Digital image analysis of liver collagen predicts clinical outcome of recurrent hepatitis C Virus 1 year after liver transplantation†
Abstract
Clinical outcomes of recurrent hepatitis C virus after liver transplantation are difficult to predict. We evaluated collagen proportionate area (CPA), a quantitative histological index, at 1 year with respect to the first episode of clinical decompensation. Patients with biopsies at 1 year after liver transplantation were evaluated by Ishak stage/grade, and biopsy samples stained with Sirius red for digital image analysis were evaluated for CPA. Cox regression was used to evaluate variables associated with first appearance of clinical decompensation. Receiver operating characteristic (ROC) curves were also used. A total of 135 patients with median follow-up of 76 months were evaluated. At 1 year, median CPA was 4.6% (0.2%-36%) and Ishak stage was 0-2 in 101 patients, 3-4 in 23 patients, and 5-6 in 11 patients. Decompensation occurred in 26 (19.3%) at a median of 61 months (15-138). Univariately, CPA, tacrolimus monotherapy, and Ishak stage/grade at 1 year were associated with decompensation; upon multivariate analysis, only CPA was associated with decompensation (P = 0.010; Exp(B) = 1.169; 95%CI, 1.037-1.317). Area under the ROC curve was 0.97 (95%CI, 0.94-0.99). A cutoff value of 6% of CPA had 82% sensitivity and 95% specificity for decompensation. In the 89 patients with hepatic venous pressure gradient (HVPG) measurement, similar results were obtained. When both cutoffs of CPA > 6% and HVPG ≥ 6 mm Hg were used, all patients decompensated. Thus, CPA at 1-year biopsy after liver transplantation was highly predictive of clinical outcome in patients infected with hepatitis C virus who underwent transplantation, better than Ishak stage or HVPG. Liver Transpl 17:178–188, 2011. © 2011 AASLD.
Evaluation of liver fibrosis remains one of the most important aspects in chronic liver disease. Histological assessment of fibrosis is still the reference standard1 for the evaluation of new antifibrotic treatments and for validation of noninvasive markers of fibrosis, as well as other established prognostic markers such as hepatic venous pressure gradient (HVPG).2, 3
Currently, all histological scoring systems use a categorical system that includes description of architectural changes as well as the location of fibrosis. They do not assess quantitative changes of fibrosis.1 Indeed, METAVIR and/or Ishak systems assign numbers to categories that are not quantitatively related, nor are they continuous variables. Applying these fibrosis stage scores as numerical data in statistics is incorrect and misleading.1 The development and use of a novel histological index that quantitates fibrosis and relates to clinical outcome would greatly improve the value of liver biopsy.
Optimal assessment of such a novel index could be performed in patients with hepatitis C virus (HCV)-derived cirrhosis after liver transplantation (LT), because often in this population there is rapid fibrosis, early cirrhosis, and decompensation.4-6 In this setting, survival is worse compared to other indications after LT.7 Because the course of HCV reinfection and decompensation is unpredictable, prediction of rapid fibrosis would be clinically useful regarding the potential to start antiviral treatment earlier and to prevent decompensation.
We have developed a method using computer-assisted digital image analysis (DIA) using sirius red–stained histological sections which can quantify liver collagen.1, 8 The quantity of PicroSirius red correlates well with morphometrically calculated hepatic fibrosis.9 Previous studies have shown that morphometric image analysis had better sensitivity than histological staging in evaluating fibrosis progression10 in 245 patients treated for chronic hepatitis C. However, when morphometric image analysis was used in the literature, the amount of collagen was not expressed as a simple proportionate area. A presumed histological section thickness was used in order to calculate the quantity of collagen.10, 11 In our method, segmentation of digital images measure the area of collagen and of tissue, producing a “fibrosis ratio” or collagen proportionate area (CPA).1, 12
More recently, we showed that CPA assessment in HCV-infected post-LT patients correlated with both Ishak stage scores and HVPG. However, CPA was a better histological correlate with HVPG than with Ishak stage, with greater percentage changes in CPA than in HVPG in early portal hypertension (HVPG > 6 mm Hg but ≤ 10 mm Hg).12 Moreover, CPA was the only independent histological variable associated with both early portal hypertension and clinically significant portal hypertension (HVPG > 10 mm Hg).12
Therefore, the aim of this study was to evaluate CPA measured in liver biopsies of patients at 1 year after transplantation for HCV-related cirrhosis, with respect to the first episode of clinical decompensation. By searching the literature, we also correlated CPA with HVPG and other known factors associated with severity of fibrosis due to recurrent HCV.
Abbreviations: ACR, acute cellular rejection; AHC, acute hepatitis C; AUROC, area under the receiver operator curve; AZA, azathioprine; BMI, body mass index; CI, confidence interval; CMV, cytomegalovirus; CPA, collagen proportionate area; CYA, cyclosporine; DIA, digital image analysis; HCV, hepatitis C virus; HLA, human leukocyte antigen; HVPG, hepatic venous pressure gradient; IQR, interquartile range; LT, liver transplantation; MMF, mycophenolate mofetil; RFH, Royal Free Hospital; SIR, sirolimus; SVR, sustained virologic response; TAC, tacrolimus; TMC, tacrolimus vs microemulsified cyclosporine.
PATIENTS AND METHODS
Between October 1988 and October 2008, 257 patients underwent transplantation at the Royal Free Hospital, London, UK, with end-stage liver disease due to HCV infection (278 transplants): 135 patients with first transplant who had a follow-up of at least 12 months were assessed for CPA at 1-year biopsy (performed between 12-15 months) and evaluated for factors that could predict clinical decompensation. Of the 122 patients excluded from our study, 41 died before 1-year post-LT biopsy, 15 had less than 12 months follow-up, 6 were followed up in other centers, 4 were lost to follow-up, 22 had insufficient stored biopsy material, and 34 were assessed for CPA but not within 12-15 months after LT. From these 135 patients, 89 had their transjugular liver biopsy combined with HVPG measurement (started in February 1999). We performed a second separate analysis for the prediction of clinical decompensation in the 89 patients with HVPG measurement.
For each patient, demographic and clinical data (listed in Tables 1 and 2), donor age and sex, cold and warm ischemia time, initial and 1-year post-LT immunosuppression, characteristics and treatment of rejection episodes, the year of transplantation (divided into 3 eras, n1 = 1988-1994, n2 = 1995-2000, n3 = 2001-2008), cytomegalovirus (CMV) post-LT infection or any other infection, histological episodes of acute hepatitis, genotype, viral load pre-LT, and 1 year post-LT, diabetes mellitus pretransplant and posttransplant, human leukocyte antigen (HLA), and blood group compatibility of donor and recipient were recorded. All of the above variables as well as liver function tests at 1-year biopsy were evaluated in the univariate analysis along with CPA, HVPG, and stage and grade according to Ishak at 1 year, for the prediction of clinical decompensation.
| Patients | Future Decompensation (27) | No Decompensation (108) | P Value | |
|---|---|---|---|---|
| Recipient | Age | 53.8 | 51.5 | NS |
| Males | 69% | 76% | NS | |
| Donor | Age | 43.7 (16-73) | 38.6 (11-69) | 0.1 |
| Year of LT | 88-94/95-00/ 01-08 (%) | 19%/44%/37% | 12%/37%/51% | NS |
| Concomitant ALD | % | 15% | 24% | NS |
| Cold/warm ischemia* | Minutes | 680/46 | 678/41 | NS |
| Diabetes pre/post-LT | % | 23%/23% | 25%/25% | NS |
| BMI pre-LT | Median (kg/m2) | 29.2 (22-34) | 28 (21-34) | NS |
| Viral load pre-LT | Median | 2.72 × 106 | 1.73 × 106 | NS |
| Viral load 1 year post-LT | Median | 4 × 106 | 2.8 × 106 | 0.1 |
| Genotype 1 | % | 55% | 42% | 0.1 |
| Patients treated | n | 13 (48%)† | 20 (19%) | 0.3 |
| SVR (censored)‡ | 3 (23%) | 8 (40%) | ||
| CMV infection | % | 21% | 13% | NS |
| Histological AHC | % | 54% | 23% | 0.015 |
| ACR episodes | 0/>2 | 35%/19% | 16%/20% | 0.1 |
| Initial immunosuppression | TAC/CYA/SIR | 62%/39%/0% (42% TAC mono) | 72%/25%/0.9% (27% TAC mono) | 0.05 |
| Steroids | 54% | 60% | NS | |
| AZA/MMF | 46%/8% | 59%/8% | NS | |
| Maintain immunosuppression | TAC/CYA/SIR | 54%/23%/11% | 67%/19%/10% | NS |
| Steroids | 11% | 5% | NS | |
| AZA/MMF | 11%/19% | 23%/22% | 0.1 | |
| Follow-up | months | 73 (14-138) | 77 (15-191) | NS |
- * Cold ischemia time: interval from donor cross-clamp to removal from cold storage/warm ischemia time: interval between removal from cold storage and venous reperfusion.
- † A total of 6 of 13 were treated after decompensation.
- ‡ Censored at time of SVR.
- P values refer to the univariate analysis.
| Patients | Future Decompensation (27) | No Decompensation (108) | P Value | |
|---|---|---|---|---|
| Ishak stage* | 0-2/3-4/5-6 (%) | 26/37/37 | 87/12/1 | <0.001 |
| Ishak grade* | 0-6/7-12/13-18 (%) | 33/63/4 | 85/15/0 | 0.001 |
| CPA* | % median (range) | 11.9 (0.7-36) | 2.9 (0.20-10) | <0.001 |
| HVPG measured | n (%) | 22 (81%) | 67 (62%) | NS |
| HVPG* | mm Hg median (range) | 7 (4-16) | 4 (1-9) | <0.001 |
Clinical decompensation was defined as the first occurrence of clinical manifestation of ascites/hydrothorax, variceal bleeding, jaundice (≥3 mg/dL, in the absence of other causes), or encephalopathy.
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki. All patients gave written informed consent for both the procedure and the histological evaluation.
Liver Biopsies
Liver biopsy samples were formalin-fixed, paraffin-embedded, and stained with hematoxylin and eosin, Gordon and Sweet staining for reticulin, and chromotrope aniline blue. Another tissue section was stained with PicroSirius red for collagen quantification and determination of CPA by DIA. For each biopsy sample, there was histological evaluation for the stage of disease (fibrosis: 0-6) and the degree of necroinflammatory activity (grade: after combining the scores for piecemeal necrosis 0-4, confluent necrosis 0-6, focal necrosis 0-4, and portal inflammation 0-4) according to Ishak et al.13
We recorded the number of liver fragments in each biopsy and its total length (lengths of each fragment summed), and the number of portal tracts per fragment and in total.14 We excluded liver biopsies less than 12 mm long. Portal tracts were defined according to Crawford et al.,15 which is “focus of connective tissue containing at least 2 luminal structures (either/or bile duct, portal vein, or hepatic artery).” A portal tract was considered complete when its full circumference was visible or when at least three-quarters of the circumference and 3 luminal structures were visible. A portal tract was considered partial when its circumference was incomplete but contained at least 2 luminal structures. Portal tracts were not counted in biopsies with severe distortion of the liver architecture, such as in cirrhosis or severe nodular expansion, because it is impossible to recognize and count individual portal tracts in such cases.
The sections of each biopsy stained with PicroSirius red were used for DIA, which was performed by 2 authors blinded to each other's results and blinded to clinical information at that time (P.M. and G.I.), which in addition was a means to assess interobserver error. Interobserver variability was assessed by repeating the CPA measurements whenever there was a difference greater than 2% between the 2 assessors. The equipment used and the CPA measurement was performed as described.12
Acute cellular rejection (ACR) was graded using the Royal Free Hospital (RFH) score.16 An RFH score ≥4 established the diagnosis of ACR; if >7, rejection was treated. Corticosteroid-resistant cellular rejection was defined if there was no histological improvement in a biopsy 5 days after the first, despite intravenous methylprednisolone administered at 1 g daily for 3 days.
Acute hepatitis C (AHC) was diagnosed when alanine aminotransferase levels increased ≥2 times upper normal limit, together with histological changes consistent with hepatitis, ie, predominantly lobular inflammation and/or scattered parenchymal apoptosis, without diagnostic features of cellular rejection, bile duct loss, or other cause of liver injury.17
Hemodynamic Studies
HVPG was measured using standard techniques14, 18 associated with transjugular biopsy in 89 patients of 135 included in our study. Clinically significant portal hypertension was defined as HVPG ≥ 10 mm Hg.19-22
Virological Assays and Antiviral Therapy
Serum samples before transplant and at the time of 1-year liver biopsies, were collected, stored at −70°C, and analyzed for HCV RNA by polymerase chain reaction; HCV genotype was evaluated as described.17 At RFH, CMV viremia was screened for by polymerase chain reaction assay initially qualitatively thrice weekly, and then quantitatively twice weekly.23 Patients were considered for off-label antiviral HCV therapy of pegylated interferon and ribavirin if stage 4 fibrosis due to recurrent HCV is reached.
Immunosuppression Regimens
In our center, maintenance immunosuppression regimens have changed over the years. Cyclosporine-based immunosuppression changed to tacrolimus-based immunosuppression following the results of the TMC study.24 Before this study, a cohort received calcineurin inhibitor monotherapy25 with either cyclosporine (CYA) or tacrolimus (TAC). After the TMC study, patients received triple immunosuppression therapy with corticosteroids, TAC and azathioprine (AZA), or TAC monotherapy while participating in a randomized trial of TAC monotherapy versus triple therapy.26 Steroids were tapered within 3 months if possible. Patients who received steroids for more than 3 months (mainly indicated because of repeated rejection episodes) were defined as those with long-term steroid maintenance therapy. Mycophenolate mofetil (MMF) was used to substitute AZA if there was intolerance to AZA or renal dysfunction.
TAC (Prograf; Fujisawa, Ltd., Killorglin, County Kerry, Ireland) administered at 0.1 mg/kg/day or CYA at 10 mg/kg/day was given nasogastrically in 2 divided doses, starting within 6 hours from transplantation. AZA was given intravenously then orally, at 1 mg/kg/day, and methylprednisolone (16 mg/day intravenously) until oral intake was established, when 20 mg/day prednisolone was used. If poor renal and/or graft function was present, TAC dosing (evaluated every other day) was adjusted according to clinical progress or occurrence of adverse effects, with the aim to maintain a whole-blood level of 5-10 ng/mL as determined by microparticle enzyme immunoassay (ImxTacrolimus II; Abbott Laboratories, Abbott Park, IL). CYA dose was adjusted in order to maintain trough whole-blood levels between 150-200 ng/mL initially and 100-150 ng/mL thereafter. AZA dose was not changed unless neutropenia developed. Prednisolone was gradually tapered from 3 weeks and then stopped between 3 and 6 months.
Acute rejection episodes were treated with 1 g daily methylprednisolone for 3 days, given intravenously. If rejection reoccurred and was not satisfactorily resolved by 1 further cycle of 1 g daily methylprednisolone for 3 days, it was considered as steroid-resistant rejection and was treated with lymphocyte antibodies Orthoclone (OKT-3) or antithymocyte globulin (ATG).
Statistical Analysis
All data were analyzed using the statistical package SPSS (version 13.0; SPSS Inc., Chicago, IL). The chi-square test was used for comparing quantitative variables. Quantitative variables normally distributed were expressed as mean values ± 1 standard deviation and non-normally distributed as median values (range). Significance testing was set to P = 0.05. Patients were censored at death, last follow-up, retransplant, or if sustained virologic response (SVR) was achieved.
Cox regression analysis was used to determine factors associated with clinical decompensation. The Kaplan-Meier method was used to plot curves for each variable that was statistically significant in the multivariate analysis. Time to decompensation was defined as the time from transplantation to the first episode of decompensation. For those with no decompensation, the interval to last follow-up or death was used as the observation interval.
We also evaluated area under the receiver operating curve (AUROC) of the prediction of clinical decompensation using CPA, stage according to Ishak, and HVPG cutoffs. The best cutoff of the curve (Youden index) was determined by the software program.
Results
Follow-up for the 135 patients was for a median of 76 months (15-191). The median recipient age was 52 years (21-66) and 101 of the patients were male (75%); median donor age was 41 years (16-70); 48% had genotype 1, 31% had genotype 3, and 6 donors had concomitant hepatitis B virus (HBV) infection. Antiviral therapy for HCV was given in 39 patients (17 within 12 months post-LT), and 11 achieved SVR (2 within 12 months post-LT). The 6 with HBV had HBV DNA completely suppressed. CMV infection was diagnosed and treated in 19 patients. A total of 39 had an episode of acute hepatitis. A total of 19% had no episodes of acute rejection, 37% had 1 episode, 24% had 2, and 20% had more than 2 episodes.
We reviewed 135 biopsies at 1 year after LT. The median length was 20 mm (mean, 21 mm; range, 12-45); 112 of the liver biopsies were 15 mm or longer. The median number of portal tracts was 12 (range, 4-22). In 5 biopsies (3.7%), the portal tracts were not counted because of cirrhosis or severe nodularity.
At 1 year, median CPA was 4.63% (range, 0.2%-36%). The stage according to Ishak was 0-2 in 101 patients, 3-4 in 23 patients, and 5-6 in 11 patients, whereas Ishak grade was 0-6 in 92 patients, 7-12 in 16 patients, and 13-18 in 1 patient.
During follow-up, 26 patients decompensated (19.3%) at a median of 61 months (range, 15-138 months); 10 patients presented with ascites and/or hydrothorax, 5 with variceal bleeding, 6 with portosystemic encephalopathy, and 5 with jaundice. Patients' characteristics with respect to compensated/decompensated groups are shown in Tables 1 and 2. Death occurred in 33 patients (24.4%) at a median of 74 months (15-165 months), 11 from causes related to decompensation and 22 unrelated.
Univariate and Multivariate Analysis
All the demographic and clinical data listed in Tables 1 and 2 were evaluated: 10 variables (P close or ≤ 0.1) which resulted from the univariate analysis were included in the Cox regression analysis for the prediction of decompensation: CPA, stage and grade according to Ishak at 1-year biopsy, ACR episodes, histological episodes of AHC, viral load at 1 year post-LT, donor age, genotype 1, initiation with TAC monotherapy, and AZA use at 1 year post-LT.
In the univariate analysis, grade (P = 0.01) and stage (P < 0.001) according to Ishak, AHC (P = 0.015), TAC monotherapy (P = 0.05), and CPA (P < 0.001) at 1-year biopsy were associated with clinical decompensation. However, multivariate analysis by Cox regression revealed that the only factor associated with the prediction of clinical decompensation was the CPA measurement (P = 0.001; Exp(B) = 1.148; 95% confidence interval [CI], 1.098-1.200). The receiver operating characteristics (ROC) curve of CPA for the prediction of decompensation is shown in Fig. 1. The AUROC was 0.965 (95%CI = 0.936-0.994). A cutoff value of 6% of CPA had an 82% sensitivity and 95% specificity for clinical decompensation and the highest Youden index. Kaplan-Meier curves of CPA ≤ 6% and CPA > 6% with respect to the prediction of decompensation are shown in Fig. 1: 102 patients had CPA ≤ 6%, 5 were decompensated (5%), whereas 22 of 33 patients with CPA > 6% were decompensated (67%).
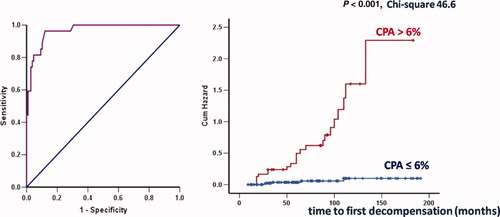
ROC curve of CPA for the prediction of clinical decompensation and Kaplan-Meier curve of CPA ≤ 6% and >6% with respect to the prediction of clinical decompensation (chi-square = 46.6, P < 0.001), in 135 patients with recurrent HCV, biopsied 1 year after liver transplantation. AUROC is 0.965 (95%CI, 0.936-0.994). At a cutoff value of 6% of CPA, there was 82% sensitivity and 95% specificity for clinical decompensation and the highest Youden index.
Relationship Between CPA and Ishak Stage
As we have already described,12 CPA values increased with worsening Ishak stage. The best cutoff of CPA was 6% for significant fibrosis (Ishak stage > 2), whereas for severe fibrosis (Ishak stage 5 and 6), the best cutoff value was 9%. Indeed, in this cohort of 135 patients, we confirmed the relationship between CPA values and the categories of stage (Fig. 2): stage 0 with median CPA values of 2% (interquartile range [IQR], 1.4%-5.35%; range, 0.40%-6.30%), stage 1 with median CPA of 2.4% (IQR, 1.6%-3.15%; range, 0.30%-18%), stage 2 with median CPA of 3.7% (IQR, 1.35%-4.7%; range, 0.20%-10%), stage 3 with median CPA of 4.7% (IQR, 2.02%-7.8%; range, 1.10%-23%), stage 4 with median CPA of 6.2% (IQR, 2.9%-11.4%; range, 2.80%-13.20%), stage 5 with median CPA of 9.9% (IQR, 7.025%-20.075%; range, 2.70%-36%) and stage 6 with median CPA of 21.7% (range, 15.80%-23%). As expected, there was a relationship between CPA values and stage with the correlation coefficient being r = 0.600, P < 0.001.
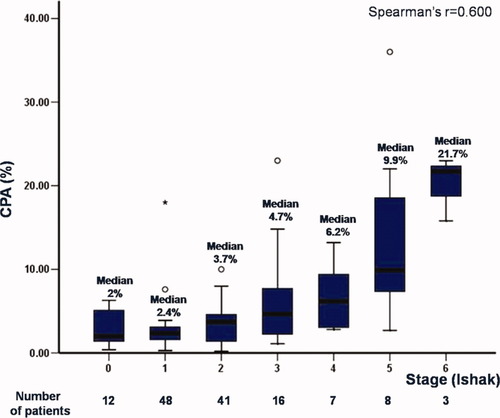
Relationship between CPA values (box plots) and stage according to Ishak in the total cohort of 135 patients.
Regarding Ishak stage, with respect to future decompensation, 4.2% (2/48) of patients decompensated with Ishak stage 1 at 1-year biopsy, 12.2% (5/41) decompensated with Ishak stage 2, 37.5% (6/16) with Ishak stage 3, 57% (4/7) with stage 4, 87.5% (7/8) with stage 5, and all the patients with stage 6 according to Ishak (Fig. 3). Stage 2 at 1-year biopsy had 83% sensitivity and 87% specificity for the prediction of clinical decompensation.
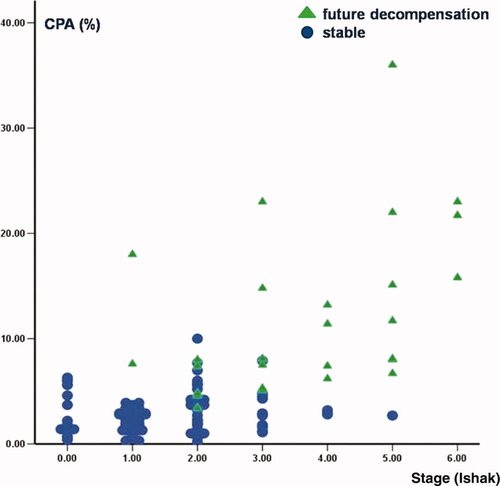
Relationship between CPA and Ishak stage with respect to prediction of future clinical decompensation.
Cohort with HVPG Measurement
HVPG was measured in 89 of 135 patients (Table 3): 22 decompensated at a median time of 65 months (12-146) and 18 patients died at a median of 61 months (14-157 months). This cohort was very similar to the whole group without any significant differences in demographic or clinical data. In the univariate analysis, we included the same variables listed in Tables 1 and 2, but with the addition of HVPG. Univariately, grade (P = 0.001) and stage (P < 0.001) according to Ishak, AHC (P = 0.005) and CPA (P < 0.001), as well as HVPG (P = 0.001) at 1-year biopsy were associated with clinical decompensation. However, in the multivariate analysis by Cox regression, only CPA independently predicted clinical decompensation (P < 0.001; Exp(B) = 1.158; 95%CI, 1.102-1.217). The ROC curves of CPA, HVPG, and categories of stage according to Ishak are shown in Fig. 4. The area under the ROC curve of CPA for the prediction of clinical decompensation was 0.962 (95%CI, 0.936-0.994), of stage 0.877 (95%CI, 0.781-0.972), and of HVPG 0.874 (95%CI, 0.790-0.959). At the cutoff value of 6% of CPA, there was 95% sensitivity and 93% specificity for the prediction of clinical decompensation. Kaplan-Meier curves of CPA ≤ 6% and CPA > 6% for this cohort with respect to the prediction of decompensation are shown in Fig. 4.
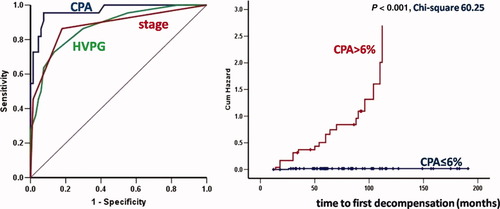
ROC curves of CPA, HVPG and categories of stage according to Ishak and Kaplan-Meier curves of CPA ≤ 6% and >6% with respect to the prediction of clinical decompensation (chi-square 60.25, P < 0.001) in the cohort of 89 patients with recurrent HCV and HVPG measurement, biopsied 1 year after liver transplantation. AUROC is 0.962 (95%CI, 0.936-0.994) for CPA, 0.877 (95%CI, 0.781-0.972) for stage and 0.874 (95%CI, 0.790-0.959) for HVPG. At a cutoff value of 6% of CPA, there was 95% sensitivity and 93% specificity for clinical decompensation and the highest Youden index.
| Patients with HVPG measured | Decompensation (22) | No Decompensation (67) | P Value | |
|---|---|---|---|---|
| Recipient | Age | 53 (21-65) | 51 (33-66) | NS |
| Males | 68% | 76% | NS | |
| Donor | Age | 44 (20-65) | 40 (16-57) | NS |
| Year of LT | 88-94/95-00/ 01-08 (%) | 13.6%/32%/ 54.4% | 7.5%/25%/ 67.5% | NS |
| Concomitant ALD | % | 13% | 28% | NS |
| Cold/warm ischemia | Minutes | 675/44 | 679/41 | NS |
| Diabetes pre/post-LT | % | 18%/27% | 20%/36% | NS |
| Viral load pre-LT | Median | 2.54 × 106 | 1.88 × 106 | NS |
| Viral load 1 year post-LT | Median | 4.2 × 106 | 2.9 × 106 | 0.1 |
| Genotype 1 | % | 57% | 47.5% | 0.1 |
| Patients treated | N | 13 | 19 | NS |
| SVR (censored) | 3 (23%) | 6 (32%) | ||
| CMV infection | % | 23% | 19% | NS |
| Histological AHC | % | 64% | 25% | 0.005 |
| ACR episodes | 0/>2 | 36%/9% | 10.6%/18% | 0.01 |
| Initial immunosuppression | TAC/CYA/SIR | 68%/32%/0% (46% TAC mono) | 79%/19%/1.5% (36% TAC mono) | 0.057 |
| Steroids | 50% | 42% | NS | |
| AZA/MMF | 45%/9% | 52%/3% | NS | |
| Maintain immunosuppression | TAC/CYA/SIR | 59%/18%/23% | 67%/15%/19% | NS |
| Steroids | 9% | 1.5% | NS | |
| AZA/MMF | 9%/18% | 18%/21% | 0.1 | |
| Follow-up | months | 72 (14-138) | 76 (15-191) | NS |
| Deaths | n | 10 | 8 | |
| Ishak stage | 0-2/3-4/5-6 (%) | 14/41/45 | 82/16/2 | 0.001 |
| Ishak grade | 0-6/7-12/13-18 (%) | 62/33/5 | 95/5/0 | 0.001 |
| CPA | % median (range) | 13.5 (3.4-36) | 3.2 (0.20-10) | 0.001 |
| HVPG | mm Hg median (range) | 8.45 (3-16) | 3.99 (1-10) | 0.001 |
Relationship Between CPA and HVPG
Previous findings suggested that CPA was a better histological correlate with HVPG than Ishak stage, showing greater numerical change when HVPG was low (<6 mm Hg).12 Indeed in the current study, there was a better relationship between CPA values and HVPG compared to CPA and Ishak stage, with the correlation coefficient being r = 0.612, P < 0.001.
With respect to clinical decompensation (Fig. 5), only 1 of 62 patients decompensated with CPA ≤ 6% (1.5%), whereas 21 of 26 (81%) patients decompensated with CPA > 6%. Considering portal hypertension (HVPG > 6 mm Hg), 11.3% (8/70) of the patients decompensated in the future when HVPG ≤ 6 mm Hg, whereas 74% (14/19) decompensated when HVPG > 6 mm Hg at 1-year biopsy. Interestingly, when both cutoff values were present (CPA > 6% and HVPG > 6 mm Hg), all of the patients above these cutoff points decompensated (Fig. 5).
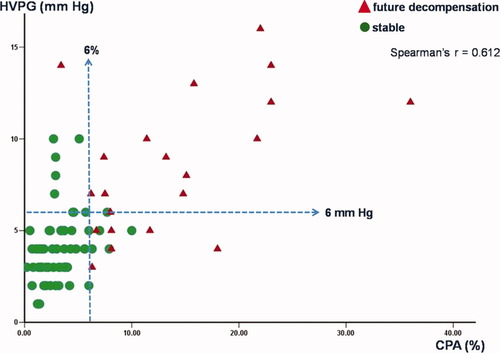
Relationship between CPA and HVPG values with respect to prediction of clinical decompensation in 89 patients with recurrent HCV who were biopsied 1 year after liver transplantation.
DISCUSSION
In this study, we retrospectively evaluated 135 patients with recurrent HCV, who underwent transplantation between 1988 and 2008 and who survived more than 12 months with a median follow-up of 76 months. We considered clinical decompensation as the endpoint in our study, and we looked at factors that could predict decompensation, comparing Ishak scoring system, CPA, and HVPG measured at the same time in biopsies at 1 year after transplantation.
We previously highlighted that quantitative assessment of liver tissue collagen must be validated against appropriate clinical outcomes and not histological stage scores alone.1 In this study, as in our previous studies, the amount of collagen was expressed as a simple proportionate area that we believe is a more representative method than that used in previous studies of computer-assisted DIA,10, 11, 27 as we have already previously noted.12
In this study, we showed that CPA measurement at 1 year post-LT was predictive of clinical decompensation in patients infected with HCV, with good sensitivity and specificity, which underscores the utility of a quantitative approach to measure fibrosis histologically. Univariately in the whole cohort, previous AHC, Ishak grade and stage, and CPA at 1-year biopsy were associated with clinical decompensation. The presence of AHC suggests that severity of necroinflammatory activity is related to rapid fibrosis progression. Multivariately by Cox regression analysis, CPA was a better discriminator for clinical decompensation than was Ishak stage. Indeed, there was considerable overlap of CPA values for the individual Ishak stages, as shown by the IQRs. At a cutoff value of 6% of CPA, there was an 82% sensitivity and 95% specificity for the prediction of clinical decompensation, representing clinically useful thresholds. Two other studies have also shown a relationship of liver collagen to outcomes. Goodman et al. measured collagen morphometrically in 535 patients randomized in the HALT-C (Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis) trial over a period of 5 years,27 but only in relation to long-term pegylated interferon in nonresponders and relapsers and not to clinical outcomes. In 53 children with biliary atresia, liver collagen at the time of a Kasai operation was a predictive marker of transplant-free survival.28 It is well established that HVPG has prognostic value in HCV-infected patients who underwent transplantation.29-31 An HVPG threshold of approximately 10 mm Hg defines clinically significant portal hypertension.32, 33 CPA in the cohort (89 patients) with HVPG measurement (Fig. 4) had a better AUROC than HVPG with respect to decompensation. CPA at the cutoff value of 6% showed a 95% sensitivity and 93% specificity. Importantly, better precision was obtained when CPA was combined with HVPG for the prediction of clinical outcome, because all patients with CPA > 6% and HVPG > 6 mm Hg at 1-year biopsy eventually decompensated (Fig. 5).
Several studies in the past have addressed the issue of clinical outcome in HCV-infected patients after transplantation, focusing on the identification of factors that were associated with worse recurrence of HCV.34-38 Factors associated with more aggressive disease recurrence were immunosuppression regimens17, 34, 39-44 and more recently treatment with TAC monotherapy compared to triple therapy with TAC, AZA, and steroids.26 Other factors have included CMV infection,45-47 HCV viral load and genotype,42, 48-50 donor age,41, 49-51 and a hepatitis flare.17, 26 Our current results are consistent with our previously published findings,17, 26 because the occurrence of AHC in the graft and TAC monotherapy were significant in the univariate analysis. However, all of the above studies including ours16, 25 are based on categorical descriptive scoring systems to grade/stage of disease recurrence without reference to quantitative changes in liver collagen.
The clinical significance of fibrosis in 1-year biopsy in HCV-infected patients who have undergone LT has been established by other groups48, 50, 52, 53 and more recently by Gallegos-Orozco et al.35 Patients with moderate fibrosis had worse outcome than patients with minimal or absent fibrosis. In our study, each Ishak stage category correlated with a range of CPA values, leading us to conclude once again12 that CPA could have additional individual prognostic value within each disease stage (Fig. 2).
In conclusion, in the current study, we confirmed at a single time point the good correlation between CPA and HVPG which had been shown previously over time.12 More importantly, we demonstrated for the first time that CPA was a good discriminator for clinical decompensation, when measured in 1-year biopsies in our cohort, and was a better discriminator than HVPG or Ishak stage. CPA predicted subsequent clinical outcomes and importantly gave added precision when combined with HVPG. Thus, CPA can be considered as the reference histological index for fibrosis in future studies in HCV-infected patients after LT, because it is both a quantitative and continuous measure of fibrosis. We believe it is a better reference standard to compare with noninvasive indices of fibrosis. However, further studies are needed to validate the significance of CPA measurement in other liver diseases and in the pretransplant setting for chronic hepatitis C.