarily virally mediated and thus may be less responsive to local irradiation. However, with new, highly effective therapies for HCV clearance, local irradiation for rejection refractory to conventional therapy may prove equally effective. Notably, among the 3 patients in this study who responded to LAI treatment, 2 had AIH, and 1 had PTLD. In addition, it appears that the timing of LAI in those with severe acute rejection may be an important factor for treatment success. The optimal treatment window for LAI may be during the early phase of rejection when there is acute transaminitis as opposed to an intervention after irreversible biliary injury and parenchymal fibrosis as noted by high bilirubinemia and biopsy changes. This case series demonstrates the use of 9 LAI treatments in 5 adult liver transplant recipients with resistant severe rejection who had exhausted conventional treatment options. Although 3 of the 5 patients ultimately succumbed to progressive liver failure, LAI was successful in treating 6 of the 9 rejection episodes.
One of the inherent limitations of this study is the small sample of patients who received LAI of the liver. The small sample is a reflection of the overall success of modern medical immunosuppression in treating acute rejection episodes. The doses used in this study were based on a previous study of liver irradiation in pediatric liver transplant recipients.14 The doses were not weight-based and consequently may have been below the optimal therapeutic dose. Because of the liver's ability to tolerate higher doses and its inherent regenerative capacity, higher fractional doses would likely be well tolerated, and additional investigation of allograft weight–based dose escalation is warranted. With limited published experience, this single-center case series with uniform immunosuppression protocols provides the best proof of concept for the beneficial use of LAI in a subgroup of liver transplant recipients with resistant severe rejection. Additionally, several of the patients suffered from a mixed picture of acute rejection and recurrence of their primary disease. Although this negatively affects the ability to draw associations, it reflects the multifactorial nature of liver allograft dysfunction and demonstrates the need to understand better the immunologic mechanisms of allograft irradiation.
In conclusion, LAI after liver transplantation can be a useful adjunct to treat severe rejection episodes in select patients for whom conventional options have been exhausted or are contraindicated. In our experience, the ability of LAI to reverse resistant allograft rejection and promote patient survival appears to be greatest in the absence of cholestatic injury to the liver. Further large, multicenter studies are needed to delineate the optimal protocols, timing, and mechanisms for LAI after liver transplantation.