Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation†
The participating members of the Bologna Liver Transplant Group are P. Andreone, G. Bianchi, M. Biselli, M. Cescon, A. Colecchia, A. Cucchetti, M. Del Gaudio, G. Ercolani, G. L. Grazi, M. Lenzi, S. Leoni, G. Mazzella, M. C. Morelli, M. Tamè, G. Verucchi, and M. Vivarelli.
Abstract
Malignant portal vein thrombosis is a contraindication for liver transplantation. Patients with cirrhosis and early hepatocellular carcinoma (HCC) may have either malignant or benign (fibrin clot) portal vein thrombosis. The aim of this study was to assess prospectively whether well-defined diagnostic criteria would enable the nature of portal vein thrombosis to be established in patients with HCC under consideration for liver transplantation. Benign portal vein thrombosis was diagnosed by the application of the following criteria: lack of vascularization of the thrombus on contrast-enhanced ultrasound and on computed tomography or magnetic resonance imaging, absence of mass-forming features of the thrombus, absence of disruption of the walls of veins, and, if uncertainty persisted, biopsy of the thrombus for histological examination. Patients who did not fulfill the criteria for benign thrombosis were not placed on the transplantation list. In this study, all patients evaluated at our center during 2001-2007 with a diagnosis of HCC in whom portal vein thrombosis was concurrently or subsequently diagnosed were discussed by a multidisciplinary group to determine their suitability for liver transplantation. The outcomes for 33 patients who met the entry criteria of the study were as follows: in 14 patients who were placed on the transplantation list and underwent liver transplantation, no malignant thrombosis was detected when liver explants were examined histologically; 5 patients who were placed on the transplantation list either remained on the list or died from causes unrelated to HCC; in 9 patients, liver transplantation was contraindicated on account of a strong suspicion, or confirmation, of the presence of malignant portal vein thrombosis; and 5 patients who were initially placed on the transplantation list were subsequently removed from it on account of progression of HCC in the absence of evidence of neoplastic involvement of thrombosis. In conclusion, for a patient with HCC and portal vein thrombosis, appropriate investigations can establish whether the thrombosis is benign; patients with HCC and benign portal vein thrombosis are candidates for liver transplantation. Liver Transpl 16:658-667, 2010. © 2010 AASLD.
Malignant portal vein thrombosis (PVT) is a well-recognized complication of hepatocellular carcinoma (HCC)1-5; this is attributable to the frequency with which HCC invades the portal venous system. This complication occurs in approximately 35% of patients with HCC6, 7; it is associated with advanced tumors and a poor prognosis.8 In most cases of HCC, local or regional treatments are contraindicated,9, 10 and systemic chemotherapy is administered.11
Liver transplantation (LT) is not advocated for HCC if there is macrovascular invasion by a tumor because of the high rate of tumor recurrence.12-16 Accordingly, the exclusion of a neoplastic cause of PVT has potentially important therapeutic implications in patients with HCC. However, differentiating between benign and malignant thrombi in portal veins is difficult without histological examination of the thrombus.
Thus, to avoid the risk of tumor recurrence following LT in patients with HCC and neoplastic vascular infiltration,12-16 PVT is often considered an absolute contraindication to LT in patients with HCC. However, this policy may be too restrictive. Benign PVT may also occur in patients with HCC, especially in those with early-stage HCC, who appear to be good candidates for LT. In such cases, if neoplastic involvement in the PVT cannot be excluded, a potentially lifesaving procedure will not be offered, and consequently, the prognosis will be poor.
In this context, differentiation between benign and malignant thrombi may be achieved by ultrasound (US)-guided fine-needle biopsy of the lesion.17, 18 However, this procedure is invasive. Furthermore, in many patients with advanced cirrhosis, biopsy of the thrombus may be contraindicated by the presence of impaired blood coagulation and/or ascites. Accordingly, it seemed desirable to evaluate the reliability of noninvasive techniques in determining the benign or malignant nature of PVT.
HCC is a hypervascular tumor. Within lesions, the distribution of arteries exhibits a highly irregular pattern. Contrast-enhanced imaging techniques can demonstrate this pattern19-25 and enable HCC to be differentiated from other focal lesions in more than 70% of patients with cirrhosis and small intrahepatic nodular lesions.26 The abnormal vascular pattern of HCC is usually maintained when the tumor invades branches of the portal vein and hence can be detected when imaging techniques are employed. This approach has facilitated significant improvements in the noninvasive assessment of PVT.
In recent years, the possibility of using color Doppler sonography,27-31 contrast-enhanced color Doppler sonography,32 computed tomography (CT),33-35 and magnetic resonance imaging (MRI)36-38 to determine the benign or malignant nature of PVT has been reported. The advent of contrast-enhanced ultrasound (CEUS) using second-generation US contrast agents has led to greater accuracy in the detection and characterization of PVT complicating HCC3, 39 than that achieved by CT.40 However, there has been a lack of prospective studies in which the nonneoplastic nature of PVT diagnosed by imaging techniques has been confirmed by pathological examination. Accordingly, we undertook a prospective study to clarify this issue. The aim of this study was to assess the validity of predefined criteria for diagnosing benign PVT in patients with HCC and to evaluate their role in the context of selecting patients for LT.
Abbreviations:
AFP, alpha-fetoprotein; CEUS, contrast-enhanced ultrasound; CT, computed tomography; CTP, Child-Turcotte-Pugh; HCC, hepatocellular carcinoma; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; MRI, magnetic resonance imaging; NS, not significant; PVT, portal vein thrombosis; SD, standard deviation; US, ultrasound.
PATIENTS AND METHODS
Patients with both HCC and PVT who were potential candidates for LT on the basis of their nodular tumor burden were enrolled in the study prospectively. A diagnosis of HCC was based on current guidelines of the European Association for the Study of the Liver or the American Association for the Study of Liver Diseases.26, 41 For patients to be considered for LT and characterization of PVT, the number and size of the HCC nodules, determined according to the modified tumor-node-metastasis staging classification of the United Network for Organ Sharing,42 should be compatible with the Milan criteria13 or, if exceeding these criteria, with the downstaging criteria employed at our center.43, 44 Briefly, the prospective downstaging protocol, applied since 2003, uses the following inclusion criteria: a single nodule 5 to 8 cm in diameter; 2 nodules, neither of which exceeds 5 cm in diameter; or 3 to 5 nodules, none of which exceeds 4 cm in diameter.43, 44 New focal liver lesions, detected during follow-up, were evaluated with imaging techniques; they were characterized as HCC if the guidelines used to make the initial diagnosis were met.26, 41
Priority for LT was based on the Model for End-Stage Liver Disease score; additional points were assigned according to the stage of the tumor, as described previously.43 The presence or absence of PVT did not influence ranking priority.
Study Design
All patients were prospectively evaluated; the tumor stage and the presence of PVT were determined. According to the protocol approved in 2001 by our local LT committee and by our hospital's institutional review board, the benign nature of PVT was established by the consensus agreement of a multidisciplinary committee, which included hepatologists, radiologists, sonographers, and surgeons. The committee convened every week at the routine liver oncology/transplant meeting; minutes of all the cases discussed were generated. After a presentation of the clinical history and examination and laboratory data and an assessment of all pertinent images, a diagnosis of benign PVT was based on the simultaneous presence of the following criteria: (1) lack of vascularization of the thrombus in the arterial or later phases of CEUS, (2) lack of vascularization of the thrombus on CT or MRI, (3) absence of mass-forming features of PVT, and (4) absence of evidence of disruption of vessel walls. If there was persistent uncertainty after these 4 criteria were applied, the multidisciplinary team adopted 2 additional criteria: (5) no features of malignancy in a biopsy sample of the thrombus and (6) stability or regression of the thrombus during follow-up. Stability or regression of the thrombus was particularly applied when tissue biopsy was contraindicated. Benign PVT is characterized by fibrin/blood clots and an absence of viable cells; it is also associated with an absence of feeding vessels and hence a lack of contrast in imaging studies (see criterion 4).
Follow-up was carried out at 3-month intervals. Whether at least 2 imaging procedures should be repeated or the patient should be readmitted was determined by the multidisciplinary team and was based on a consideration of all the available clinical, laboratory, and imaging findings. Examples of these diagnostic criteria are given in Figs. 1-3. Patients placed on the transplantation list entered the routine follow-up program employed at our center; specifically, clinical, US, and CT or MRI evaluations were undertaken every 3 months and then every 6 months for patients who met the Milan criteria and every 3 months for those included in the downstaging protocol.44
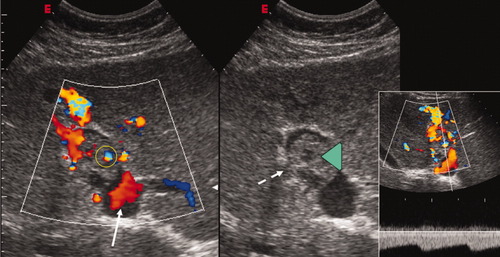
Conventional and color Doppler sonography of a neoplastic thrombus occupying part of the lumen of the left portal vein (green arrowhead, central/right panel) with a mass-forming aspect. Disruption of the vessel walls is evident, as indicated by the dashed-line arrow. Color Doppler sonography shows both vessels within the thrombus (yellow circle) and hepatopetal flow in the remaining patent lumen upstream from the thrombus (white arrow). Doppler flowmetry (right panel) confirms that circulation within the thrombus is characterized by low-resistance pulsatile arterial flow and is, therefore, not due to portal recanalization.
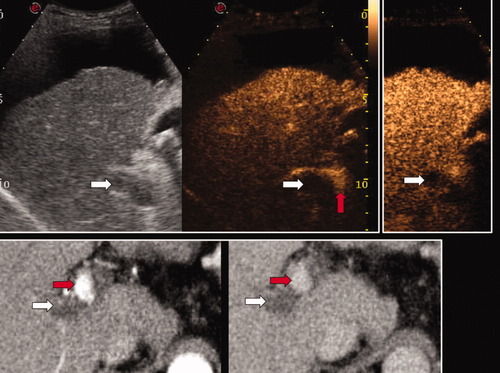
Patient with decompensated cirrhosis and subcapsular bifocal HCC who developed PVT while on the transplantation list. The right portal vein appeared to be subtotally occluded by echoic material, which, however, showed neither a mass-forming aspect nor disruption of vessel walls (white arrow, left panel of the upper row). In CEUS, the thrombosed part of the portal venous system did not take up contrast (white arrows, arterial phase in the middle panel and late phase in the right panel of the upper row), whereas the contrast material could be seen in the distal portal trunk and in the adjacent hepatic artery (red arrow, middle panel) in this intercostal US scan. Similar findings were confirmed by CT (lower row, arterial phase in the left panel and venous phase in the right panel; the white arrows indicate the absence of contrast uptake by the portal thrombus, whereas the red arrows indicate arterial and residual patent portal venous lumen proximal to the thrombus). Abundant ascites and subcapsular HCC prevented biopsy of the thrombus, which was considered to be benign on the basis of imaging techniques. The patient subsequently underwent LT; a pathological examination of the liver explant confirmed the nonmalignant nature of the thrombus.
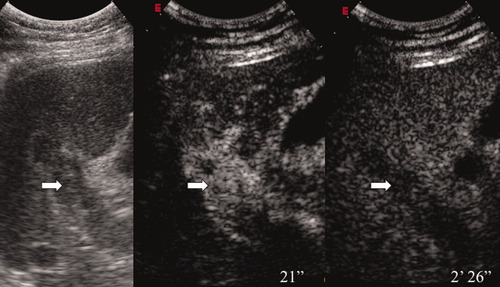
Complete PVT investigated by a right intercostal approach. The left panel shows the appearance of the thrombus by conventional B-mode US (the diagnosis is uncertain, although it is suspicious). The middle panel shows CEUS during the arterial phase of contrast enhancement (21 seconds after contrast injection; 2.4 mL of SonoVue), and the right panel shows CEUS in the late phase (2 minutes 26 seconds after injection). The thrombus appears hyperechoic (enhanced) during the arterial phase (hypervascular appearance) and washes out the contrast in the late phase (hypoechoic); this indicates a malignant nature.
Patients who did not fulfill the aforementioned criteria for benign PVT either at the initial evaluation or during follow-up were removed from the transplantation list. Those removed included patients in whom PVT had previously been judged to be benign but in whom the criteria became less certain during follow-up. In patients who underwent LT, the precise nature of the PVT was determined by histological examination of the explanted liver.
Imaging Techniques
All US examinations were undertaken by experienced operators. CEUS was conducted after the administration of 2.4 mL of a second-generation US blood pool contrast agent (SonoVue, Bracco Spa, Milan, Italy); dedicated US technology using contrast-specific software that operated at a low acoustic pressure (mechanical index = 0.04-0.07) and in real time was employed.24
The arterial and later phases of contrast perfusion were studied. All CT scans were multiphasic and included baseline, arterial, and venous phases. MRI, using gadolinium-based contrast agents, was undertaken to explore both the arterial and venous dynamic phases.
When needle biopsy of the thrombus was indicated, a US-guided procedure was used. Tissue specimens were obtained with Menghini-modified 19- to 21-gauge needles (Biomol, Hospital Service, Latina, Italy).
Pathological Examination
Microscopic and macroscopic evaluations of HCC nodules in liver explants were carried out by experienced pathologists. Sections of liver tissue 5 μm thick were prepared. The following variables were recorded: tumor location, size and number of the lesions, pathological stage of the tumor (pT1-pT4) according to the modified tumor-node-metastasis staging classification of the United Network for Organ Sharing,42 histological stage based on the Edmondson criteria,6 and presence or absence of microvascular or macrovascular invasion. When multiple nodules were present in the explanted liver, the highest histological stage was assigned.
Statistical Analysis
Characteristics of patients considered to have malignant PVT were compared to those of patients considered to have benign PVT with the criteria specified in the Study Design section. Serum alpha-fetoprotein (AFP) levels were classified as being below or above a cutoff value of 30 ng/mL; they were related to the number and maximum diameter of HCC lesions. Univariate analysis was undertaken with the Mann-Whitney test. A P value <0.05 was considered significant.
RESULTS
Forty-six patients with HCC and PVT who were potential candidates for LT were prospectively entered into the study between September 2001 and December 2007. Thirteen of these patients had a history of PVT; in most of them, the duration of this history was >12 months, and recanalization of portal veins had occurred by the time of the diagnosis of HCC. Because the development of PVT preceded the occurrence of HCC in this subgroup of patients, the likelihood of malignant PVT appeared to be negligible. Accordingly, the study focused on the other 33 consecutive patients (Table 1), in whom a diagnosis of PVT was first made at the same time as or after the diagnosis of HCC. Clinical developments in these 33 patients included the following: 14 patients were placed on the transplantation list and underwent LT; 2 were placed on the list and in May 2009 were still awaiting LT; 5 were removed from the list because progression in the size of the tumor exceeded the limits specified by our center,44 but signs of malignant PVT were not evident; 3 died from causes unrelated to HCC; and 9 patients were not placed on the transplantation list because of the development of malignant PVT. Of this last group of 9 patients, 2 were not placed on the transplantation list because malignant PVT was diagnosed at the first assessment, and the remainder were removed from the list when, after an initial evaluation had been negative, malignant PVT subsequently developed.
| Male/female | 28/5 |
| Age [years; mean (range)] | 55.8 (35-64) |
| Etiology of liver disease (number of patients) | |
| Viral | 24 |
| Alcoholic | 8 |
| Other | 1 |
| AFP [ng/mL; median (range)] | 10 (1-7837) |
| PVT diagnosis (number of patients) | |
| Simultaneously with HCC | 10 |
| After HCC, before listing, or while on the waiting list | 23 |
In all patients who underwent LT, subsequent pathological examinations confirmed the benign nature of the PVT. For the 14 patients for whom the final reference standard was available, specifically pathological examination of the liver explant, the diagnostic approach adopted before LT had predicted the presence of benign PVT in patients with HCC and cirrhosis reliably.
PVT and HCC were detected simultaneously in 10 patients; PVT was detected after HCC had been detected in 23 patients, 20 of whom had been placed on the transplantation list (Table 1).
The 13 patients who were not included in the final analysis because PVT had been diagnosed before the presentation of HCC were all placed on the transplantation list: 2 are still on the list; 2 died from causes unrelated to HCC; 2 were removed from the list because of tumor progression, without signs of malignant PVT being evident; and in 7 who underwent LT, the diagnosis of benign PVT was subsequently confirmed by pathological examination of the liver explant.
Table 2 shows the results of the univariate analysis. There were trends, which did not reach statistical significance, for some variables to be associated with malignant PVT. These findings might have been influenced by the limited number of patients in at least 1 of the 2 groups analyzed. The data on AFP levels appeared to show clear trends.
| Variable | Malignant PVT (n = 9) | Benign PVT (n = 24) | P* |
|---|---|---|---|
| AFP (ng/mL; median) | 50 | 9 | 0.189 (NS) |
| AFP ≥ 30 ng/mL (number of cases) | 5 | 6 | 0.214 (NS) |
| Multifocal HCC [number of cases (%)] | 7 (78%) | 10 (42%) | 0.145 (NS) |
| Number of lesions (mean ± SD) | 2.6 ± 1.3 | 2.0 ± 1.4 | 0.277 (NS) |
| Maximum diameter (mm; mean ± SD) | 31.6 ± 11.1 | 30.1 ± 11.3 | 0.748 (NS) |
| PVT synchronous with HCC [number of cases (%)] | 2 (22%) | 8 (33%) | 0.847 (NS) |
- * The P value was assessed with the Mann-Whitney U test.
Extent of Thrombosis and HCC Stage
PVT involved the main portal vein in 19 patients (6 with complete thrombosis and 13 with partial thrombosis), the right portal vein in 26 (10 with complete thrombosis and 16 with partial thrombosis), and the left portal vein in 11 (3 with complete thrombosis and 8 with partial thrombosis). In 3 patients, the splenic vein was involved, and in 4 patients, mesenteric veins were involved. PVT was exclusively intrahepatic in 14 patients, was extrahepatic in 5, and was both intrahepatic and extrahepatic in 14. Malignant PVT was intrahepatic in 67% of cases and was also extrahepatic in 33%; no case of malignant PVT was exclusively extrahepatic.
Data on the staging of HCC at the time of placement on the transplantation list and when PVT was subsequently diagnosed (23 patients) are shown in Table 3. Fourteen patients (45.2%) were in Child-Pugh class A, 11 (35.5%) were in class B, and 6 (19.3%) were in class C.
| Staging of HCC at Listing (n = 31)* | Number of Patients | CTP Score† | MELD Score | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | ≤12 | 13-19 | ≥20 | ||
| T0 | 0 | — | — | — | — | — | — |
| T1 | 5 | 1 | 2 | 2 | 1 | 4 | — |
| T2 | 21 | 8 | 9 | 4 | 9 | 11 | 1 |
| T3 (downstaging) | 2 | 2 | — | — | 2 | — | — |
| T4a (downstaging) | 3 | 3 | — | — | 2 | 1 | — |
| T4b | — | — | — | — | — | — | — |
| Staging of HCC at the PVT Diagnosis in Patients Who Developed PVT After HCC (n = 23)¶ | Number of Patients | ||||||
| T0 | — | ||||||
| T1 | 2 (benign PVT) | ||||||
| T2 | 9 (benign PVT) | ||||||
| T3 (downstaging) | 2 (benign PVT) | ||||||
| T4a (downstaging) | 3 (benign PVT) | ||||||
| T4b | 7 (malignant PVT)‡ | ||||||
| Latest HCC Staging Before Transplantation (n = 14) | Number of Patients | ||||||
| T0 | — | ||||||
| T1 | 2 | ||||||
| T2 | 9 | ||||||
| T3 (downstaging) | 3§ | ||||||
| T4a (downstaging) | — | ||||||
| T4b | — | ||||||
| Pathological Staging on Explanted Livers (n = 14) | Number of Patients | ||||||
| pT0 | 1 | ||||||
| pT1 | 1 | ||||||
| pT2 | 7 | ||||||
| pT3 | 3 | ||||||
| pT4a | 2 | ||||||
| pT4b | — | ||||||
- * Two patients were evaluated for LT but were not included on the list because of the diagnosis of malignant PVT.
- † Child class A = 5-6; Child class B = 7-9; Child class C = 10-15.
- ‡ Of the 7 patients who developed neoplastic PVT, 1 was at stage T1 at listing, 4 were at stage T2, 1 was at stage T3, and 1 was at stage T4a.
- § With only viable tumors considered, these patients fulfilled the Milan criteria.
- ¶ Seven patients developed neoplastic PVT and were taken off the waiting list.
Transplanted Patients
The 14 patients who were placed on the transplantation list and underwent LT showed no evidence of malignant PVT in explanted liver tissue. PVT was diagnosed simultaneously with HCC in 4 patients and after the diagnosis of HCC in 10 patients. Histological confirmation that PVT was benign was also obtained by needle biopsy of the lesion in 1 of these 14 cases.
A diagnosis of benign PVT was established with the criteria specified in the Study Design section. In 1 case, CT evidence of benign PVT was equivocal, but CEUS and the other criteria were consistent with benign PVT. In accordance with the protocol, this patient underwent fine-needle biopsy of the thrombosed vessel, which confirmed an absence of malignant cells. In another patient, an assessment of the thrombus mass was equivocal; CEUS and CT were negative. Because biopsy was contraindicated, a management decision on this patient was delayed. During follow-up, no change in the size of the thrombus was observed, and imaging techniques using contrast were repeatedly negative for thrombus perfusion. This patient was placed on the transplantation list and underwent successful LT.
In patients who underwent LT, the median serum AFP concentration at the time of the diagnosis of PVT was 7.5 ng/mL (range = 1-7837 ng/mL, mean = 627.4 ng/mL); high values (>100 ng/mL) were present in 2 patients (7837 and 810 ng/mL). In the patient with an initial AFP level of 7837 ng/mL, levels returned to normal (<20 ng/mL) after effective treatment of HCC nodules, whereas in the other patient with a high initial value, levels remained consistently high until LT.
Data on the most recent staging of HCC before LT and pathological staging of HCC in explanted livers are shown in Table 3. No evidence of HCC was detected in 1 patient (pT0, radiological T1). This patient has been included in the analysis, however, because preoperatively he was considered to have active HCC and was discussed and managed accordingly.
According to the histological stage based on the Edmondson criteria, pathological examinations revealed the following findings: 2 patients were G2, 8 were G3, and 1 was G4; in 2 cases, the histological stage could not be assessed because of complete tumor necrosis following the previous treatment. Microvascular invasion by a tumor was detected in 6 cases. One patient was not included in the evaluation of the stage and vascular invasion because of an absence of evidence of HCC (pT0).
The mean and median times that patients spent on the transplantation list were 283 and 285 days, respectively (range = 1-960 days). The mean follow-up period after LT was 3 years.
Two patients (14%) developed tumor recurrence after LT and died; both had microvascular invasion by a tumor and Edmondson grade 3 HCC in the explanted liver. These 2 patients included the one in whom the AFP concentration had been high initially (810 ng/mL) and had remained high while the patient was on the transplantation list.
Patients with Malignant PVT
Nine patients either were not placed on the transplantation list (n = 2) or were removed from it (n = 7) on account of a strong suspicion or confirmation of a diagnosis of malignant PVT. Malignant PVT was diagnosed simultaneously with HCC in the 2 patients and after HCC had been diagnosed in the other 7 patients. The median AFP level at the time of the diagnosis of malignant PVT was 50 ng/mL (range = 2-392 ng/mL, mean = 104 ng/mL).
In 7 patients, the diagnosis of malignant PVT was made by imaging techniques (ie, noninvasive diagnosis). In each of these cases, at least 3 of the previously specified criteria for benign PVT were lacking. All of these 7 patients died within 6 months of the diagnosis of malignant PVT.
In 2 patients, imaging studies were not considered to be conclusive. Imaging techniques did not reveal perfusion of the thrombus with contrast or disruption of the walls of portal veins, but its mass-forming aspect was of uncertain significance. The AFP level in both of these patients was <10 ng/mL. In one of them, PVT developed soon after treatment of an HCC nodule with a percutaneous injection of ethanol. In accordance with our protocol, they underwent fine-needle biopsies of the lesion, which showed apparently malignant cells. As a result, they were excluded from the transplantation list. These 2 patients were still alive 34 and 48 months after presumed malignant PVT had been detected.
Patients Currently on the Transplantation List
Two patients in whom the diagnosis of PVT was made after that of HCC are still on the transplantation list; the mean follow-up period of 1095 days after the diagnosis of PVT is consistent with a diagnosis of benign PVT. The mean AFP level at the time of the diagnosis of PVT was 6.5 ng/mL. In 1 patient, complete thrombosis of a minor branch of the portal vein and an extension of mural thrombosis to the right portal vein developed after partially effective transarterial chemoembolization of a single nodule of HCC (diameter = 3.7 cm). The segmental thrombosis was associated with a contrast pattern of uncertain significance on CT, but all other imaging variables were consistent with benign PVT. A biopsy sample of the thrombus was subsequently obtained and revealed no evidence of malignancy. The nodule was then resected; pathological examination of the resected specimen showed an absence of infiltration of portal vein branches by a tumor. During follow-up after surgery, the residual thrombosis underwent complete regression; the patient was alive, without evidence of tumor recurrence, 33 months after the resection.
Other Patients
Five patients who were placed on the transplantation list were subsequently removed from it because the size of the tumor exceeded the threshold specified in our center, although evidence of malignant PVT had not developed. The median AFP level at the time of the diagnosis of PVT was 18 ng/mL (range = 4-977 ng/mL, mean = 228.8 ng/mL).
Three patients died from causes unrelated to HCC while on the transplantation list; in 2, the cause was rapid deterioration of hepatocellular function due to end-stage chronic liver disease, and in the remaining case, the cause was an aggressive lymphoma. The median AFP level at the time of the diagnosis of PVT was 14 ng/mL (range = 4-100 ng/mL, mean = 39.3 ng/mL).
The frequency of LT was similar for patients in whom PVT and HCC were diagnosed simultaneously and for patients in whom the diagnosis of PVT was made after that of HCC (range = 40%-43.5%; Table 4).
| PVT Diagnosis Simultaneouslywith HCC Diagnosis (n = 10) | Number of Patients |
|---|---|
| Dropout | 6 (60.0%) |
| Malignant PVT | 2 (20.0%)† |
| HCC growth* | 2 |
| HCC-unrelated death | 2 |
| Transplanted | 4 (40.0%) |
| Still waiting | 0 (0%) |
| PVT Diagnosis After HCC Diagnosis (n = 23) | Number of Patients |
| Dropout | 11 (47.8%) |
| Malignant PVT | 7 (30.4%)‡ |
| HCC growth* | 3 |
| HCC-unrelated death | 1 |
| Transplanted | 10 (43.5%) |
| Still waiting | 2 (8.7%) |
- * Progression in the size of HCC beyond the threshold allowed in the center.
- † Two patients were evaluated for LT but were not included on the list on account of malignant PVT.
- ‡ Seven patients were removed from the waiting list for LT because of suspected or confirmed malignant PVT.
DISCUSSION
In this prospective study, the use of well-defined criteria for the diagnosis of benign PVT in patients with cirrhosis and HCC has enabled malignant PVT to be excluded and thereby has facilitated the selection of patients for LT who do not have a subsequently increased risk of recurrence of HCC.
Previously, the presence of PVT was considered in most centers to be an absolute contraindication to LT in patients with HCC because of the possibility that the tumor had invaded portal veins and the high risk of recurrence of HCC when LT is undertaken in patients with malignant PVT. In contrast, benign PVT is not a contraindication to LT, provided that any associated technical problems can be overcome, as has been demonstrated in a series of patients with cirrhosis in the absence of HCC.45
Patients with cirrhosis and early HCC may develop either benign or malignant PVT.1-5 Differentiating benign and malignant PVT may be difficult. Indeed, the challenging nature of this differentiation was confirmed in a recent study in which 42% of 12 patients who underwent LT were found to have neoplastic invasion of the portal vein.46 This problem has led to PVT being considered a contraindication to LT in patients with HCC. Fine-needle biopsy of the thrombus has the potential of clarifying the nature of PVT.17, 18 However, this procedure is invasive and is associated with a high risk of complications in patients with impaired blood coagulation, such as those with decompensated cirrhosis. Furthermore, in patients with HCC, seeding of the tumor along the needle track may occur. Therefore, needle biopsy of the thrombus may not always be feasible and may be contraindicated. In this study, the reliability of imaging techniques in establishing the benign or malignant nature of PVT in potential candidates for LT was evaluated prospectively.
In recent years, contrast-enhanced imaging techniques, which had already been accepted as reliable tools in the diagnosis of malignant hepatic nodules in patients with cirrhosis,19-27, 41 have also been shown to be useful in the characterization of PVT. Intrathrombus vascularity, observed in the arterial phase of imaging studies after the administration of contrast, has been reported to be a sign that is specific for malignant PVT on both CT33-35 and MRI,36-38 whereas, in contrast, a lack of vascularization of the thrombus could be considered to imply the benign nature of PVT. However, it was unclear how reliable these procedures were in confirming the presence of benign PVT in patients who presented with a malignancy. In particular, in the context of LT, a high degree of specificity in the recognition of benign thrombosis is required to avoid false-positive results in the diagnosis of benign PVT, which would lead to the inappropriate use of liver allografts.
In this context, the range of sensitivities of the various techniques available for the diagnosis of malignant PVT has been reported to be 40% to 90%.3, 28, 29, 32 Procedures that may improve sensitivity are contrast-enhanced color Doppler sonography, as described by Ricci et al.,32 and, more recently, CEUS, which combines high-contrast sensitivity and spatial resolution. The role of CEUS for this purpose has been assessed with respect to color Doppler sonography and CT. CEUS was shown to be more sensitive and reliable than color Doppler sonography, especially in the detection of small PVT, in studies in which the clinical course or tissue biopsy samples were used to establish correct diagnoses.3, 39 CEUS was reported recently to produce significantly better results than CT in the detection and characterization of PVT in 316 patients with hepatic malignancies.40
These results and similar findings at our center led us to hypothesize that imaging techniques might be sufficiently sensitive and specific to establish a diagnosis of benign PVT in candidates for LT. The findings in the present study are consistent with this hypothesis. The results obtained with imaging techniques, after discussion by a multidisciplinary team, enable benign PVT to be identified in patients with HCC who are potential candidates for LT. Such patients with benign PVT can be selected for LT without a subsequently increased risk of recurrence of HCC and hence wastage of liver allografts. Our 14 patients with HCC in whom a diagnosis of benign PVT was made at the same time as or after the diagnosis of HCC were placed on the transplantation list and underwent LT; in each of these cases, the absence of malignant thrombosis was subsequently confirmed by histological examination of the explanted liver. Recurrence of HCC was detected in 2 patients (14%) of this group after a median follow-up of 9 months after LT. This recurrence rate is comparable to that for patients with HCC without PVT who have undergone LT at our center.44 The 2 patients in our study group in whom the tumor recurred had the highest AFP levels, even though they were diagnosed as having nonneoplastic PVT. Indeed, in both of these cases, the benign nature of the thrombus was confirmed by pathological examination of liver explants; recurrence of the tumor appeared to be related to its aggressiveness (poor differentiation and microvascular invasion on pathological examination of the liver explant).
In this study, histological confirmation of the nature of PVT, as a result of fine-needle biopsy or autopsy, was not possible in all of the patients considered to have malignant PVT or in all of those considered to have benign PVT who were removed from the transplantation list on account of progression of the HCC or hepatocellular failure. Thus, a precise calculation of the positive and negative predictive values of the diagnostic criteria used in this study could not be made.
The fact that the data were not completely comprehensive in these particular respects might imply that there could have been some erroneous diagnoses in some of the patients who did not undergo LT. Because the availability of liver allografts is limited, it is mandatory that their allocation be optimal. When there is substantial suspicion of the presence of malignant PVT (multiple positive imaging studies or positive tissue biopsy), it is very difficult to disprove this diagnosis with sufficient certainty to enable LT to be advocated with the prospect of a satisfactory outcome.
Our findings indicate that when PVT develops in a patient with HCC already on the transplantation list, the probability that the PVT is neoplastic is high (30%; Table 3), whereas if a diagnosis of PVT is made at the same time as that of HCC, the probability that the PVT is malignant is lower (20%).
In conclusion, the results of this study indicate that PVT should no longer be regarded as an absolute contraindication to LT in patients with cirrhosis and HCC. In this context, the PVT should be critically evaluated by imaging techniques, and our criteria for diagnosing benign PVT should be applied, before discussion of all the available clinical, laboratory, and imaging data by a multidisciplinary team. This approach allows the appropriate selection of patients with HCC and PVT for LT.




