The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment†
The contents of this article were presented in part at the 15th Annual Congress, International Liver Transplantation Society, July 8-11, 2009, New York, NY.
Abstract
To expand the donor liver pool, ways are sought to better define the limits of marginally transplantable organs. The Donor Risk Index (DRI) lists 7 donor characteristics, together with cold ischemia time and location of the donor, as risk factors for graft failure. We hypothesized that donor hepatic steatosis is an additional independent risk factor. We analyzed the Scientific Registry of Transplant Recipients for all adult liver transplants performed from October 1, 2003, through February 6, 2008, with grafts from deceased donors to identify donor characteristics and procurement logistics parameters predictive of decreased graft survival. A proportional hazard model of donor variables, including percent steatosis from higher-risk donors, was created with graft survival as the primary outcome. Of 21,777 transplants, 5051 donors had percent macrovesicular steatosis recorded on donor liver biopsy. Compared to the 16,726 donors with no recorded liver biopsy, the donors with biopsied livers had a higher DRI, were older and more obese, and a higher percentage died from anoxia or stroke than from head trauma. The donors whose livers were biopsied became our study group. Factors most strongly associated with graft failure at 1 year after transplantation with livers from this high-risk donor group were donor age, donor liver macrovesicular steatosis, cold ischemia time, and donation after cardiac death status. In conclusion, in a high-risk donor group, macrovesicular steatosis is an independent risk factor for graft survival, along with other factors of the DRI including donor age, donor race, donation after cardiac death status, and cold ischemia time. Liver Transpl 16:874–884, 2010. © 2010 AASLD.
As attempts have been made to expand the donor pool, defining the limitations of marginal organs for liver transplantation has become more refined and more critical. Many investigators have examined the variables associated with patient and graft outcome after liver transplantation.1-28 The variables can be categorized into: donor factors, procurement logistics, recipient factors, and operative factors. Having a clear understanding of the donor factors and procurement logistics factors can improve recipient selection, organ allocation, and potentially patient and graft survival.
The Donor Risk Index (DRI) developed in 2006 by Feng et al.29 contains 7 donor and 2 procurement characteristics (including cold ischemia time [CIT]) that predict an increased risk of graft failure. The objective and quantitative nature of the DRI enables frank discussion between transplant staff and potential recipients regarding the risks involved with prospective donor organs. Importantly, the factors required to determine the DRI are known, and the CIT can be estimated at the time of the organ offer, allowing responsible assessment by transplant staff and honest discourse with the potential recipient(s). Given that the most severely ill patients have the highest risk of dying and derive the most survival benefit from liver transplantation, an appreciation of the risks inherent in a particular donor allograft should help direct an organ to the neediest recipient who is able to tolerate the physiologic challenge(s) brought by that organ.30 One drawback of the DRI is that, in its development, steatosis of the donor graft was excluded from the analysis.29
Although not included in the DRI formula, we hypothesized that increased donor hepatic steatosis is an independent risk factor that further compromises graft survival. Additional reports, and our own institutional experience, have suggested that donor liver steatosis and its association with CIT might have an important influence on transplant outcomes.31-44 Therefore, we predicted that liver biopsies requested in donors are from the higher-risk donors. In high-risk donors, we hypothesized that the additional factor of hepatic steatosis, and possible association with CIT, would be shown as independent factors associated with survival with other factors of the DRI.
Abbreviations:
DRI, Donor Risk Index; CIT, cold ischemia time; DBD, donation after brain death; DCD, donation after cardiac death; CVA, cerebrovascular accident; HCV, hepatitis C virus; MELD, model for end-stage liver disease; RR, relative risk; UNOS, United Network for Organ Sharing;
PATIENTS AND METHODS
Study Population
After approval by the University of Washington Institutional Review Board, we conducted a retrospective review of the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research files from October 1, 2003, through February 6, 2008, based on Organ Procurement and Transplantation Network data as of February 6, 2008. Data were analyzed for all adult (≥18 years old) patients undergoing orthotopic liver transplantation using livers from deceased donors. Multiple organ transplants were excluded. There were 21,777 patients involved in this study. Graft survival was assessed according to donor risk factors and procurement logistics at the time of transplantation.
Donor factors analyzed included age, sex, height, weight, race, type of donor (donation after brain death [DBD] or donation after cardiac death [DCD]), split or reduced liver, cause of donor death (anoxia, cerebrovascular accident [CVA] or stroke, head trauma or central nervous system carcinoma, or other), presence of any type of diabetes mellitus (as recorded in the UNOS records), hypertension (requiring medication), history of any type of cancer, cardiac arrest prior to procurement, requirement for inotropic support at time of procurement (as recorded in the UNOS files), hepatitis C virus (HCV) status, presence of pulmonary infiltrate, and percentage of macrovesicular and microvesicular fat in donor liver biopsy. Only donors with complete data for macrovesicular fat were included. The following laboratory values were collected prior to procurement (all measured in milligrams per deciliter): serum creatinine, total bilirubin, serum glutamic oxaloacetic transaminase, and serum glutamic pyruvic transaminase.
Procurement logistics factors analyzed from the procurement included allocation source of donor liver (local, regional, or national), distance in miles from the donor liver to the transplant center, and CIT in hours (time period from excision of the donor liver and placement on ice until removal from ice at transplantation).
To isolate the significant donor factors for graft survival, adjustments for recipient factors were made. Recipient data collected included age, sex, body mass index, race, and etiology of liver disease (as listed in the UNOS records), including fulminant hepatic failure, HCV (including HCV together with any other diagnosis), other noncholestatic liver disease, cholestatic liver disease (primary biliary cirrhosis or primary sclerosing cholangitis), metabolic liver disease (α-1-antitrypsin deficiency, Wilson's disease, hemochromatosis, and others), presence of tumor (any type) as the primary diagnosis, and other liver diseases. Other recipient factors collected included presence of hepatocellular carcinoma with other liver disease at time of transplant, medical condition at time of transplant (in the intensive care unit, hospitalized not in the intensive care unit, and not hospitalized), on life support (ventilator, aortic balloon pump, etc.), previous liver transplant, presence of portal vein thrombosis, previous abdominal surgery, on dialysis at time of transplant, and presence of any form of diabetes mellitus as noted in the UNOS records. Laboratory factors collected included serum albumin (mg/dL), and the laboratory model for end-stage liver disease (MELD) score was recorded for each at the time of transplant.
Statistical Analysis
All missing data for each variable were noted. There was no data imputation for statistical analysis. Continuous variables were given as the mean ± standard deviation, and categorical variables were presented as percentages. The Cox proportional hazards model was used to determine univariable and multivariable factors predicting 1-year graft survival. Graft loss was determined by time from transplant to graft loss as determined by either retransplantation or recipient death, similar to the calculations of the DRI.29 For all of the analyses, a P value ≤ 0.05 was considered significant. The data were evaluated using the JMP version 8.0.1 statistical software package (SAS Institute, Cary, NC).
RESULTS
Study Population
From October 1, 2003, through February 6, 2008, there were 21,777 adult primary liver transplants or retransplants performed in the United States, as shown by the UNOS Standard Transplant Analysis and Research files. There were 5051 patients with records that included the percentage of macrovesicular fat found on donor liver biopsy (23.2% of all donors). Comparing the 16,726 donors whose livers did not undergo biopsy to the 5051 donors whose livers were biopsied revealed that the biopsied donors were a higher-risk group (Table 1). This group of donors had a higher DRI, was older, had a higher body mass index, and had a higher incidence of death from anoxia and CVA/stroke, and a lower incidence of death from head trauma. The biopsied donors also had an increased incidence of diabetes mellitus, hypertension, HCV-positive status, and were shared nationally. This higher-risk donor group became our study population.
| Donor Factors | Not Biopsied (n = 16,726) | Biopsied (n = 5051) | P Value |
|---|---|---|---|
| Donor Risk Index* | 1.52 ± 0.4 | 1.73 ± 0.43 | <0.0001 |
| Age (years) | 38 ± 17.1 | 49.6 ± 15.6 | <0.0001 |
| Body mass index | 25.8 ± 5.2 | 28.3 ± 6.7 | <0.0001 |
| Donation after cardiac death | 4.90% | 4.00% | 0.004 |
| Cause of death | |||
| Anoxia | 10.20% | 15.70% | <0.0001 |
| Cerebrovascular accident/stroke | 40.40% | 56.70% | <0.0001 |
| Head trauma | 43.40% | 25.00% | <0.0001 |
| Central nervous system tumor | 0.80% | 0.70% | 0.4 |
| Other | 1.80% | 2.00% | 0.2 |
| Split liver | 1.60% | 0.30% | <0.0001 |
| Race | |||
| African American | 15.30% | 15.50% | 0.7 |
| Other | 14% | 9.10% | <0.0001 |
| Diabetes | 7.30% | 16.60% | <0.0001 |
| Hypertension | 40.40% | 57.90% | <0.0001 |
| History of cancer | 3.00% | 5.50% | <0.0001 |
| Inotropic support | 59.70% | 61.80% | 0.003 |
| Creatinine (mg/dL) | 1.4 ± 1.5 | 1.6 ± 1.6 | <0.0001 |
| Bilirubin (mg/dL) | 0.98 ± 1 | 0.94 ± 0.93 | 0.01 |
| SGOT (mg/dL) | 78.8 ± 147 | 82.9 ± 163 | 0.07 |
| SGPT (mg/dL) | 64.7 ± 148 | 67.2 ± 141 | 0.2 |
| Hepatitis C virus positive | 1.20% | 6.70% | <0.0001 |
| Cold ischemia time (hours) | 7.3 ± 3.3 | 8 ± 3.6 | <0.0001 |
| Distance (miles)† | 142 ± 239 | 188 ± 296 | <0.0001 |
| Regional | 23.90% | 22% | 0.01 |
| National | 5.10% | 13.80% | <0.0001 |
- * Donor Risk Index (DRI) as calculated by the following formula:
- DRI = exp[(0.154 if 40 ≤ age <50) + (0.274 if 50≤ age <60) + (0.424 if 60≤ age <70) + (0.501 if 70 ≤ age) + (0.079 if COD = anoxia) + (0.145 if COD = CVA) + (0.184 if COD = other) + (0.176 if race = African American) + (0.126 if race = other) + (0.411 if DCD) + (0.422 if partial/split) + (0.066[{170 − height}/10]) + (0.105 if regional share) + (0.244 if national share) + (0.010 × cold time)].
- † Distance in miles between the hospital where the donor is located and the transplanting center. COD, cause of death; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Donor, Procurement Logistics, and Recipient Factors
There were 846 (16.7%) allograft failures among the 5051 transplants that had findings of macrovesicular fat on donor liver biopsy. The mean follow-up interval was 388 days ± 335 days.
The donor and procurement logistics factors are listed in Table 2. Approximately 75% of the donors were ≥ 40 years old, 9.7% of the donors were ≥ 70 years old, 72.5% of the donors were white, and 4.2% were DCD donors. The largest cause of donor death was CVA or stroke (56.6%). Inotropic support was required by 61.1% of the donors. The majority of the macrovesicular and microvesicular steatosis results from the donor liver biopsies were reported in increments of 5% (Fig. 1). These categories of 5% increments were retained for analysis. With respect to procurement logistics, 65.5% of the donors were local, with the donated livers traveling a mean of 183.5 ± 289.4 miles, and the mean CIT was 7.9 ± 3.2 hours. Only 7 factors had missing data. Other than the factors of microvesicular fat and CIT, the missing data comprised ≤ 1.2% of each data point. Missing data for CITs were noted as a result and used in the analysis.
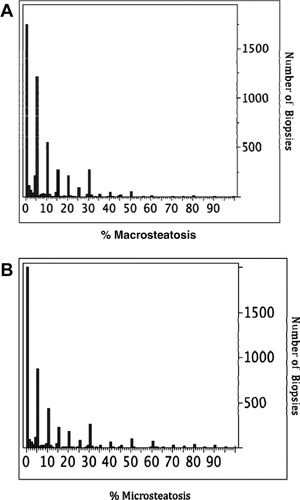
Distribution of the actual number of clinical reports of steatosis in the donor liver biopsies. Most results are given in 5% increments. (A) Distribution of the actual number of clinical reports of macrovesicular steatosis. (B) Distribution of actual number of clinical reports of microvesicular steatosis.
| Donor Factors | Number | Percent | Missing Data |
|---|---|---|---|
| Age | None | ||
| 0-17 | 104 | 2.0% | |
| 18-39 | 1069 | 21.2% | |
| 40-49 | 1190 | 23.6% | |
| 50-59 | 1339 | 26.5% | |
| 60-69 | 860 | 17.0% | |
| 70+ | 489 | 9.7% | |
| Male | 2680 | 53.1% | None |
| Height (cm) | 171.1* | ±10.5† | None |
| Weight (kg) | 83.7* | ±21.4† | None |
| Donor race | None | ||
| Asian | 98 | 1.9% | |
| African American | 769 | 15.2% | |
| Hispanic | 466 | 9.2% | |
| Other | 56 | 1.2% | |
| White | 3662 | 72.5% | |
| Donation after cardiac death | 212 | 4.2% | None |
| Partial/split | 17 | 0.3% | None |
| Cause of donor death | None | ||
| Anoxia | 786 | 15.6% | |
| Cerebrovascular accident/stroke | 2860 | 56.6% | |
| Head trauma | 1280 | 25.3% | |
| Central nervous system carcinoma and Other | 125 | 2.5% | |
| Diabetes | 830 | 16.5% | 0.6% |
| Hypertension | 2423 | 48.4% | 0.9% |
| History of cancer | 290 | 5.7% | 0.3% |
| Cardiac arrest | 233 | 4.7% | 1.2% |
| Inotropic support | 3071 | 61.1% | 0.4% |
| Creatinine (mg/dL) | 1.58* | ±1.57† | None |
| Bilirubin (mg/dL) | 0.94* | ±0.93† | 0.2% |
| Macrovesicular steatosis categories | None | ||
| 0% | 1729 | 34.3% | |
| 5% | 1634 | 32.3% | |
| 10% | 609 | 12% | |
| 15% | 313 | 6.2% | |
| 20% | 223 | 4.3% | |
| 25% | 91 | 1.8% | |
| 30% | 299 | 5.6% | |
| >30% | 153 | 3% | |
| Microvesicular steatosis categories | 7.5% | ||
| 0% | 1957 | 41.9% | |
| 5% | 1139 | 24.4% | |
| 10% | 502 | 10.7% | |
| 15% | 244 | 5.2% | |
| 20% | 171 | 3.7% | |
| 25% | 63 | 1.3% | |
| 30% | 269 | 5.8% | |
| >30% | 326 | 6.9% | |
| Logistics factors | |||
| Location | None | ||
| Local | 3309 | 65.5% | |
| Regional | 1057 | 20.9% | |
| National | 685 | 13.6% | |
| Distance (miles) | 183.5* | ±289.4† | None |
| Cold ischemia time (hours) | 7.9* | ±3.2† | 9.6% |
- * Mean;
- † Standard deviation.
For statistical analysis, donor age was categorized in a similar manner as in Feng et al.29 Macrovesicular steatosis results had 8 categories of 5% increments: 0%, 5%, 10%, 15%, 20%, 25%, 30%, and all the increments above 30% (which were in one category due to small numbers with >30% steatosis). Using 0% as the reference group, a univariable Cox regression analysis was performed for 1-year graft survival (P = 0.03). From this analysis, we were able to recategorize the macrovesicular steatosis biopsy results into 3 groups: the categories 0%, 5%, 10%, and 15% were combined into 1 group (relative risk [RR] = 1, 1.03, 0.95, and 0.94, respectively). The categories 20%, 25%, and 30% (RR = 1.37, 1.38, and 1.3, respectively) were combined into the second group. All levels >30% (RR = 1.61) formed a third group. Microvesicular steatosis results had 8 categories (like macrovesicular steatosis) of 5% increments each, and all biopsy results >30% were placed into one group. Using 0% as the reference group, univariable Cox regression analysis was performed for 1-year graft survival (P = 0.7). None of the categories (5%, 10%, 15%, 20%, 25%, 30%, and >30%, with RR = 0.89, 1.06, 0.82, 1.19, 1.04, 0.99, and 0.97, respectively) were significantly different from the reference category. CITs were stratified into 15 categories with 0 to 4 hours into 1 category, then each succeeding hour into a separate category until 16 hours, and finally, all ischemia times of >16 hours into one category (Table 3). The group with unknown ischemia times (9.6% of the donors) was a separate category. More than three-fourths (>78.5%) of all CITs were ≤ 11 hours. Univariable Cox regression analysis of CITs for 1-year graft survival allowed grouping CIT into 4 distinct groups. With similar RR values, the 0-hour to 4-hour reference group and the 5-hour group formed 1 group. Groups having ischemia times > 5 hours but ≤ 11 hours had similar RR values and formed a second group. A marked increase in RR occurred in groups with ischemia times > 11 hours; these formed a third group. The group with unknown ischemia times formed a fourth group, with a RR of 2.69.
| Hourly Categories | Number | Percent | Relative Risk* | P Value |
|---|---|---|---|---|
| 0-4 hours | 387 | 7.7% | Reference | |
| >4-5 hours | 438 | 8.7% | 1.04 | 0.9 |
| >5-6 hours | 604 | 11.9% | 1.65 | 0.007 |
| >6-7 hours | 626 | 12.4% | 1.92 | 0.0002 |
| >7-8 hours | 682 | 13.5% | 1.68 | 0.004 |
| >8-9 hours | 512 | 10.1% | 1.61 | 0.01 |
| >9-10 hours | 436 | 8.6% | 1.73 | 0.005 |
| >10-11 hours | 284 | 5.6% | 1.54 | 0.05 |
| >11-12 hours | 117 | 2.3% | 2.31 | <0.0001 |
| >12-13 hours | 262 | 5.2% | 2.1 | 0.003 |
| >13-14 hours | 89 | 1.8% | 3.52 | <0.0001 |
| >14-15 hours | 54 | 1.1% | 2.2 | 0.03 |
| >15-16 hours | 25 | 0.5% | 2 | 0.17 |
| >16 hours | 50 | 1.0% | 2.14 | 0.06 |
| Unknown | 485 | 9.6% | 2.69 | <0.0001 |
- * Univariable Cox proportional hazards model.
Recipient factors were collected to allow for adjustments for statistical analysis (Table 4). The average age was 52.7 ± 11.5 years. At the time of transplantation, approximately 25% of the recipients had HCV, and 20.8% had hepatocellular carcinoma. Only 8.1% of the recipients were in the intensive care unit, and 4.1% required some form of life support at transplantation. The mean calculated MELD score was 19.9 ± 9. Only 6 factors had missing data; previous abdominal surgery had 6% missing values, whereas the other factors had ≤ 2% missing values.
| Recipient Factors | Number | Percent | Missing Data |
|---|---|---|---|
| Age (years) | 52.7* | 11.5† | None |
| Male | 3534 | 70.00% | None |
| Body mass index | 28.8* | ±5.8† | 0.20% |
| Recipient race | None | ||
| Asian | 214 | 4.20% | |
| African American | 420 | 8.30% | |
| Hispanic | 508 | 10.00% | |
| Other | 51 | 1.00% | |
| White | 3858 | 76.50% | |
| Diagnosis | None | ||
| Fulminant hepatic failure | 252 | 5.00% | |
| Hepatitis C | 1258 | 24.90% | |
| Other noncholestatic liver disease | 1993 | 39.50% | |
| Cholestatic liver disease | 423 | 8.40% | |
| Metabolic liver disease | 117 | 2.30% | |
| Malignant liver disease | 770 | 15.20% | |
| Other | 238 | 4.70% | |
| Hepatocellular carcinoma at transplant | 1053 | 20.80% | None |
| Medical condition | 0.20% | ||
| In intensive care unit | 407 | 8.10% | |
| Hospitalized, not in intensive care unit | 741 | 17.70% | |
| Not hospitalized | 3891 | 77.20% | |
| On life support | 209 | 4.10% | None |
| Previous liver transplant | 39 | 0.80% | None |
| Portal vein thrombosis | 258 | 5.20% | 2.00% |
| Previous abdominal surgery | 1766 | 35.00% | 6% |
| On dialysis | 358 | 7.10% | 0.05% |
| Diabetes mellitus | 1138 | 23.00% | 2.00% |
| Albumin (mg/dL) | 2.93* | ±0.7† | None |
| Model for End-Stage Liver Disease (MELD) | 19.9* | 9† | None |
- * Mean;
- † Standard deviation.
Univariable Analysis of Donor Risk and Procurement Logistics Factors for Graft Loss
Examination of the univariable analysis for 1-year graft survival (Table 5) demonstrated 10 variables prognostic for allograft survival. Similar to the findings of Feng et al.,29 our univariable analysis also found 3 donor demographic characteristics (age, race, and height) and 2 characteristics of donor death (CVA/stroke and DCD) to be significant. Compared to the reference group of donor age 18 to 39, increasing age was associated with a significant increase in the risk of graft failure. Donor age ≥ 40 but < 50 had a RR of 1.34 (P = 0.01) for graft failure. Donor age ≥ 50 but < 70 had a RR of about 1.54 (P < 0.01) for graft loss. Donor age ≥ 70 had the strongest risk factor for graft failure at 1 year (RR = 1.83, P < 0.0001). Livers from African American and Hispanic donors had an approximately 30% higher risk of graft failure at 1 year compared to white donors (RR > 1.3; P < 0.02). The parameters of donor size were assessed: the association of graft failure with height was stronger than, and independent of, the association with weight. Each 10 cm decrease in height resulted in increased graft loss, with a RR of 1.08 (P = 0.02). Compared to trauma as a cause of death, CVA/stroke was associated with a 22% risk of graft failure (RR = 1.22; P < 0.02). DCD donors were even more significantly associated with graft failure at 1 year (RR = 1.62; P = 0.002). The presence of either diabetes mellitus or hypertension in the donor was also associated with graft failure at 1 year. The location of the donor was associated with graft loss. Compared to local donors, the use of regional donor livers resulted in a 22% increase in graft loss (RR = 1.22, P = 0.02), and the use of national donors produced a 34% increase in graft loss (RR = 1.34, P = 0.003). Compared to CIT of 0 to 5 hours, the CIT > 5 hours but ≤ 11 hours was associated with a 67% increased risk of graft failure at 1 year (RR = 1.67, P < 0.0001). CIT of > 11 hours increased the risk of graft failure at 1 year by 135% (RR = 2.35, P < 0.0001).
| Donor Factors | 1-Year Univariable Analysis | |
|---|---|---|
| Relative Risk | P Value | |
| Age (years) | ||
| 0-17 | 0.92 | 0.8 |
| 18-39 | Ref | |
| 40-49 | 1.34 | 0.01 |
| 50-59 | 1.54 | <0.0001 |
| 60-69 | 1.53 | 0.0003 |
| 70+ | 1.83 | <0.0001 |
| Male | 1.01 | 0.9 |
| Height (cm), per 10 cm decrease | 1.08 | 0.02 |
| Weight (kg) | 0.99 | 0.7 |
| Donor race | ||
| Asian | 1.27 | 0.3 |
| African American | 1.31 | 0.004 |
| Hispanic | 1.32 | 0.02 |
| Other | 0.68 | 0.3 |
| White | Ref | |
| Donation after cardiac death | 1.62 | 0.002 |
| Partial/split | 1.72 | 0.3 |
| Cause of donor death | ||
| Anoxia | 1.01 | 0.9 |
| Cerebrovascular accident/stroke | 1.22 | 0.02 |
| Head trauma | Ref | |
| Central nervous system carcinoma and Other | 1.1 | 0.7 |
| Diabetes | 1.21 | 0.03 |
| Hypertension | 1.31 | <0.0001 |
| History of cancer | 1.24 | 0.1 |
| Cardiac arrest | 1.04 | 0.8 |
| Inotropic support | 112 | 0.1 |
| Log creatinine | 1.02 | 0.7 |
| Log bilirubin | 0.99 | 0.8 |
| Macrovesicular steatosis groups | ||
| 0%, 5%, 10%, and 15% | Ref | |
| 20%, 25%, and 30% | 1.27 | 0.02 |
| >30% | 1.61 | 0.008 |
| Logistics Factors | ||
| Distance (miles) per 100 miles | 1.01 | 0.6 |
| Location | ||
| Local | Ref | |
| Regional | 1.22 | 0.02 |
| National | 1.34 | 0.003 |
| Cold ischemia time (hours) | ||
| ≤5 | Ref | |
| >5≤11 | 1.67 | <0.0001 |
| >11 | 2.35 | <0.0001 |
| Unknown | 2.64 | <0.0001 |
- Ref, reference value.
Building on the original DRI study by Feng et al.,29 we found that percent macrosteatosis of the donor liver was significantly associated with graft failure at 1 year. Using the group of 0%, 5%, 10%, and 15% macrosteatosis as the reference, the 20%, 25%, and 30% macrosteatosis group increased the risk of graft failure by 27% (RR = 1.27, P = 0.02). The >30% macrosteatosis group increased the risk of graft failure by >60% (RR = 1.62, P = 0.008).
Multivariable Analysis of Donor Risk and Procurement Logistics Factors for Graft Loss
In both the unadjusted and adjusted multivariable analysis (adjusted by recipient characteristics shown in Table 4), 5 factors were found to be significant for graft loss at 1 year after transplantation. Increasing donor age was associated with increasing risk of graft loss (Table 6). Donor age ≥ 40 but < 50 produced a 40% increased risk of graft loss (RR = 1.4, P = 0.003), while donor age ≥ 50 but < 70 gave a 60% increase in graft loss (RR = 1.6, P < 0.0001). Donor age ≥ 70 gave the largest increased risk of graft loss at 110% (RR = 2.1, P < 0.0001). Livers from African American and Hispanic donors resulted in >30% increase in graft loss (RR = 1.36 and 1.32, respectively,) compared to the reference group. Liver grafts from DCD donors produced a 110% increased risk of graft loss (RR = 2.1, P < 0.0001) at 1 year. With respect to macrovesicular steatosis, the 20%, 25%, and 30% macrovesicular steatosis group was not independently associated with increased graft loss; however, this 20%, 25%, and 30% steatosis group statistically interacted with the CIT > 11 hours group. This interaction between the 20%, 25%, and 30% steatosis group and the > 11 hour CIT group was associated with a 54% increase in graft loss (RR = 1.54, P = 0.03). There was no interaction between the >11 hour CIT group and either the 0%, 5%, 10%, 15% group or the >30% steatosis group. The group with >30% macrovesicular steatosis was independently associated with an increased risk of graft loss of 71% (RR = 1.71, P = 0.007). CIT of > 5 but ≤ 11 hours was associated with a 66% increase in graft loss at 1 year (RR = 1.66, P < 0.0001), while CIT > 11 hours was associated with an 87% increase in graft loss at 1 year (RR = 1.87, P = 0.002).
| Donor Factors | 1-Year Unadjusted Multivariable Analysis | 1-Year Adjusted* Multivariable Analysis | ||
|---|---|---|---|---|
| RR | P Value | RR | P Value | |
| Age, years (ref: 18-39) | ||||
| 40-49 | 1.36 | 0.006 | 1.4 | 0.003 |
| 50-59 | 1.55 | <0.0001 | 1.6 | <0.0001 |
| 60-69 | 1.61 | <0.0001 | 1.6 | <0.0001 |
| 70+ | 2.01 | <0.0001 | 2.1 | <0.0001 |
| Donor race (ref: white) | ||||
| African American | 1.38 | 0.0006 | 1.36 | 0.002 |
| Hispanic | 1.37 | 0.007 | 1.32 | 0.03 |
| Donation after cardiac death | 1.94 | <0.0001 | 2.1 | <0.0001 |
| Macrovesicular steatosis (ref: 0%,5%, 10%, and 15%) | ||||
| 20%, 25%, and 30% | 0.99 | 0.9 | 0.95 | 0.8 |
| 20%, 25%, and 30% interaction with cold ischemia time >11 hours | 1.51 | 0.03 | 1.54 | 0.03 |
| >30% | 1.69 | 0.004 | 1.71 | 0.007 |
| Logistics factors | ||||
| Cold ischemia time (hours) (ref: 0-5) | ||||
| >5≤11 | 1.66 | <0.0001 | 1.66 | <0.0001 |
| >11 | 1.82 | 0.003 | 1.87 | 0.002 |
| Unknown | 2.54 | <0.0001 | 2.32 | <0.0001 |
- Only significant multivariables are shown.
- * Adjusted for all recipient factors in Table 4. Ref, reference value.
DISCUSSION
Our study is the largest to date that analyzes macrovesicular and microvesicular steatosis as a possible factor for increased liver graft loss within 1 year after transplantation. In a high-risk donor group, microvesicular steatosis is not a risk factor for graft loss, whereas >30% macrovesicular steatosis is an independent risk factor together with other factors in the DRI. We also report that macrovesicular steatosis in the 20%, 25%, and 30% group interacts with CIT > 11 hours and is associated with poorer graft survival.
The DRI developed by Feng et al.29 was based on data derived from liver transplants that were reported in the Scientific Registry of Transplant Recipients from January 1, 1998, through December 31, 2002.29 Our multivariable analysis of significant donor and procurement logistics factors was developed through analysis of high-risk donor livers on which biopsy was obtained at the time of procurement in a later era of liver transplantation. Our work and the DRI29 reveal similar significant donor and procurement logistics factors associated with poorer graft survival.
Our adjusted multivariable analysis determined 6 factors to be associated with higher-risk donors, with 4 of these factors matching the 9 factors of the DRI, plus 2 new factors (donor liver >30% macrosteatosis, and donor liver with 20%, 25%, or 30% macrosteatosis in conjunction with > 11 hours CIT). Similar to the DRI,29 we demonstrated donor age ≥ 40 in 10-year increments, African American and Hispanic donor race (the DRI does not code for Hispanic donors but codes for “other”29), DCD donation, and CIT to be associated with increased risk of graft loss. We have added > 30% macrovesicular steatosis in the donor liver as an independently associated risk factor for graft loss. We have also added the use of a donor liver with macrovesicular steatosis levels of 20%, 25%, or 30% in conjunction with > 11 hours of CIT as a second independently associated risk factor for graft loss. In contrast to the DRI by Feng et al.,29 our multivariable analysis does not include donor height, any cause of donor death, partial/split donor liver, or sharing outside the local area.
It is debatable whether race should be used as a significant donor factor for graft loss. As pointed out by Kim et al.,45 donor race has no known biological basis for affecting organ quality. Likewise, Kim et al.45 determined that African American race was confounded by poorly performing transplant centers. Certain donor characteristics such as race that have been significantly associated with graft failure may be confounders. The true contribution to donor risk is more likely seen in the objective pathology of the liver rather than in donor physical or racial attributes. We acknowledge that both the DRI and our multivariable analysis found donor race to be significant factors predicting graft loss, and confounding could not be determined in our analysis.
In potentially higher-risk donor livers that require a biopsy at the time of procurement, 4 risk factors from our analysis show the highest association with graft loss and should be considered in donor selection. Donor age >70 years and the use of DCD donor livers each have the highest level of RR at 2.1. At one time, there was limited use of liver grafts from donors ≥ 50 years old.1, 2, 9, 27 However, other authors have demonstrated that the use of older donor organs without additional risk factors results in survival similar to that from the use of younger donor organs.3, 5, 6, 27 Some liver programs categorically do not accept organs from donors ≥ 70 years old, whereas other programs, with careful selection, do use liver grafts from donors ≥ 70 years old.46 If a donor >70 years old is selected, we recommend that this donor have no other risk factors.
The use of DCD donor livers has the same highest RR of 2.1 as donor age > 70 years. Focusing only on DCD liver donors, Mateo et al.18 showed that donor age > 60 years, warm ischemia time > 30 minutes, and CIT > 10 hours were donor factors predicting poor graft survival. Chan et al.24 reported that donor weight > 100 kg and total ischemia time ≥ 9 hours in donors > 50 years old predict ischemic cholangiopathy and, therefore, impending graft loss. Our report, which reviewed all donors from multiple centers, results in more conservative guidelines. This data may include the learning curve for using DCD donors at several centers. Our data suggest that the DCD donor liver should be used in a manner similar to that of a > 70-year-old donor liver when there are no other risk factors. However, experienced individual transplant centers may use more aggressive criteria.
Prolonged CIT is a known risk factor for poor graft survival and the only possible modifiable risk factor in donor selection.2, 14, 16, 27, 29 Our data demonstrates that the optimal CIT is < 5 hours, followed by > 5 but ≤ 11 hours. We recommend that if the CIT is >11 hours that other donor risk factors be at a minimum and the percent macrovesicular steatosis should be ≤15% (see below).
In our analysis of high-risk donors, macrovesicular steatosis levels of >30% had a RR of 1.71 and must be taken into account in donor selection. Increasing percentages of donor liver steatosis have a negative impact on graft survival.33 Due to the small numbers, we were not able to evaluate livers with > 60% macrovesicular steatosis. However, it has been suggested that without other additional risk factors, livers with > 30% but ≤ 60% macrovesicular steatosis can be safely used.44 Other authors state that livers with > 60% macrovesicular steatosis are universally not acceptable for transplantation,31, 42 whereas still other authors suggest that the use of livers with steatosis > 60% in well-controlled cases can be successful.42 Our data suggest that donor livers with > 30% macrovesicular steatosis can be successfully used if the other donor risk factors are kept to a minimum. The principle of limiting risk in the use of severely steatotic donor livers is evident throughout the collective experience of the United States liver transplant community. This is shown by the fact that in our study population, the average donor age and CIT decreased as the percent steatosis in the biopsy increased (data not shown). We have demonstrated that efforts can be made to utilize steatotic livers by limiting the CIT. Some authors have suggested that longer CIT allows less tolerance of even lower amounts of steatosis.32, 39 We confirmed this suggestion by our result showing that donor livers with 20%, 25%, and 30% steatosis in conjunction with CIT > 11 hours is associated with an increased risk of graft loss.
There are potential problems with our study. This is the largest study to consider steatosis found in donor liver biopsy to be integral to the donor risk evaluation for transplant outcome. Admittedly, the evaluation of donor macrovesicular steatosis through use of frozen section liver biopsies can be both difficult and subjective,36, 37, 47 a problem often compounded by freeze-artifact, and/or more junior faculty on service, a different pathologist at the time of organ procurement, and even problems inherent in intraobserver reliability. Multiple transplant centers report the percent of macrovesicular steatosis, and there are no standard criteria for reporting. We cannot confirm the reliability or reproducibility of the percent of macrovesicular steatosis. We reported the results as they are used clinically in the process of liver procurement. We did find 3 large groups of macrovesicular steatosis associated with particular different risk levels for graft loss, and we grouped the biopsy results into generally separate categories, similar to prior studies.39-44
Approximately 20% of liver transplant recipients in the UNOS Standard Transplant Analysis and Research file had a donor liver biopsy recorded, and the corresponding group of donors became our study population. Even though some programs conduct protocol donor liver biopsies, most are performed because of potential abnormal donor or liver graft characteristics. We report that the donor livers that were biopsied are from a higher-risk population than the donor livers that were not biopsied. This was supported first by their DRI being significantly higher, and by comparing our population with that of Feng et al.29 We found a higher percentage of the older age groups in our study. In our study, 17% of the population was in the 60-69 age group compared to 9.5% of the population in the study by Feng et al. Likewise, for the >70 age group, our study included 9.7% of this age group, compared with 4.3% in the Feng et al. study. Our study group had 4.2% DCD donors compared with 1.1% for the group in the Feng et al. study. The percent of our population receiving split/reduced livers grafts was 0.3%, compared with 2% in the Feng et al. study. Trauma was the cause of death in 25.3% of our population, compared with 44.6% in the population in the Feng et al. study. We had results similar to Feng et al.29 regarding sex, serum creatinine and bilirubin levels, and donor height.
We also acknowledge that there might have been different practice patterns among the different eras of liver transplantation.48 However, our conclusions are based on our study population, who received a liver biopsy at the time of procurement during the most recent era of liver transplantation. Most importantly, it is precisely this higher-risk population that is most difficult to evaluate for allocation issues. Our large study population of >5000 subjects would suggest an adequate volume of potentially higher-risk donors for developing significant factors associated with graft loss. Finally, our significant donor risk factors are similar to those described by Feng et al.29 It therefore appears that the measure of percent macrovesicular steatosis should be added to the evaluation of high-risk donors.
Finally, as with most studies, other potentially important factors might not have been collected and thus made available for analysis. It is interesting to note that increased CIT time, increased distance between donor and recipient hospitals, and national sharing were all significantly different between the high-risk biopsied donor livers and the donor livers not biopsied. These donor livers might have been turned down locally due to their biopsy results, or these may have been considered marginal grafts due to other factors that were not captured as risk factors.
The increasing shortage of donor organs, combined with the increasing numbers of patients on waiting lists, requires an objective discrimination of the risks of particular donor organs, with the goal of minimizing the combination of negative prognostic factors. Additionally, although the use of many “marginal” livers ultimately results in good outcomes, it has been recognized that there are often significantly higher costs associated with caring for recipients of less than optimal grafts.49, 50 A formal cost study is necessary on outcomes with the “fatty liver.” Finding the appropriate balance in donor variables by minimizing the combination of age, macrosteatosis, and ischemia time should be reflected in better outcomes and perhaps lower costs to the patient and medical system.
In conclusion, the validity of the variables used in the DRI has been demonstrated. The DRI continues to provide an efficient and objective evaluation for liver donor risk. Importantly, we have shown that in high-risk donors, macrovesicular steatosis is an independent variable, additional to the variables of the DRI, that is useful in evaluating donor risk. Given the urgent nature of liver transplantation, some compromises with these donor risk factors are likely necessary, but would significantly affect graft outcome.
Acknowledgements
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors thank Marilyn Carlson for her editorial assistance.




