Timing of hepatitis C antiviral therapy in patients with advanced liver disease: A decision analysis model†‡
No author has a conflict of interest to declare with respect to the content of this study. No part of this study was funded by the pharmaceutical industry.
See Editorial on Page 697
Abstract
Antiviral therapy for the treatment of hepatitis C virus (HCV) infection is used before and after liver transplantation. The objective of this study was to determine the most cost-effective timing for pegylated interferon/ribavirin therapy in patients with advanced liver disease infected with genotype 1 HCV. A Markov model was constructed to compare treatment strategies: (1) no treatment, (2) antiviral therapy in patients with compensated cirrhosis, (3) antiviral therapy in patients with decompensated cirrhosis, and (4) antiviral therapy in patients with progressive fibrosis due to recurrent HCV post-transplantation. Outcomes of interest included the total cost per patient, number of quality-adjusted life years (QALYs) saved, cost per QALY saved, number of deaths and hepatocellular carcinomas (HCCs), and number of transplants required. Compared to the no–antiviral treatment strategy, treatment during compensated cirrhosis increased QALYs by 0.950 and saved $55,314. Treatment during decompensated cirrhosis increased QALYs by 0.044 and saved $5511. Treatment during posttransplant advanced recurrence increased QALYs by 0.061 and saved $3223. Treatment of patients with compensated cirrhosis resulted in 119 fewer deaths, 54 fewer HCCs, and 66 fewer transplants with respect to the no-treatment strategy. The model was sensitive to the rate of graft failure in patients with and without sustained virological response. The model was otherwise robust to all variables tested in sensitivity analysis. In conclusion, the treatment of patients with compensated cirrhosis was found to be the most cost-effective strategy and resulted in improved survival and decreased cost in comparison with all other strategies. This study provides pharmacoeconomic evidence in support of treating HCV in patients with compensated cirrhosis before progression to more advanced liver disease. Liver Transpl 16:748-759, 2010. © 2010 AASLD.
Hepatitis C virus (HCV) infection is the most common indication for liver transplantation in the United States.1 Unfortunately, post–liver transplantation outcomes are worse for those who undergo transplantation for HCV than for those with many other etiologies of liver disease.2-4 Reinfection of the graft is universal,5-7 and progression to graft cirrhosis and its subsequent complications can be rapid and may compromise patient and graft survival.8
Antiviral therapy for HCV can eliminate the virus,9 arrest the development of fibrosis,10-16 and reduce the incidence of complications of progressive disease both before and after transplantation.17-22 A number of strategies have emerged for antiviral treatment of patients with HCV cirrhosis awaiting liver transplantation. Unfortunately, antiviral treatment of patients with cirrhosis, especially those with decompensated disease, is often limited by side effects, and early discontinuation is common. Alternatively, treatment can be administered post-transplant once reinfection has occurred. This strategy is also limited by significant side effects, with high rates of dose reductions (73%) and discontinuations of treatment (27.6%).21
Combination therapy with pegylated interferon/ribavirin (PEG/RBV) is the standard of care of antiviral therapy for chronic HCV.23 Unfortunately, rates of sustained virological response (SVR) among PEG/RBV-treated patients with cirrhosis pre-transplant and among those treated after liver transplantation are lower than rates among noncirrhotic HCV patients.24 In this study, a Markov model was used to compare the cost effectiveness of HCV treatment at different stages of advanced HCV disease over 17 years in a cohort of 4000 patients entering the model with compensated cirrhosis.
Abbreviations:
AFP, alpha-fetoprotein; CT, computed tomography; EVR, early virological response; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; IFN/RBV, interferon/ribavirin; NA, not applicable; PEG/RBV, pegylated interferon/ribavirin; QALY, quality-adjusted life year; SVR, sustained virological response.
PATIENTS AND METHODS
Decision Model
We compared 3 strategies for administering antiviral therapy using PEG/RBV in patients with HCV genotype 1. All patients entered the model with the diagnosis of compensated cirrhosis. A 4000-patient entry cohort of 55-year-old patients was evenly divided into 4 different treatment strategies (Fig. 1). In the control strategy, no antiviral treatment was administered (Fig. 1A). In the first treatment strategy, antiviral treatment was initiated in patients with compensated HCV cirrhosis (Fig. 1B). In the second treatment strategy, patients were treated only after the development of decompensated liver disease (Fig. 1C). Decompensated cirrhosis was defined as clinical evidence of ascites, hepatic encephalopathy, or variceal bleeding. Patients without a history of these complications and also without hepatocellular carcinoma (HCC) were defined as having compensated cirrhosis.19, 25, 26 In the third treatment strategy, patients were treated only after the development of histological evidence of advanced fibrosis due to HCV recurrence post-transplantation based on annual protocol graft biopsies (Fig. 1D). Advanced fibrosis due to recurrent HCV was defined histologically as a METAVIR fibrosis score ≥2 on protocol graft biopsy in accordance with recommendations from a large meta-analysis of treatment of posttransplant HCV.21 Patients assigned to treatment strategies received antiviral therapy only upon reaching the natural history state at which they were designated to be treated. The goal duration of therapy was 48 weeks. The mean duration of therapy in the treatment during decompensation strategy was 30 weeks.25, 26 Rates of dose discontinuation due to side effects and absence of early virological response (EVR) were derived from a literature review (Table 1).19, 21, 24-27 Patients were not eligible for repeated courses of antiviral therapy.
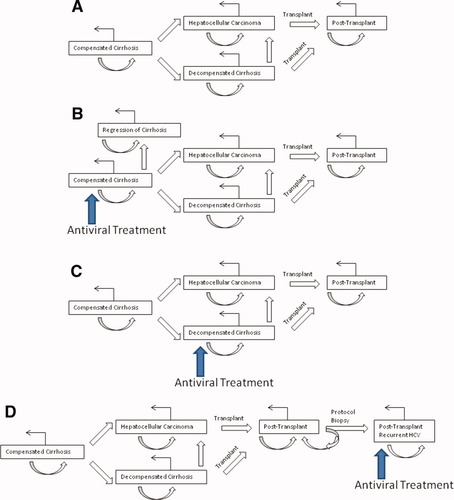
Schematic of the Markov chain and antiviral treatment strategies. The treatment strategies include (A) no antiviral treatment, (B) treatment during compensated cirrhosis, (C) treatment during decompensated cirrhosis, and (D) treatment during posttransplant advanced histological recurrence. At each stage, patients can (1) remain at the current stage (curved arrow), (2) progress to the next stage (straight arrow), or (3) die (right-angle arrow). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Variable | Baseline | Range | References |
|---|---|---|---|
| SVR | |||
| Compensated cirrhosis | 0.30 | 0.15-0.6 | 19, 58 |
| Decompensated cirrhosis | 0.143 | 0.072-0.286 | 25, 26 |
| Post-transplant | 0.255 | 0.128-0.51 | 15, 22, 46-48, 59-65 |
| EVR | |||
| Post-transplant (measured) | 0.642 | 0.321-1 | 15, 59, 61-63, 66 |
| EVR/SVR ratio post-transplant | 2.07 | 21 | |
| Compensated cirrhosis (calculated) | 0.549 | 0.275-1 | |
| Decompensated cirrhosis (calculated) | 0.296 | 0.148-0.592 | |
| Post-transplant (calculated) | 0.523 | 0.262-1 | |
| Discontinuation of treatment due to side effects | |||
| Compensated cirrhosis | 0.088 | 0.044-0.176 | 12, 19, 27 |
| Decompensated cirrhosis | 0.163 | 0.082-0.326 | 25, 26 |
| Post-transplant | 0.218 | 0.109-0.436 | 21 |
| Discontinuation of treatment due to failed EVR and side effects | |||
| Compensated cirrhosis | 0.539 | 0.27-1 | 12, 19, 27 |
| Decompensated cirrhosis | 0.867 | 0.434-1 | 25, 26 |
| Post-transplant | 0.695 | 0.348-1 | 21 |
| Use of filgrastim | |||
| Compensated cirrhosis | 0.26 | 0.13-0.52 | 12, 19, 27 |
| Decompensated cirrhosis (PEG/RBV and IFN/RBV) | 0.324 | 0.162-0.648 | 25, 26, 67, 68 |
| Post-transplant | 0.473 | 0.237-0.946 | 15, 22, 45, 48, 63 |
| Use of epoetin | |||
| Compensated cirrhosis | 0.205 | 0.103-0.52 | 12, 19, 27 |
| Decompensated cirrhosis (PEG/RBV and IFN/RBV) | 0.396 | 0.198-0.792 | 25, 26, 68, 69 |
| Post-transplant | 0.46 | 0.23-0.92 | 15, 22, 44-47, 62 |
| Decompensation | |||
| Ascites as initial decompensation event | 0.477 | 0.239-0.954 | 33 |
| Liver transplantation | |||
| Transplant rate | 0.37 | 0.185-0.74 | 32 |
| Transplant rate, HCC | 0.14 | 0.07-0.28 | 87-90 |
| Rate of rejection, general | 0.03 | 0.015-0.06 | 70 |
| Rate of rejection during antiviral treatment | 0.05 | 0.025-0.1 | 15, 44-48, 60-65, 71-74 |
| Liver biopsy | |||
| Complication rate | 0.003 | 0.0015-0.006 | 75 |
| Mortality from biopsy | 0.00018 | 0.00009-0.00036 | 75 |
A Markov model was constructed with the TreeAge Pro Health Module (2006; TreeAge Software, Williamstown, MA). Patients were followed through the model for 17 years or until death with a mean life expectancy of 72 years. Life expectancy was derived with the declining exponential approximation of life expectancy method.28-31 Outcomes of interest included the total cost per patient, number of quality-adjusted life years (QALYs) saved, cost per QALY saved, number of deaths and HCCs, and number of transplants required. The commonly used incremental cost-effectiveness ratio (ICER) threshold of $50,000 per QALY saved was implemented.
Natural History
In our model, patients had an annual probability of progressing to a more advanced disease state based on assumptions guided by an extensive review of the published literature (Table 2). The transplantation rate of patients with decompensated cirrhosis on the waiting list was estimated from rates published in the United Network for Organ Sharing database from 2007.32
| Variable | Baseline | Range | References |
|---|---|---|---|
| Annual transition probabilities | |||
| Compensated cirrhosis with SVR to | |||
| Decompensated cirrhosis | 0.001 | 0.00005-0.002 | 17-19, 34 |
| HCC | 0.008 | 0.004-0.016 | 17, 18, 33, 34 |
| Compensated cirrhosis without SVR to | |||
| Decompensated cirrhosis | 0.031 | 0.016-0.062 | 17, 18, 76 |
| HCC | 0.027 | 0.014-0.054 | 17, 18, 33, 34, 76 |
| Decompensated cirrhosis to | |||
| HCC | 0.07 | 0.035-0.14 | 77 |
| Post-transplant with SVR to | |||
| Graft failure | 0.06 | 0.03-0.12 | 4 |
| Post-transplant without SVR to | |||
| Histological recurrence (METAVIR ≥ 2) | 0.15 | 0.075-0.30 | 6, 78, 79 |
| Graft failure | 0.08 | 0.04-0.16 | 4, 5, 38, 80 |
| Post-transplant with advanced histological recurrence to | |||
| Graft failure | 0.08 | 0.04-0.16 | 78 |
| Post-transplant without advanced histological recurrence to | |||
| Graft failure | 0.06 | 0.03-0.12 | 78 |
| Mortality rate | |||
| General population, 55 years old | 0.0006295 | 0.00315-0.0126 | 81 |
| Compensated cirrhosis with SVR | 0.008 | 0.004-0.016 | 17, 18, 34 |
| Compensated cirrhosis without SVR | 0.021 | 0.011-0.042 | 17, 18, 33, 34, 76 |
| Decompensated cirrhosis | 0.138 | 0.069-0.276 | 33, 77 |
| Costs | |||
| PEG/RBV | 37,904 | 18,952-75,808 | 51 |
| Filgrastim, 11 months | 10,885 | 5442-21,770 | 51 |
| Epoetin, 11 months | 27,731 | 13,866-55,462 | 51 |
| Norfloxacin | 138 | 69-276 | 51 |
| Clinic visits/labs during antiviral treatment | 2505 | 1253-5010 | 50 |
| AFP | 25 | 13-50 | 50 |
| Abdominal ultrasound | 153 | 77-306 | 50 |
| CT scan of chest | 308 | 154-616 | 50 |
| CT scan of abdomen/pelvis | 341 | 171-682 | 50 |
| Liver biopsy averaged for complications | 1370 | 685-2740 | 51 |
| Radiology-guided liver biopsy averaged for complications | 3133 | 1567-6266 | 50, 51 |
| Rejection | 6943 | 3472-13,886 | 82 |
| Compensated cirrhosis | 1072 | 536-2144 | 83 |
| Decompensated cirrhosis | 13,848 | 6924-27,696 | 29 |
| HCC | 43,034 | 21,517-86,068 | 83 |
| Liver transplantation (first year) | 141,724 | 70,862-283,448 | 83 |
| Liver transplantation (subsequent years) | 24,750 | 12,375-49,500 | 83 |
| Death | 50,825 | 25,413-101,650 | 84 |
- NOTE: Transition probabilities are expressed as rates per year. Costs have been adjusted to 2009 US dollars. Utilities are expressed in quality-adjusted life years.
Treatment Response
Response to treatment was classified as SVR or no SVR. SVR was defined as an undetectable HCV viral load at 6 months after completion of treatment. In the model, patients who achieved SVR had lower rates of progression to more advanced disease states (Table 2).15, 17, 18, 21, 33-36 In all treated patients, the HCV viral load was estimated 3 months after treatment to determine whether EVR occurred. EVR was defined as a ≥2-log decrease in the HCV viral load compared to the baseline value.23
Model Assumptions
- 1
Repeat liver transplantation is not available.
- 2
Patients who fail to achieve SVR have a prognosis similar to the prognosis of those who do not receive treatment.
- 3
The natural history of liver transplant recipients who achieve SVR is the same as that of patients who undergo transplantation for hepatitis B virus (i.e., a patient population with rates of disease recurrence and graft failure lower than those of patients who undergo transplantation for HCV without SVR).4
- 4
Progression from compensated cirrhosis to decompensated cirrhosis is irreversible.
- 5
The finding of fibrosis by annual posttransplant protocol biopsy correlates with a high risk for worsening recurrent graft disease and ultimate graft failure.8, 37-39
- 6
Patients with HCC who are not transplant candidates have an average lifespan of 1 year.
- 7
Patients who do not achieve EVR discontinue therapy early as they are unlikely to achieve SVR.
- 8
Patients enter the study without contraindications to therapy and are assumed to be candidates for treatment during the entirety of the study unless they develop HCC.
HCC
In the pretransplant setting, all patients underwent semiannual screening for HCC using abdominal ultrasound and serum alpha-fetoprotein (AFP).40-42 Patients with evidence of HCC based on ultrasound or elevated AFP underwent computed tomography (CT) of the abdomen/pelvis followed by CT-guided biopsy to confirm the diagnosis of HCC. Patients who underwent transplantation for HCC were screened for recurrent HCC with semiannual chest CT scans and serum AFP measurements for 2 years post-transplant and then annually thereafter (the institutional practice of the University of California at Los Angeles).
Growth Factors
Growth factors were used for the management of anemia and neutropenia at rates derived from published estimates (Table 1). Erythropoietin was initiated for hemoglobin levels less than 10 g/dL,15, 22, 26, 43-47 and filgrastim was initiated when the absolute neutrophil count descended below 750 mm3.15, 26, 43, 45, 48 When used, growth factors were initiated 1 month after the initiation of therapy and were continued throughout treatment. The dosage of antiviral therapy was reduced for patients requiring growth factors. Spontaneous bacterial peritonitis prophylaxis with norfloxacin was provided to all treated patients with decompensated cirrhosis who had ascites.49
Costs
Estimations of cost were obtained from published studies, from Medicare reimbursement rates for 2009, and from Red Book 2009.50, 51 The costs of administration of antiviral therapy, including costs of antiviral medications, growth factors, and monthly clinic visits and laboratory tests during treatment, were calculated and adjusted for rates of premature discontinuation of therapy due to side effects or absence of EVR. In the posttransplant setting, the costs of performing annual protocol liver biopsies and managing subsequent complications were included, as well as the costs of managing rejection during antiviral treatment. Cost estimates were converted to 2009 US dollars with the Bureau of Labor Statistics consumer price index inflation calculator (Table 2).52 All costs were discounted by 3% per year.28, 29 The study perspective was one of a third-party payer. Quality-of-life estimates were obtained with weighted averages from studies using the Short Form 36 Health Survey for quality-of-life estimation (Table 3).53-57
Sensitivity Analysis
Univariate sensitivity analysis was performed to assess whether the results of the model were robust. All variables used in the model for clinical assumptions, annual transition probabilities, and costs (Tables 1 and 2) were tested over a wide range of values in 1-way sensitivity analysis to determine their impact on cost effectiveness. All values ranged from 50% of the baseline literature values to 200% of the literature values. The model was considered to be sensitive to a variable if modification of that variable resulted in an ICER of less than $50,000/QALY in comparison with the preferred strategy.
RESULTS
Our model demonstrated that antiviral therapy for patients with compensated cirrhosis led to the best outcomes. The treatment of patients with compensated cirrhosis resulted in 119 fewer deaths, 54 fewer HCCs, and 66 fewer transplants with respect to patients in the no-treatment strategy group. In addition, treatment resulted in cirrhosis regression in 44 patients. On the other hand, antiviral therapy in patients with decompensated liver disease led to 10 fewer deaths with respect to the no-treatment strategy. Antiviral therapy in liver transplant recipients with advanced fibrosis due to recurrent hepatitis C disease led to 12 fewer deaths in comparison with the no-treatment cohort. All patients in the compensated treatment strategy group received antiviral treatment, whereas 204 and 101 patients were treated in the decompensated and posttransplant recurrence strategy groups, respectively.
Despite the additional cost of treating a higher number of patients with antiviral therapy, treatment during compensated cirrhosis was associated with lower costs than the other antiviral strategies. The total cost per patient in the compensated cirrhosis treatment cohort was $300,159. In contrast, the cost per patient was similar between patients in the treatment during decompensated cirrhosis cohort and patients in the treatment during posttransplant recurrence cohort ($349,962 and $352,250, respectively). The cost per patient for untreated patients was $355,473, which represents the cost of medical care incurred by complications of worsening cirrhosis without antiviral therapy (Table 4).
| Antiviral Strategy | Total Cost per Patient | Incremental Cost | QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| No antiviral treatment | 355,473 | NA | 9.175 | NA | NA |
| Treatment during compensated cirrhosis | 300,159 | −55,314 | 10.125 | 0.950 | Compensated cirrhosis dominates |
| Treatment during decompensated cirrhosis | 349,962 | −5511 | 9.219 | 0.044 | Decompensated cirrhosis dominates |
| Treatment during posttransplant recurrence | 352,250 | −3223 | 9.236 | 0.061 | Posttransplant recurrence dominates |
- NOTE: Each treatment strategy is compared to the no-treatment strategy. Costs are expressed in US dollars.
With respect to the other strategies, treatment was cost-saving when it was administered during compensated cirrhosis. Compared to controls, treatment during compensated cirrhosis increased QALYs by 0.950 and saved $55,314. To a lesser extent, treatment during decompensated cirrhosis and during posttransplant recurrence was also cost-saving in comparison with untreated controls. Treatment during decompensated cirrhosis increased QALYs by 0.044 and decreased costs by $5511. Treatment during advanced posttransplant recurrence increased QALYs by 0.061 and decreased costs by $3223 (Table 4).
In cost-effectiveness analysis, treatment during compensated cirrhosis dominated all other strategies as it yielded the greatest survival benefit and the lowest cost. Treatment during decompensated cirrhosis and treatment during advanced posttransplant recurrence were both more cost-effective than no treatment and showed dominance over the no-treatment strategy in cost-effectiveness analysis (Table 4). Posttransplant treatment was less cost-effective than treatment during decompensation; an ICER greater than $50,000/QALY was yielded in a direct comparison between the 2 strategies.
The cost-effectiveness results were robust to all variables tested in sensitivity analysis, with the exception of the annual rate of graft failure in patients with and without SVR (Table 5). The literature-based annual rate of graft failure in patients with SVR was 6.0% (Table 2). Decreasing the rate of graft failure in patients with SVR to less than 3.6% resulted in treatment during decompensated cirrhosis replacing treatment during compensated cirrhosis as the most cost-effective strategy, with an ICER of less than $50,000/QALY (Fig. 2A). The literature-based annual rate of graft failure in patients without SVR was 8.0% (Table 2). Decreasing the rate of graft failure in patients without SVR to less than 4.1% resulted in the no-treatment strategy replacing treatment during compensated cirrhosis as the most cost-effective strategy (Fig. 2B). The analysis of all other transition probabilities, clinical assumptions, and costs did not alter the results of our cost-effectiveness analysis (Table 5), and for the majority of variables, the compensated strategy dominated all other strategies over the entire range of the sensitivity analysis.
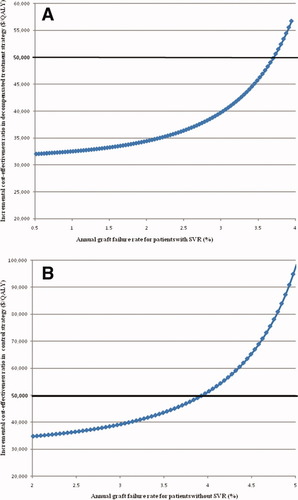
One-way sensitivity analysis showing effects of varying annual graft failure rates. Decompensated treatment strategy becomes most cost-effective strategy when graft failure rate in patients with SVR is sufficiently diminished (A). Control strategy becomes most cost-effective strategy when graft failure rate in patients without SVR is diminished (B). ICER calculated based on comparison with dominant (compensated) treatment strategy using a threshold of $50,000/QALY. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Variable | Most Cost-Effective Strategy |
|---|---|
| Baseline assumptions | |
| SVR | |
| Treatment during compensated cirrhosis | Compensated strategy |
| Treatment during decompensated cirrhosis | Compensated strategy |
| Treatment post-transplant | Compensated strategy |
| Use of filgrastim | |
| Treatment during compensated cirrhosis | Compensated strategy |
| Treatment during decompensated cirrhosis | Compensated strategy |
| Treatment post-transplant | Compensated strategy |
| Use of epoetin | |
| Treatment during compensated cirrhosis | Compensated strategy |
| Treatment during decompensated cirrhosis | Compensated strategy |
| Treatment post-transplant | Compensated strategy |
| Liver transplantation | |
| Transplantation rate, decompensated cirrhosis | Compensated strategy |
| Transplantation rate, HCC | Compensated strategy |
| Transition probabilities | |
| Compensated cirrhosis with SVR to decompensated cirrhosis | Compensated strategy |
| Compensated cirrhosis with SVR to HCC | Compensated strategy |
| Compensated cirrhosis without SVR to decompensated cirrhosis | Compensated strategy |
| Compensated cirrhosis without SVR to HCC | Compensated strategy |
| Decompensated cirrhosis to HCC | Compensated strategy |
| Post-transplant with SVR to graft failure | The decompensated strategy was most cost-effective when the probability was less than 0.036. Above this probability, the compensated strategy was most cost-effective. |
| Post-transplant without SVR to advanced histological recurrence (METAVIR ≥ 2) | Compensated strategy |
| Post-transplant without SVR to graft failure | No treatment was most cost- effective when the probability was less than 0.041. Above this probability, the compensated strategy was most cost-effective. |
| Post-transplant with advanced histological recurrence to graft failure | Compensated strategy |
| Post-transplant without advanced histological recurrence to graft failure | Compensated strategy |
| Mortality rate, compensated cirrhosis with SVR | Compensated strategy |
| Mortality rate, compensated cirrhosis without SVR | Compensated strategy |
| Mortality rate, decompensated cirrhosis | Compensated strategy |
| Costs | |
| Antiviral treatment, compensated cirrhosis | Compensated strategy |
| Antiviral treatment, decompensated cirrhosis | Compensated strategy |
| Antiviral treatment, post-transplant | Compensated strategy |
| Filgrastim, 11 months | Compensated strategy |
| Epoetin, 11 months | Compensated strategy |
| Liver biopsy | Compensated strategy |
| Radiology-guided liver biopsy | Compensated strategy |
| Compensated cirrhosis | Compensated strategy |
| Decompensated cirrhosis | Compensated strategy |
| HCC | Compensated strategy |
| Liver transplantation (first year) | Compensated strategy |
| Liver transplantation (subsequent years) | Compensated strategy |
| Death | Compensated strategy |
- NOTE: Variables ranged from 50% to 200% of the baseline value. A strategy was determined to be the most cost-effective strategy if it yielded an incremental cost-effectiveness ratio of less than $50,000 per quality-adjusted life year saved in comparison with the baseline preferred strategy.
DISCUSSION
We found treatment during compensated cirrhosis to be the most cost-effective strategy for antiviral administration in the setting of advanced HCV-related liver disease. This strategy yielded the greatest survival benefit with the lowest associated cost by reversing cirrhosis and reducing the incidence of decompensation, HCC, transplantation, and death. Treatment after the development of decompensation or in the posttransplant setting was also found to be cost-effective, but these patients derived less survival benefit at greater cost in comparison with patients treated during compensated cirrhosis. The sensitivity analysis revealed the cost-effectiveness assessment to be robust, with only variations of graft failure rates influencing the model. Given the results of this study, we strongly recommend expeditious administration of antiviral therapy to patients with compensated cirrhosis before their disease advances. Antiviral treatment for patients with compensated cirrhosis due to genotype 1 HCV is clearly cost-saving, and if these patients are allowed to develop more advanced disease, their prognosis worsens considerably, and the benefits that they can potentially derive from antiviral therapy diminish considerably.
In this study, antiviral therapy for patients with decompensated HCV cirrhosis was less cost-effective than treatment of patients with compensated cirrhosis. The most obvious limitation to the efficacy of treatment in this population is the low SVR rate (14.3%), which is lower than that for patients with compensated cirrhosis (30.0%) and for patients with posttransplant recurrence (25.5%). The high rates of early discontinuation of treatment due to the absence of EVR and severe side effects clearly diminish the chance of obtaining SVR in these patients (Table 1). Indeed, a recent study recommended against antiviral treatment for patients with advanced cirrhosis (Model for End-Stage Liver Disease score >18) because of a lack of SVR and a high risk of developing bacterial infections during treatment.49 Despite the aforementioned limitations, however, treatment during decompensated cirrhosis was found to be cost-effective in comparison with no treatment. Two studies to date have reported acceptable levels of safety and efficacy of antiviral treatment with PEG/RBV in patients exclusively with decompensated HCV cirrhosis.25, 26 It should be noted, however, that these studies included few patients, and some had non–genotype 1 disease.
Antiviral treatment for patients with advanced posttransplant recurrent HCV was found in this study to be less cost-effective than treatment during compensated cirrhosis, although it was more cost-effective than no treatment. Similar to the treatment of patients with decompensated cirrhosis, the treatment of these patients is limited by poor efficacy and high rates of early discontinuation of treatment. In addition, posttransplant therapy with PEG/RBV can heighten the risk of rejection episodes, and in this model, these patients underwent yearly protocol biopsy, which exposed them to the accompanying costs and complications of this procedure.
Patients with decompensated cirrhosis and those with posttransplant recurrent disease present a management dilemma. The finding in this study that treatment strategies involving these patient populations were cost-effective versus no treatment suggests that providing antiviral therapy for these patients may be beneficial. Comparing the 2 strategies showed that the posttransplant treatment strategy was associated with higher total costs ($2288 more per patient) but also yielded a slight improvement in QALYs (0.017) in comparison with the decompensated treatment strategy (Table 4). In a comparative cost-effectiveness analysis of the 2 strategies, the posttransplant strategy was less cost-effective than the decompensated strategy and yielded an ICER of $134,588/QALY. Indeed, the sensitivity analysis demonstrated that if the rate of graft failure in patients with SVR can be reduced by more than 42%, treatment during decompensation becomes more cost-effective than all other strategies, including the treatment during compensated cirrhosis strategy. At a minimum, our study has demonstrated that further randomized controlled trials are justified to better understand the safety and efficacy of treatment during decompensation and post-transplant and are indeed necessary before universal recommendations regarding these strategies can be made.
This is the first study to assess the cost effectiveness of antiviral treatment for patients in the general population with compensated cirrhosis and with decompensated cirrhosis. The only similar study assessing the cost effectiveness of antiviral treatment for patients with compensated cirrhosis focused only on prison inmates. Tan et al.29 found the treatment of incarcerated male patients with compensated cirrhosis to be cost-saving and to improve the quality of life in patients that were 40 to 70 years old. In that study, in accordance with our findings, treatment dominated no treatment in patients with genotype 1 HCV compensated cirrhosis.
One study28 has demonstrated that interferon-based antiviral treatment was cost-effective and efficacious in patients with posttransplant HCV recurrence. In that study, the ICER for posttransplant treatment was found to be $29,100/QALY. That study, however, differed from the current study in a number of ways. It did not incorporate quality-of-life data, it did not focus exclusively on the treatment of patients with genotype 1 disease, and it was published at a time when studies of the long-term natural history of posttransplant HCV were more limited than they are today. Also, the histological definition of graft fibrosis in the patients treated with posttransplant recurrence was not specified; therefore, these patients may not be able to be compared directly to the patients in this study.
It is generally agreed that antiviral treatment in patients with chronic HCV prior to cirrhosis is more efficacious and better tolerated than treatment administered to patients with advanced liver disease. A number of studies have confirmed the cost effectiveness of PEG/RBV combination therapy in patients with genotype 1 chronic HCV without cirrhosis. In 2003, Salomon et al.83 demonstrated an ICER of $26,000-64,000/QALY for the treatment of 40-year-old patients with chronic HCV in the United States. In 2004, Shepherd et al.85 found an ICER of $15,378/QALY for the treatment of patients with chronic HCV in the United Kingdom. In 2007, Gerkens et al.86 published an ICER of $31,300/QALY for the treatment of 45-year-old patients with mild HCV in Belgium. In 2008, Tan et al.29 found treatment to dominate no treatment in US prison inmates that were 50 to 59 years old, whether they had no fibrosis, portal fibrosis, or bridging fibrosis. Although the current study did not evaluate the treatment of patients without cirrhosis, in comparison with the aforementioned studies, our results could suggest that treatment during compensated cirrhosis compares favorably to treatment prior to cirrhosis from a cost-effectiveness perspective. Comparisons among these cost-effectiveness studies, however, are significantly limited by differences in the methodologies employed, target populations used, and availability of natural history data.
Given their potential benefit and frequent use in clinical practice, growth factors were implemented in this study. The most common side effects related to antiviral treatment, especially in patients with advanced liver disease, are cytopenias. The use of growth factors may decrease the rate of premature discontinuation of therapy due to cytopenias. The benefit of growth factor use has not been firmly established, however, and indications for growth factor initiation are variable in the literature. In this study, epoetin was used for 205, 81, and 46 patients in the compensated, decompensated, and posttransplant treatment strategy groups, respectively. Filgrastim was used for 260, 66, and 48 patients in the compensated, decompensated, and posttransplant strategy groups, respectively. Growth factors were associated with substantial increases in costs, and because their benefit is unproven, these increased costs may have resulted in an underestimation of the potential benefit of treatment, particularly in the compensated treatment group, in which the highest number of patients received these therapies. Regardless, our results remained consistent even when broad ranges were used for growth factor usage and costs in sensitivity analysis. More studies on the cost effectiveness of and indications for the administration of growth factors in the setting of antiviral treatment for HCV are needed.
Similar to the use of growth factors, CT-guided biopsy for diagnosing HCC has somewhat uncertain clinical benefit and increases costs. In this study, this intervention was used in 217 patients in the compensated treatment cohort and in 281 patients in each of the other cohorts. The costs associated with this intervention could have led to an underestimation of the cost effectiveness of treatment, particularly in the decompensated treatment, posttransplant treatment, and control strategies. The costs of CT-guided biopsy as well as rates of HCC were tested in sensitivity analysis, however, and were not found to alter the results of our cost-effectiveness analysis.
In conclusion, the treatment of patients with compensated cirrhosis is the most cost-effective and efficacious antiviral strategy in patients with advanced liver disease due to genotype 1 HCV. Antiviral treatment during compensated cirrhosis results in decreased costs and improved survival in comparison with the treatment of patients with decompensated disease or with advanced fibrosis due to recurrent HCV post-transplant. Survival benefit is achieved through regression of cirrhosis and a reduction in the incidence of decompensation, HCC, liver transplantation, and death. The optimal antiviral treatment strategy for decompensated patients and posttransplant patients with advanced recurrent disease has yet to be elucidated, although the current study suggests that treatment during decompensated cirrhosis and, to a lesser extent, during advanced posttransplant recurrence is more cost-effective than no antiviral treatment. Further studies of antiviral treatment in these populations are needed.