Continuous development of arrhythmia is observed in swedish transplant patients with familial amyloidotic polyneuropathy (amyloidogenic transthyretin Val30Met variant)
Abstract
In patients with familial amyloidotic polyneuropathy (FAP), heart complications are prognostic factors for mortality and morbidity after liver transplantation (LT). However, only a few studies have analyzed the development of arrhythmia in transplant patients with FAP. We investigated the development of arrhythmia requiring pacemaker insertion (PMI) in Swedish transplant patients with FAP, and we related the findings to gender, age at disease onset, and survival. One hundred four transplant patients with the amyloidogenic transthyretin Val30Met mutation were included in the study. Twenty-six (25%) received a pacemaker during the observation period (a median of 11 years after disease onset). This frequency was comparable to that noted in a previous study describing the natural course of FAP. No significant differences in PMI between early-onset cases (<50 years old) and late-onset cases (≥50 years old) or between genders were observed. PMI was not significantly related to patient survival. Our study confirms our previously reported short-time observation: LT does not prevent the development of heart arrhythmia necessitating PMI. The development of arrhythmia is unrelated to gender or age at disease onset, and the yearly risk does not appear to decrease with time after LT. Liver Transpl 17:122–128, 2011. © 2011 AASLD
Familial amyloidotic polyneuropathy (FAP) is a hereditary systemic amyloidosis caused by a point mutation in the transthyretin (TTR) gene. The most common neuropathic mutation is caused by an exchange of valine for methionine in position 30 (ie, TTR Val30Met). The disease is characterized clinically by the systemic deposition of amyloid fibrils, which results in multiple-organ dysfunction and ultimately death.1, 2 The clinical manifestations are polyneuropathy, autonomic and gastrointestinal dysfunction, and cardiac complications such as cardiomyopathy and conduction disturbances.3-5
Liver transplantation (LT) has been shown to halt the progression of FAP. The rationale for LT is that mutant TTR is mainly produced by the liver, so an exchange of the mutant TTR–producing liver with one producing a wild type should cease only the production of amyloidogenic transthyretin (ATTR) and stop amyloid formation.6 According to the Familial Amyloidotic Polyneuropathy World Transplant Registry (http://www.fapwtr.org), approximately 110 FAP patients on average undergo transplantation worldwide each year. Previous studies have revealed that LT improves the survival of FAP patients, and this is especially true for females and individuals with early-onset disease (<50 years old).7-9
Although LT can halt the progression of the disease, unforeseen complications have emerged. Heart complications are the main cause of posttransplant death after LT, and an increasing amount of evidence points to the continuous deposition of amyloid fibrils derived from wild-type TTR in the myocardium.10, 11 Thus, LT does not prevent the development of heart complications in FAP.
Echocardiographic studies of Swedish and Japanese transplant patients with the ATTR Val30Met mutation have shown that cardiomyopathy arises in some patients after LT.12, 13 However, only a few studies have focused on cardiac arrhythmia in transplant patients with FAP. The continuous development of arrhythmia after LT [revealed by a Holter electrocardiogram (ECG)] has been reported.14 To elucidate the long-term development of arrhythmia that necessitates pacemaker insertion (PMI) in transplant patients with FAP, we studied 108 transplant patients followed for a median period of 11 years from the onset of the disease. Special attention was directed to gender and age at disease onset, which are factors with a proven relationship with cardiomyopathy in Swedish FAP patients.15
Abbreviations:
ATTR, amyloidogenic transthyretin; AV, atrioventricular block; BBB, bundle branch block; ECG, electrocardiogram; FAP, familial amyloidotic polyneuropathy; LT, liver transplantation; PMI, pacemaker insertion; SA, sinoatrial block; TTR, transthyretin; VT, ventricular tachycardia.
PATIENTS AND METHODS
One hundred eight FAP patients who were evaluated at Umeå University Hospital (Umeå, Sweden) from 1990 to 2008 and subsequently underwent LT were available for the study. In all cases, the diagnosis was based on typical clinical symptoms and findings of amyloid deposits in biopsy specimens, and it was confirmed by the identification of an ATTR mutation via genetic testing. All patients except 3 carried the TTR Val30Met mutation. Patients with other mutations (TTR Phe33Leu, Ala45Ser, or Leu55Gln) were excluded from the study. In addition, 1 patient with cardiomyopathy at the time of diagnosis who also suffered from hemochromatosis, a disorder in which cardiac involvement is common, was excluded. Thus, 104 patients were included in the study.
All patients underwent transplantation between 1990 and June 2008. In addition, all patients were followed for at least 6 months after the procedure. LT was performed at Karolinska University Hospital (Huddinge, Sweden) or at Sahlgrenska University Hospital (Gothenburg, Sweden). The patients' clinical details are listed in Table 1.
| Total | Early-Onset Cases (<50 Years Old) | Late-Onset Cases (≥50 Years Old) | P Value* | |
|---|---|---|---|---|
| Patients (n) | 104 | 65 | 39 | |
| Gender: male/female (n) | 57/47 | 31/34 | 26/13 | 0.06† |
| Age at onset (years) | 45 (22-67) | 36 (22-49) | 59 (50-67) | |
| Duration of disease at LT (years) | 3 (0-12) | 3 (0-12) | 2 (1-9) | 0.026 |
| Follow-up from the onset of disease (years) | 11 (2-25) | 12 (2-25) | 7 (2-15) | <0.01 |
| Deceased patients [n (%)] | 29 (28) | 14 (22) | 15 (38) | 0.06 |
- * Between early-onset cases and late-onset cases.
- † The number of females in the late-onset group tended to be smaller than the number in the early-onset group.
Medical records were scrutinized for information regarding the duration of the disease and clinical outcomes after LT. The date of onset of the disease was defined as the date of onset of symptoms related to amyloid disease (most commonly polyneuropathy). All patients underwent standard 24-hour Holter ECG. The presence of cardiac conduction and rhythmic disturbances was determined on the basis of standard 24-hour ambulatory ECG recordings. All recording were performed with a standard recorder unit (Tracker II, Reynolds Medical, Ltd., United Kingdom, or Braemer, Inc., Burnsville, MN). The ECG recordings were automatically analyzed with a personal computer–based Holter system (Holter reply unit, Danica, Borlänge, Sweden), and then they were examined and edited by an investigator (R.H.). Cardiac readings from 24-hour Holter recordings were analyzed for (1) rhythm disturbances, which included supraventricular arrhythmia (the presence of a series of more than 5 consecutive supraventricular beats and ventricular arrhythmia), the presence of 3 or more ventricular beats in series, more than 10 ventricular extrasystoles per hour, or some combination thereof,16 and (2) conduction disturbances, which included first- to third-degree atrioventricular block (AV), sinoatrial block (SA)/sinus arrest, and complete right bundle branch block (BBB) or left BBB. Patients without the aforementioned Holter ECG abnormalities were regarded as normal.
The patients were divided into 2 groups: those with early-onset disease (<50 years old) and those with late-onset disease (≥50 years old). The age of 50 years has been used to separate early-onset and late-onset cases in several previous studies.17, 18 In addition, the impact of gender was analyzed.
Differences between groups were tested with the Mann-Whitney U test and the chi-square test. Survival analysis was performed with Kaplan-Meier analysis, and differences between group survival rates were tested with the Cox-Mantel test. The hazard rate was calculated with life tables. For statistical analysis, SPSS 17.0 (SPSS, Inc., Chicago, IL) was used. A P value less than 0.05 was accepted as statistically significant.
RESULTS
The clinical differences between the early-onset and late-onset groups are shown in Table 1. The number of females in the late-onset group tended to be smaller than the number in the early-onset group. The duration of disease at LT and the duration of follow-up of the early-onset group were longer than those of the late-onset group. In comparison with the early-onset group, the frequency of death tended to be higher in the late-onset group (P = 0.06).
Twenty-six of the 104 patients underwent PMI (25%; Table 2). The median duration of the disease at PMI was 8 years (range = 2-18 years). In 26 patients with PMI, there was no statistical significance between the early-onset and late-onset groups with respect to the duration of disease at LT. The follow-up duration from the onset of disease in the early-onset group with PMI was longer than that in the late-onset group.
| All PMI Cases | Early-Onset Cases (<50 Years Old) | Late-Onset Cases (≥50 Years Old) | P Value* | |
|---|---|---|---|---|
| PMI cases/all cases [n (%)] | 26/104 (25) | 18/65 (28) | 8/39 (21) | 0.24 |
| Gender: male/female (n) | 12/14 | 7/11 | 5/3 | 0.25 |
| Age at onset (years) | 43.5 (26-63) | 37.5 (29-49) | 58 (51-63) | |
| Duration of disease at LT (years) | 3.5 (1-19) | 4.5 (2-9) | 2.5 (1-8) | 0.18 |
| Follow-up from the onset of disease (years) | 13 (7-22) | 17 (9-22) | 11.5 (7-15) | 0.022 |
| Duration of disease at PMI (years) | 8 (2-18) | 9.5 (2-18) | 6.5 (4-13) | 0.24 |
- * Between early-onset cases with PMI and late-onset cases with PMI.
The clinical differences between the patients with PMI and the patients without PMI are shown in Table 3. No differences between males and females were noted for the number of PMIs. The age at onset for patients treated by PMI and the duration of their disease at LT were not significantly different from those for patients not treated by PMI. The follow-up was significantly longer for patients with PMI versus those without PMI.
| Patients With PMI | Patients Without PMI | P Value | |
|---|---|---|---|
| Patients (n) | 26 | 78 | |
| Gender: male/female (n) | 12/14 | 33/45 | 0.3 |
| Age at onset (years) | 43.5 (26-63) | 45 (22-67) | 0.8 |
| Duration of disease at LT (years) | 3.5 (1-9) | 3 (0-12) | 0.19 |
| Follow-up from the onset of disease (years) | 13 (7-22) | 10 (2-25) | <0.001 |
| Deceased patients [n (%)] | 6 (23) | 23 (29) | 0.53 |
In Table 4, details concerning PMI are outlined. Seven of 26 patients were pacemaker-treated before LT, and the time from PMI to LT was 8 months (range = 2-12 months). Two of these patients received PMI before the transplantation procedure after a request from the transplant center (Sahlgrenska University Hospital). The causes of PMI for the remaining 5 patients were a high degree of AV, SA/sinus arrest, or both.
| Time Between LT and PMI (Months) | Arrhythmia | Age at the Onset of Disease (Years) | Gender | Duration of Disease at LT (Years) | Follow-Up From the Onset of Disease (Years) | Dead or Alive | Age at PMI (Years) |
|---|---|---|---|---|---|---|---|
| PMI before LT | |||||||
| −12 | AV II, left BBB | 31 | Female | 8 | 18 | Alive | 38 |
| −10 | Preventable treatment | 37 | Female | 9 | 9 | Dead | 45 |
| −8 | SA | 45 | Female | 6 | 12 | Dead | 50 |
| −8 | Preventable treatment | 40 | Female | 5 | 12 | Alive | 45 |
| −4 | AV I and II | 61 | Male | 8 | 15 | Alive | 69 |
| −2 | AV I and II, SA | 50 | Female | 2 | 17 | Alive | 52 |
| −2 | AV I, left BBB | 36 | Male | 7 | 21 | Dead | 43 |
| PMI after LT | |||||||
| 17 | AV I and II, SA, BBB | 57 | Female | 4 | 12 | Dead | 63 |
| 20 | AV I and II, SA | 43 | Female | 2 | 10 | Alive | 47 |
| 20 | AV I and II | 63 | Male | 4 | 10 | Alive | 69 |
| 24 | AV II, SA | 46 | Female | 2 | 11 | Alive | 50 |
| 34 | AV II | 53 | Female | 2 | 12 | Alive | 58 |
| 40 | AV II, SA | 38 | Female | 6 | 14 | Alive | 48 |
| 47 | Atrial fibrillation/flutter | 62 | Male | 1 | 7 | Alive | 66 |
| 59 | AV I and II, nonconducted supraventricular beats | 53 | Male | 2 | 7 | Alive | 60 |
| 61 | AV II | 44 | Female | 5 | 17 | Alive | 54 |
| 61 | AV I and II, BBB, VT | 36 | Male | 5 | 19 | Alive | 46 |
| 79 | SA | 60 | Female | 2 | 11 | Alive | 69 |
| 85 | AV I and II | 48 | Male | 4 | 18 | Alive | 59 |
| 87 | AV I and II, SA | 27 | Female | 2 | 12 | Alive | 37 |
| 95 | AV I and II, SA | 50 | Male | 3 | 11 | Dead | 60 |
| 99 | AV I, SA | 37 | Male | 3 | 20 | Alive | 49 |
| 115 | AV III | 31 | Male | 6 | 22 | Alive | 47 |
| 121 | Atrial fibrillation | 51 | Male | 3 | 15 | Dead | 65 |
| 148 | AV I, II, and III | 33 | Female | 2 | 18 | Alive | 48 |
| 184 | AV II | 26 | Male | 3 | 20 | Alive | 44 |
Nineteen patients developed arrhythmia necessitating PMI after LT. The time of disease from LT to PMI was 61 months (range = 17-184 months). Fifteen of 19 patients developed cardiac conduction block (eg, AV, SA/sinus arrest, or both). The causes for the remaining 4 patients were as follows: bradycardia, heart failure, and atrial fibrillation/flutter in 2 patients; a conduction disturbance with first-degree AV and nonconducted supraventricular beats with extended RR intervals in 1 patient; and a conduction disturbance with BBB and ventricular tachycardia (VT) in 1 patient, who received an intracardiac defibrillator.
A Kaplan-Meier PMI plot for all patients is shown in Fig. 1A. The requirement for PMI appeared to be fairly constant during the first 18 years after disease onset. The estimate of the risk (hazard rate) of PMI increased year by year (Fig. 1B). There was a similar tendency in the frequency of PMI after LT (Fig. 2).
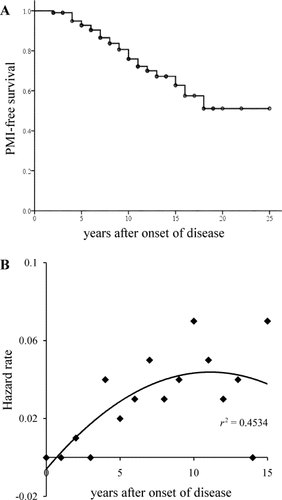
(A) Overall PMI-free survival and (B) yearly risk (hazard rate) of PMI in Swedish transplant patients. The time is from the onset of the disease and not from transplantation; PMI-free survival (not overall patient survival) is presented.
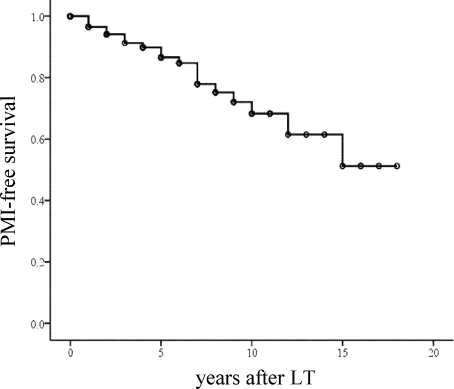
Overall PMI-free survival after LT. Seven patients who underwent PMI before LT are excluded from this figure; the total number of patients is 97.
In Fig. 3, the influence of the age at disease onset on PMI is displayed. No significant difference was noted between early-onset and late-onset groups. We also analyzed the association between gender and PMI, and no significant difference between the groups was observed (Fig. 3A,B).
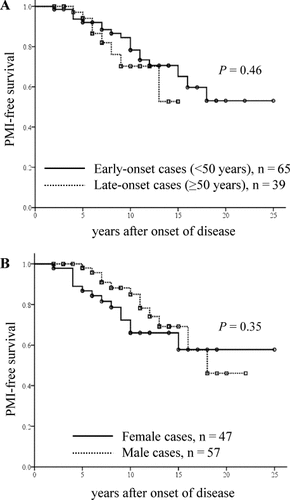
PMI-free survival in transplant patients: the influence of (A) age at disease onset [early onset (<50 years) and late onset (≥50 years)] and (B) gender on PMI. The time is from the onset of the disease and not from transplantation.
In addition, we investigated the long-term outcomes of patients with PMI and patients without PMI (Fig. 4). There was no statistical difference between the 2 groups, although pacemaker-treated patients tended to have a better survival rate than non–pacemaker-treated patients. The 10- and 15-year survival rates were 95% and 74%, respectively, for the patients with PMI and 79% and 56%, respectively, for those without PMI. In the PMI group, 6 of 26 patients died during follow-up. All 6 patients died from the progression of FAP disease and its complications. In the non-PMI group, 23 of 78 patients died. Heart-related death during or shortly after LT was the cause of death in 4 patients; this included circulatory collapse during the operation in 2 patients. In addition, 1 patient died suddenly 32 months after LT, and another died suddenly 89 months after LT; both deaths were probably due to heart arrhythmia.
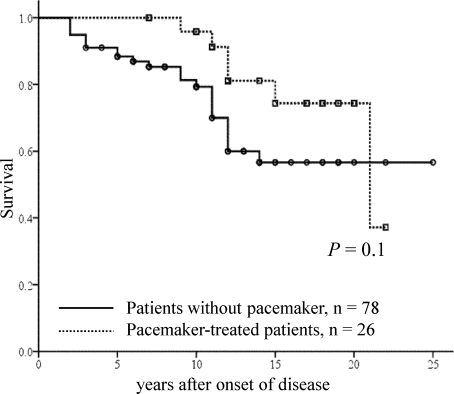
Kaplan-Meier survival plots for patients with or without PMI: the influence of PMI on the long-term outcomes of transplant patients with the ATTR Val30Met mutation. The time is from the onset of the disease and not from transplantation.
DISCUSSION
The present patient sample consists of all LT patients with FAP from our center, that is, practically all Swedish LT patients with FAP. The sample includes patients from the early period (1990-1995) when patients with severe disease were accepted for LT. Twenty-six of 104 patients (25%) underwent PMI. The proportion steadily increased to approximately 50% 18 years after disease onset. The hazard rate of PMI increased year by year, and even though it appeared to decrease after 15 years, the number of patients at risk was too limited to substantiate any declining risk. This frequency of PMI in the present transplant patient population was comparable to that noted for nontransplant patients (19/61, 31%) in a previous report.19 Several studies have shown that the development of conduction disturbances continues after LT.14, 20 Thus, LT does not prevent the development of heart arrhythmia necessitating pacemaker treatment; and the risk does not appear to diminish with time after the procedure.
We recently presented our investigation of the outcome of heart rate variability in transplant patients with FAP and controls.21 We were unable to detect any improvement, and the follow-up of the patients was limited, mainly by the development of subtle supraventricular arrhythmia not detectable by routine examination or computerized ECG analysis. Thus, conduction disturbances and supraventricular arrhythmia steadily develop in FAP patients after LT, and this makes heart rate variability unreliable for follow-up of autonomic neuropathy in FAP patients.
Conduction disturbances were the most common causes of PMI and occurred in 22 of 26 cases (85%). The indication for the remaining 2 patients was bradycardia with atrial fibrillation/flutter. In the present series, several patients had rhythm disturbances (eg, supraventricular beats in series and episodes of VT) in addition to conduction disturbances as indications for PMI. This contrasts with previous studies in which PMI was performed solely as a treatment for conduction disturbances.14, 20 Cardiac arrhythmia was previously noted as a complication in FAP patients in a Portuguese study; the authors noted a high prevalence of intermittent atrial and ventricular arrhythmias, including tachycardia (6%).22 A Japanese study of the natural course of the disease revealed that the frequency of VT was 8%.23 A study of Swedish FAP patients showed that the occurrence of ventricular late potential was common in older patients and was associated with unsustained ventricular arrhythmia.24 These observations suggest that a number of life-threatening arrhythmias, such as VT and fibrillation, in transplant patients with FAP may develop over time. Regular follow-ups with Holter ECG recordings are needed to detect not only conduction disturbances but also arrhythmias such as VT.
According to Fig. 1A,B, the risk of developing arrhythmia requiring PMI decreases with time. However, the number of patients with more than 15 years of follow-up is limited, and the apparent decrease probably reflects a decreased number of patients at risk.
In a previous study, we found that the survival of LT patients with late-onset FAP was similar to the survival of nontransplant patients. In addition, the survival of late-onset female patients was superior to the survival of late-onset males.8 Cardiomyopathy in FAP is related to a late onset of the disease and male gender.25, 26 It resembles that of senile systemic amyloidosis caused by wild-type TTR. In a histopathological study of senile systemic amyloidosis, heavy amyloid deposits were found in the myocardium. In the conduction system, the amount of amyloid was considerably less than that in the septum wall.27, 28 Our results raise the possibility that the amounts of amyloid deposition in the conduction system after LT are similar for early-onset and late-onset patients and likewise for females and males. It deserves to be mentioned that conduction disturbances were unrelated to septal hypertrophy in a series of Swedish nontransplant patients,15 and the frequency of pacemaker-requiring conduction disturbances was similar to that found in the present series of transplant patients.19
Autonomic disturbances with often marked orthostatic hypotension are common and serious complications of FAP. A defect in the autonomic regulation of the blood pressure and heart rate makes FAP patients more prone to serious arrhythmia when they develop conduction disturbances and especially nightly bradyarrhythmia because sudden death at night has been observed.29 Therefore, the indication for PMI is different for FAP patients versus other patients with arrhythmia; this can be seen from the indications for PMI displayed in Table 4. Our indication for pacemaker treatment remained constant during the observation period: a single episode of bradycardia depending on SA and/or atrioventricular disturbances and other extended RR intervals (eg, atrial fibrillation) with Adams-Stokes attacks in combination with reduced heart rate variability.
PMI was not associated with increased mortality. On the contrary, a trend of increased survival was noted. This may be related to sudden death occurring among our patients in the early series (1990-1995), for whom Holter ECG was not regularly checked after LT. Our findings indicate that PMI is an appropriate treatment for cardiac arrhythmia and that the yearly risk of developing arrhythmia is fairly constant after transplantation for a patient.
In conclusion, LT does not prevent the development of heart arrhythmia necessitating pacemaker treatment. The development of arrhythmia is not related to the age at disease onset or gender, and the risk does not appear to decrease with time after LT.