T Cell-mediated biliary epithelial-to-mesenchymal transition in liver allograft rejection†
This study was financed by the Roche Organ Transplantation Research Fund. Karolina A. Rygiel is supported by a European Union FP6 Marie Curie Fellowship for Early-Stage Research Training (Regulation of Organ Survival After Transplantation project). Joseph D. P. Willet is supported by a British Transplantation Society Ph.D. studentship. John A. Kirby receives support from an award of the Biomedical Research Centre for Ageing and Age-Related Disease (United Kingdom National Institute for Health Research) to the Newcastle upon Tyne Hospitals NHS Foundation Trust.
Abstract
Loss of bile duct epithelium is characteristic of early chronic rejection following liver transplantation. Recent studies have suggested that intrahepatic biliary epithelial cells can transform into myofibroblasts. This study examines the induction and molecular regulation of this transition during allograft rejection. Immortalized human cholangiocytes were stimulated with either transforming growth factor β1 (TGFβ1) or a T cell line, and they were examined for morphological, proteomic, and functional features. Posttransplant liver biopsy sections were also examined. Treatment of cholangiocytes with TGFβ1 or TGFβ-presenting T cells induced a bipolar morphology, reduced expression of E-cadherin and zona occludens 1 (ZO-1), and increased vimentin, fibronectin, matrix metalloproteinase 2 (MMP-2), MMP-9, and S100 calcium binding protein A4 (S100A4); treated cells invaded a model basement membrane. Chemokines induced T cell penetration of 3-dimensional, cultured bile duct-like structures and bile ducts in liver biopsy sections. A spatial association was observed between duct-infiltrating T cells and cholangiocyte expression of mesenchymal markers, including S100A4. Inhibition of S100A4 expression in vitro blocked TGFβ1-mediated loss of E-cadherin and ZO-1 but did not reduce induction of fibronectin, MMP-2, or MMP-9. This study demonstrates the potential for T cells to induce an intrahepatic biliary epithelial-to-mesenchymal cell transition during chronic rejection. Furthermore, S100A4 expression by cholangiocytes was identified as a crucial regulator of this transition. Liver Transpl, 2010. © 2010 AASLD.
We have previously demonstrated that the biliary epithelial-to-mesenchymal cell transition (EMT) may contribute to the ductopenia characteristic of a number of chronic inflammatory liver diseases.1 Histological parallels have been drawn between the ductopenia of chronic liver disease and that observed during chronic rejection of liver allografts.2 It is well established that acute cellular rejection of liver allografts is associated with the infiltration of T cells and that chronic rejection is associated with multiple episodes of acute rejection. The exact underlying mechanism or mechanisms by which EMT is induced remain unclear.
Numerous in vitro studies have characterized the ability of terminally differentiated epithelial cells to acquire a fibroblast-like phenotype after exposure to cytokines.3 Defining features of this process are the loss of epithelial markers, such as zona occludens 1 (ZO-1), E-cadherin, and specific cytokeratins (CKs), and the gain of S100 calcium binding protein A4 (S100A4), vimentin, matrix metalloproteinases (MMPs), α-smooth muscle actin, interstitial collagens, and transforming growth factor β (TGFβ).4 These observations have been confirmed in murine models of kidney,5 lung,6 and liver fibrosis7 by the application of cell lineage tracing techniques. Although this approach has not been directly possible in humans, the identification of mesenchymal markers, such as S100A4 in cells that retain expression of E-cadherin, has been used to demonstrate that the EMT seen in murine models is also present in human organs such as the kidneys,8 lungs,9 and liver1, 10 either having chronic disease or undergoing rejection. The underlying function of these markers also supports the hypothesis that they represent an observed EMT.11, 12 S100A4 is involved in promoting cell migration and is constitutively expressed by macrophages and various metastatic cancer cells, although it is not tumorigenic by itself.13, 14 The murine homologue of S100A4, fibroblast specific protein-1, is expressed during epithelial-mesenchymal transformations in early development and later during repair and remodeling in response to injury.11 S100A4 has no known enzymatic activity, so interaction with other intracellular proteins is critical to its function.14 It is thought that S100A4 influences cell motility by interacting with the cytoskeleton, and several reports have identified proteins that can associate with S100A4 to enhance cell migration; these include nonmuscle tropomyosin15 and myosin heavy chain IIA.16
Although cytokines such as the fibroblast growth factors and epidermal growth factor play a role in EMT,17 TGFβ is the most important driving factor. The downstream regulators of the TGFβ signaling pathway have been observed during EMT; these include the nuclear localization of phosphorylated (activated) Smad protein 2 or 3 (or both) during the TGFβ-mediated induction of EMT in cultured human renal tubular epithelial18 and biliary epithelial cells.1 These phospho-proteins have also been demonstrated in the nuclei of epithelial cells undergoing EMT in clinical renal allograft biopsy sections19 and bile ducts of patients with primary biliary cirrhosis (PBC).1
Normal human biliary epithelial cells (HBECs) express low levels of messenger RNA (mRNA) encoding TGFβ1, but this is increased in liver biopsy tissue from PBC patients.1 However, epithelial cells are not the only source of this growth factor during episodes of portal tract inflammation. Activated T cells can express and present a membrane-bound form of active TGFβ. These cells include some regulatory CD4+CD25+ T cells20 and a potentially overlapping subpopulation of T cells that is induced by TGFβ to express the αE(CD103)β7 integrin.21 The only defined ligand for this integrin is E-cadherin, which allows CD103+ T cells to bind epithelial cells specifically.22 It is known that CD103+ lymphocytes are present within the portal tract infiltrate of the PBC liver,23 in which they may penetrate and bind cells of the biliary epithelium. This pathway represents one potential method by which the T cell infiltrate seen in both chronic liver diseases and allograft rejection could mediate the observed EMT process.
The current study was designed to examine the potential of TGFβ-presenting T cells to induce biliary EMT both in vitro and in vivo during human liver allograft rejection. Further experiments were performed to assess the cell migration and EMT regulatory functions of S100A4 expression in HBECs.
Abbreviations:
3D, 3-dimensional; CCL, chemokine (C-C motif) ligand; CK, cytokeratin; CTACK, cutaneous T cell-attracting chemokine; CXCL, chemokine (C-X-C motif) ligand; CXCR, chemokine (C-X-C motif) receptor; DAB, diaminobenzidine; DAPI, 4′,6-diamidino-2-phenylindole; DDAO-SE, 7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridin-2-one succinimidyl ester; EMT, epithelial-to-mesenchymal cell transition; ENA-78, epithelial cell-derived neutrophil-activating peptide 78; FITC, fluorescein isothiocyanate; HBEC, human biliary epithelial cell; IBEC, immortalized biliary epithelial cell; IL, interleukin; IP-10, interferon-inducible protein 10; MCP-1, monocyte chemotactic protein 1; MIP-1β, macrophage inflammatory protein 1β; MMP, matrix metalloproteinase; mRNA, messenger RNA; NAP, nucleosome assembly protein; PBC, primary biliary cirrhosis; PDC, pyruvate dehydrogenase complex; RT-PCR, reverse-transcriptase polymerase chain reaction; S100A4, S100 calcium binding protein A4; shRNA, short hairpin RNA; TGF, transforming growth factor; WT, wild type; ZO-1, zona occludens 1.
MATERIALS AND METHODS
Immunohistochemical Investigation of Liver Tissue
Formalin-fixed liver transplant tissue (n = 14; all after moderate cellular rejection) was obtained from the Newcastle upon Tyne Teaching Hospitals NHS Trust in accordance with approval from the local research ethics committee of Newcastle and North Tyneside. Time zero biopsies were used for normal controls.
All immunohistochemical protocols were based on those previously described.1 Sections were incubated with polyclonal rabbit anti-human MMP-9 (Dako, United Kingdom) or pSmad2/3 (Santa Cruz, CA) or monoclonal mouse anti-human CD4 (Novocastra, United Kingdom), CD8, or CD3 (Dako). Appropriate irrelevant immunoglobulins were used as controls. The secondary antibodies were biotinylated swine anti-rabbit immunoglobulins (Dako) or rabbit anti-mouse immunoglobulins (Dako). Bound antibodies were visualized with streptavidin-biotin-peroxidase complex (Vector, United Kingdom) with diaminobenzidine (DAB; brown: MMP-9 and E-cadherin) or nickel DAB (black: pSmad2/3; both from Sigma, United Kingdom). Subsequently, CD3-, CD4-, and CD8-labeled (nickel DAB) sections were incubated overnight with rabbit anti-human S100A4 and mouse anti-human CK-7 primary antibodies (both from Dako), which were applied simultaneously. CK labeling was developed first with indirect immunoperoxidase (horse anti-mouse horseradish peroxidase) and DAB and finally with S100A4 with biotinylated swine anti-rabbit immunoglobulin and streptavidin-biotin-alkaline phosphatase complex (Vector) and Vector red. Dual labeling with E-cadherin and S100A4 was carried out like triple labeling with the omission of the initial T cell phenotyping step.
Three-Dimensional (3D) Culture of Immortalized Biliary Epithelial Cells (IBECs) with TGFβ or T Cells
The simian vacuolating virus 40-transformed adult intrahepatic HBEC line H-69 was provided by Dr. D. M. Jefferson (Tufts University School of Medicine, Boston, MA).24 This IBEC line was cultured in Dulbecco's modified Eagle's medium/Ham's F12 nutrient mixture (3:1) supplemented with 1.8 × 10−4 M adenine, 2 × 10−9 M tri-iodothyronine, 5.5 × 10−6 M epinephrine, 1 μM hydrocortisone, 10% fetal calf serum, 100 U/mL penicillin and streptomycin (all from Sigma), and insulin-transferrin-selenium X supplement (10 mg/L insulin, 5.5 g/L transferrin, 2.0 g/L ethanolamine, and 6.7 μg/L sodium selenite; Invitrogen, United Kingdom). Once expanded, IBECs were cultured on a 0.5-mm layer of Matrigel (Becton Dickinson, United Kingdom) at a density of 200,000 cells/cm2; after 48 hours, 3D structures were formed within the Matrigel.
Lymphoblastoid human T cells25 were labeled with CellTrace Far Red 7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridin-2-one succinimidyl ester (DDAO-SE) dye (Molecular Probes, Invitrogen; 8 μM). TGFβ1 (5 ng/mL) or labeled T cells (150,000 cells/cm2) were added to 3D cultures of IBECs in Matrigel. After incubation for another 72 hours, the cultures were incubated with dispase (Becton Dickinson), fixed, permeabilized, and immunofluorescence-labeled for E-cadherin (Dako) with secondary fluorescein isothiocyanate (FITC)-labeled antibody. The structures were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
The labeled 3D structures were analyzed with a Leica TCS SP2 UV laser scanning confocal microscope (Leica GMBH, Germany). Appropriate areas were scanned for fluorochrome-labeled cells (FITC excitation = 488 nm, emission = 510-535 nm, far-red excitation = 633 nm, emission = 670-680 nm), and a Z series of optical sections was collected at 1-μm intervals to define T cell penetration of the epithelial structures.
Chemokine Array
A human anti-chemokine antibody array (RayBiotech, Inc., United States) was used in accordance with the manufacturer's protocol. Briefly, the membranes were incubated with a conditioned medium from IBEC cultures, and bound chemokines were detected with a biotinylated antibody cocktail. The membranes were then treated with horseradish peroxidase-conjugated streptavidin and developed, and the signal was detected with X-ray film. The intensity and area of the spots were quantified and normalized to a positive control (at 100%) with Intelligent Quantifier-11 software (Bio Image Systems, Inc., United States).
T Cell Chemotaxis Assay
The potential of T cells to respond to chemokines produced by biliary epithelial cells was assessed by the measurement of chemotactic migration.26 Briefly, 106 T cells were added to the upper chamber of 24-well-format Transwell filters (5-μm pore diameter; Fisher Scientific, United Kingdom) and placed in wells containing a complete medium or a medium conditioned by culture of IBECs. The migrant cells were counted after incubation for 2.5 hours at 37°C.
Monolayer Culture of IBECs and Primary HBECs
IBECs were cultured as described previously, and after reaching 70% confluency, the cells were added to chamber slides (Permanox Lab-Tek, Nunc, Denmark) and cultured for 48 hours to allow firm attachment. The medium in selected wells was then supplemented with TGFβ1 at 5 ng/mL (R&D Systems, United Kingdom). Lymphoblastoid human T cells were added to the epithelial cell cultures in some chambers at a density of 8.3 × 104 cells/cm2. Each culture was examined after incubation for another 72 hours. For some experiments, primary HBECs (ScienCell, United States) were expanded in a proprietary medium and treated as IBECs. Before being used, the primary cells were validated to be >95% pure on the basis of CK-19 expression; these cells were guaranteed to be shipped at passage 3 and were not used after 5 population doublings.
Immunocytochemical Characterization of IBECs
IBEC or primary biliary epithelial cell monolayer cultures were antibody-labeled to detect epithelial and mesenchymal antigens as described previously.1 Briefly, the cells were fixed in 4% phosphate-buffered paraformaldehyde, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline, and treated with 5% bovine serum albumin (Sigma) in phosphate-buffered saline before incubation with primary antibodies specific for E-cadherin (Becton Dickinson), vimentin, or ZO-1; an irrelevant antibody was used as a control. Appropriate FITC-conjugated secondary antibody reagents were then applied, and the labeled cells were counterstained with DAPI (Sigma) before being mounted in a fluorescence mounting medium (Dako). Immunofluorescence-labeled preparations were examined with laser scanning confocal microscopy as described previously.
TGFβ Presentation by T Cells
Bioactive TGFβ on the surface of the T cells was assessed by culture with the MFB-F11 reporter cell line.27 These cells were mixed either with a medium conditioned by T cells or at a ratio of 1:1 with T cells. After 48 hours, the production of secreted alkaline phosphatase was measured with a chromogenic reaction (para-nitrophenylphosphate substrate; Sigma). The results were compared with a standard curve generated with defined concentrations of TGFβ1.
S100A4 Gene Knockdown in IBECs
A set of plasmids (Sigma) carrying short hairpin RNA (shRNA) sequences complementary to different regions of human S100A4 and a nontargeting control were used to transfect IBECs. Briefly, the cells were cultured to 70% to 80% confluence and separately transfected with each construct with Effectene (Qiagen, United Kingdom); this was followed by selection with puromycin (0.25 μg/mL). Several clones were expanded, and the expression of S100A4 was measured by quantitative, real-time reverse-transcriptase polymerase chain reaction (RT-PCR; Applied Biosystems, United Kingdom); 2 knockdown clones (KD1 and KD2) and 1 nontargeting control clone (C1) were selected for further experiments.
Western Blotting
Selected IBEC transfectants (KD1, KD2, and C1) and wild-type (WT) cells were cultured to confluence; some cultures were stimulated with TGFβ1 at 5 ng/mL for 72 hours. The cells were harvested, resuspended in a lysis buffer, boiled for 10 minutes, and sonicated for 10 seconds. After centrifugation, the protein extract was supplemented with a buffer containing urea (300 mg/mL), sodium dodecyl sulfate (50 mg/mL), trishydroxymethylaminomethane (50 mM, pH 6.8), dithiothreitol (0.1 M), glycerol (10%), and bromophenol blue (0.05 g); 10% β-mercaptoethanol was added, and the samples were boiled for another 10 minutes before being loaded for polyacrylamide gel electrophoresis and western blotting with anti-S100A4 antibody (Dako). All blots were reprobed with anti-pyruvate dehydrogenase complex (PDC) antibodies to control for an equal loading.
Matrigel Migration/Invasion Assay
Epithelial cells were seeded at a density of 4 × 104 cells/cm2 onto Matrigel invasion chambers with 8-μm pores (BioCoat, Becton Dickinson) and cultured for 24 hours. The noninvading cells were removed from the upper surface, and the membranes were stained with Diff-Quick (Merck, United Kingdom). Cells on the lower surface of the membranes were counted by microscopy.
Zymography
Supernatants from cultured IBECs were mixed with a loading buffer (Invitrogen) and loaded onto 8% polyacrylamide gels containing 0.1% gelatin. After electrophoresis, the gels were washed and incubated at 37°C for 18 hours in a buffer containing 20 mM trishydroxymethylaminomethane (pH 7.8), 10 mM CaCl2, and 1% Triton X-100. After staining with Coomassie blue (Sigma), the area of gelatin digestion was recorded.
Data Analysis and Statistical Methods
The presentation of the data and Student t test analysis were performed with Prism 3 software for Windows (GraphPad Software, United States).
RESULTS
Immunohistochemistry of Liver Biopsy Sections
Biopsy sections from acute rejection biopsies showed immune cell infiltration, with CD3+ T cells generally restricted to the portal tracts (Fig. 1A); in many cases, the biliary epithelium (CK-7+) was also infiltrated. Further phenotyping of allograft biopsy sections undergoing moderate acute cellular rejection (n = 14) revealed large accumulations of CD4+ T cells in the portal tract infiltrate (Fig. 1B). T cells infiltrating the bile ducts were predominantly CD8+ (Fig. 1C). Individual epithelial cells within the ducts frequently coexpressed CK-7 and S100A4; these cells were often in close proximity to the infiltrating immune cells (Fig. 1A,C), and this indicated that epithelial cells could undergo the first stages of EMT in this environment. A number of biliary epithelial cells showing S100A4 expression were not associated with immune cells. Bile ducts in a normal liver did not express S100A4, and only small populations of lymphocytes were observed (not shown).
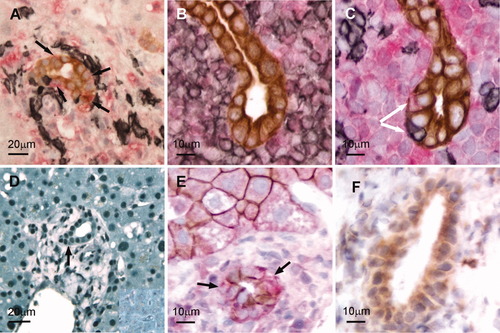
(A-C) Immunohistochemical examination of T cell interactions with biliary epithelial cells in vivo. (A) Invasion of the bile duct epithelium (CK-7: brown) by T cells (black) in a biopsy of a liver with moderate acute rejection. S100A4-expressing biliary epithelial cells are dual-labeled (brown/red and black arrows) (B,C) Accumulations of CD4+ and CD8+ T cells (black) surrounding bile ducts (CK-7: brown) in serial sections from a rejection biopsy. Some CD8+ infiltrating cells penetrated the bile duct (arrow), and adjacent epithelial cells expressed S100A4 (red) with marginated CK-7. (D-F) TGFβ and EMT markers in biliary epithelial cells in vivo. (D) Nuclear accumulation of pSmad2/3 in acute cellular rejection. Most cells in the portal tract (and parenchyma), including the bile duct (arrow), showed a high nuclear accumulation of pSmad2/3 in comparison with a normal liver (inset). (E) E-cadherin (brown) in the biliary epithelium (and hepatocytes) during allograft rejection. In the biliary epithelium, E-cadherin expression was noncontinuous, and some bile duct cells coexpressed S100A4+ (brown/red and arrows). (F) MMP-9 expression (brown) in a bile duct during allograft rejection.
Hepatocytes, biliary epithelial cells, and infiltrating cells all showed a nuclear accumulation of pSmad2/3 in rejection in comparison with a normal liver (Fig. 1D). Biliary epithelial cells (and hepatocytes) expressed E-cadherin (Fig. 1E); some S100A4-expressing biliary epithelial cells showed reduced E-cadherin labeling (Fig. 1E, arrows). A proportion of ducts in transplant biopsies showing moderate acute cellular rejection also expressed MMP-9 (Fig. 1F); this enzyme was not present in normal bile ducts (not shown).
T Cell Infiltration of 3D Cell Cultures and Chemokine Production by IBECs
IBECs cultured on Matrigel formed branched 3D structures (Fig. 2A) that expressed E-cadherin (Fig. 2B) and specifically recruited T cells (Fig. 2B), which readily penetrated the 3D structure to become localized between individual epithelial cells (Fig. 2C).
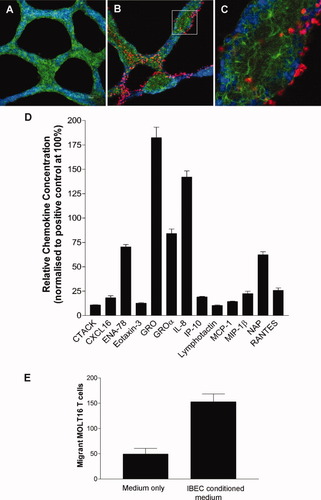
Examination of T cell interactions with biliary epithelial cells in vitro. (A) The 3D structure formed by IBECs grown on Matrigel was labeled to detect E-cadherin (green) with a nuclear counterstain (DAPI; blue). (B) Coculture of 3D structures (E-cadherin: green) with T cells (DDAO-SE dye: red). A Z-section analysis was performed with laser scanning confocal microscopy. (C) The rectangle delineated in panel B, at a higher magnification, reveals penetration by T cells, which were lying between adjacent E-cadherin-expressing epithelial cells. (D) Expression of functional chemokines by cultured IBECs. Results from a protein array show the expression of a range of chemokines by unstimulated cells, including some chemokines known to be T cell chemoattractants; the data have been normalized to a positive control protein (100%). (E) A chemokine-containing medium, conditioned by culture with unstimulated cholangiocytes, induced chemotactic migration of T cells. The mean data are representative of 3 similar assays; error bars show the standard error of the mean.
An antibody array demonstrated constitutive expression by IBECs of a range of chemokines, with GRO [chemokine (C-X-C motif) ligand 1 (CXCL1)], interleukin 8 (IL-8; CXCL8), and epithelial cell-derived neutrophil-activating peptide 78 (ENA-78; CXCL5) present at the highest concentrations and with lower but easily detectable levels of the specific T cell chemokines RANTES [chemokine (C-C motif) ligand 5 (CCL5)], macrophage inflammatory protein 1β (MIP-1β; CCL4), and interferon-inducible protein 10 (IP-10; CXCL10) also present (Fig. 2D). A medium conditioned by cultured IBECs induced a significantly increased chemotactic migration of T cells (P < 0.01; Fig. 2E).
Effect of TGFβ1 or T Cells on the Phenotype of IBEC Monolayers
Resting IBEC monolayers had a uniform epithelioid morphology and strong expression of CK-7 and CK-19 (not shown). As previously described in primary HBECs,1 IBECs also expressed E-cadherin (Fig. 3A) and ZO-1 (Fig. 3D) with strong and unbroken expression along the lateral contacts between confluent cells; the antigen was expressed at a very low level in the cytoplasm. There was a low level of expression of the mesenchymal markers S100A4 (Fig. 4C) and vimentin (not shown). Treatment with TGFβ1 (5 ng/mL) for 72 hours caused reduced expression of E-cadherin and ZO-1 at the cell junctions (Fig. 3B,E). Coculture with T cells also reduced the expression of cell-surface E-cadherin and ZO-1, although a slight increase in cytoplasmic staining was observed for E-cadherin (Fig. 3C,F). There was also an increase in the expression and organization of vimentin (not shown).
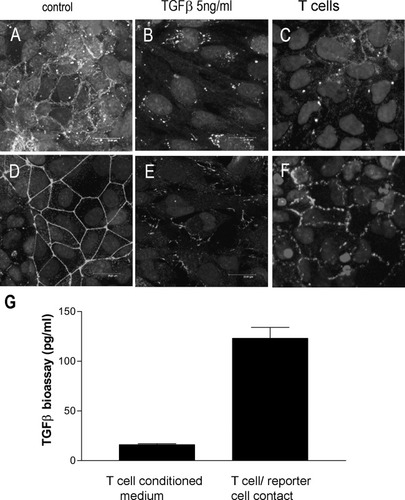
Immunocytochemical labeling of cultured IBECs. The figure shows (A,D) unstimulated cells, (B,E) cells stimulated with 5 ng/mL TGFβ1 for 72 hours, and (C,F) cells cocultured with T cells (8.3 × 104 cells/cm2) for 72 hours. Cells were labeled by indirect immunofluorescence to detect the epithelial markers (A-C) E-cadherin and (D-F) ZO-1. Representative results from 3 separate experiments are shown. (G) A TGFβ-responsive reporter cell line demonstrated the presentation of active TGFβ by T cells during an assay with cell-to-cell contact. The mean data (n = 3) are representative of 3 similar assays; error bars show the standard error of the mean.
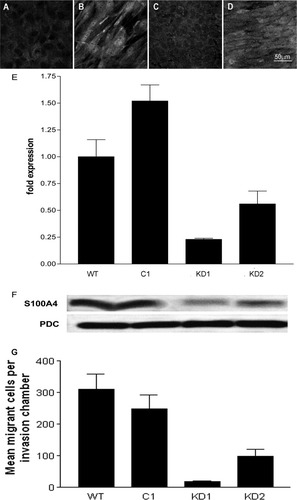
(A-F) Examination of S100A4 expression by primary HBECs, WT IBECs, and shRNA-transfected cells. (A,C) S100A4 expression by resting primary HBECs and resting IBECs, respectively. (B,D) S100A4 expression after stimulation of the primary and immortalized cells (IBECs) with TGFβ1 for 72 hours. A bipolar morphology and increased expression of S100A4 were observed after stimulation with TGFβ. (E) Relative levels of S100A4 mRNA expressed by WT IBECs and after stable transfection with the nontargeting control (C1) and S100A4-specific shRNA (KD1 and KD2). The mean values are shown together with the standard error of the mean. (F) The knockdown of mRNA encoding S100A4 in clones KD1 and KD2 was confirmed by western blotting; the 12-kDa band indicates monomeric S100A4. An equal protein loading was confirmed by reprobing to detect PDC. (G) Motility of WT IBECs and shRNA-transfected cells. The graph shows that WT IBECs and cells transfected with the control (C1) readily migrated through a Matrigel-coated membrane, but S100A4-specific shRNA-transfected cells (KD1 and KD2) showed reduced motility. The total numbers of cells that migrated to the lower surface of duplicate invasion filters were counted.
The human T cell line was shown to present biologically active TGFβ during coculture with the MFB-F11 reporter cell line in comparison with TGFβ produced by a T cell-conditioned medium (P < 0.01; Fig. 3D). The amount of active TGFβ released by T cells into the medium (Fig. 3G) was not greater (P > 0.05) than the total level of this growth factor found in the basal medium by enzyme-linked immunosorbent assay (<50 pg/mL; data not shown).
S100A4 Knockdown in Immortalized IBECs
Resting cultures of primary HBECs expressed little S100A4 (Fig. 4A), but this was greatly increased by culture with TGFβ1 (5 ng/mL) for 72 hours (Fig. 4B). In common with many transformed cells, resting IBECs constitutively expressed a low level of S100A4 (Fig. 4C): stimulation with TGFβ1 (Fig. 4D) enhanced this to a level comparable to that of stimulated primary cells.
Real-time RT-PCR was performed to quantify S100A4 expression by WT IBECs (normalized to 1) or cells transfected with the nontargeting control sequence (C1) or the S100A4-targeting sequence. Two clones (KD1 and KD2) with reduced S100A4 expression (in all cases, P < 0.01 versus C1) were selected for further study (Fig. 4E). This specific knockdown was confirmed by western blotting for monomeric (12-kDa) S100A4 protein (Fig. 4F).
The functional significance of reduced S100A4 expression was assessed by an examination of the potential of WT IBECs and C1, KD1, and KD2 transfectants to invade an artificial basement membrane coated on porous supports. The WT and C1 cells showed similarly high levels of invasion, whereas the 2 clones with reduced expression of S100A4 showed very little invasion. The numbers of invading cells are expressed in graphical form in Fig. 4G, which shows that the invasive potential of the S100A4 knockdown clones was 22% (KD2) and 6% (KD1) of the control cell (C1) level (Fig. 4G).
Relationship Between S100A4 Knockdown and EMT
Cells from the nontargeting control (C1; Fig. 5A,B) and the S100A4 knockdown clone (KD1; Fig. 5C,D) were examined by immunocytochemistry for evidence of EMT following the addition of TGFβ1 (5 ng/mL) for 72 hours. Treatment with TGFβ1 markedly reduced the expression of E-cadherin by the control cells (Fig. 5B), with some evidence of a redistribution of the remaining E-cadherin to the cytoplasm. In contrast, clone KD1 maintained a normal expression of E-cadherin after stimulation with TGFβ1 (Fig. 5D).
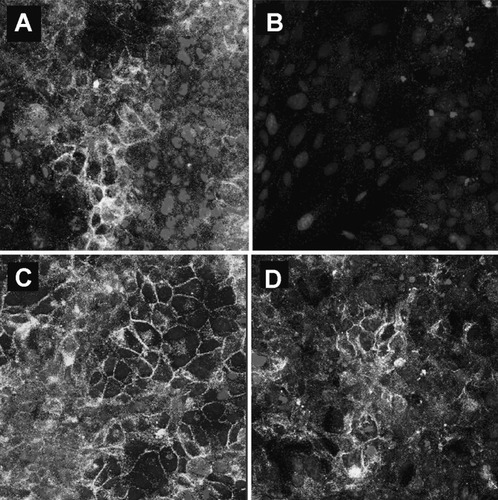
E-cadherin expression before and after the TGFβ1 treatment of IBECs transfected with (A,B) the nontargeting control (C1) or (C,D) S100A4-specific shRNA (KD1). (B) As in WT IBECs, E-cadherin expression was reduced in C1, but (D) KD1 cells retained the expression of E-cadherin after stimulation with TGFβ1 for 72 hours.
Zymographic analysis of MMP-2 and MMP-9 expression in a medium conditioned by WT IBECs or the C1, KD1, and KD2 clones showed an increase after stimulation with TGFβ1 (Fig. 6A). Mean densitometric values from 3 separate experiments showed significantly increased pro-MMP-9 expression by all the TGFβ1-treated cell lines (Fig. 6B; P < 0.005).

MMP-2 and MMP-9 expression after the treatment of WT and shRNA-transfected IBECs. (A) Zymogram showing MMP-2 and MMP-9 production. Both assays show the analysis of WT IBECs, control cells (C1), and shRNA transfectants (KD1 and KD2); the cells were either unstimulated or treated with 5 ng/mL TGFβ1 (T5) for 72 hours before analysis. (B) Mean results for the densitometric analysis of pro-MMP-9 levels from 3 separate experiments; error bars show the standard error of the mean. The dotted line indicates the normalized level for WT cells.
DISCUSSION
This study was performed to assess the potential of T cells to induce pathogenic biliary EMT resulting in bile duct loss and portal fibrosis. A coculture model was developed with IBECs, which have phenotypic attributes similar to those of primary biliary epithelial cells1 and a human T cell line known to express the αE(CD103)β7 integrin allowing adhesion to E-cadherin on epithelial cells.26 Initial experiments demonstrated that these T cells expressed latency associated peptide-associated TGFβ on their surface and that this cytokine was presented in an active form to reporter cells.1, 27 The presence of T cell-surface TGFβ has been reported previously and is associated with expression of the αEβ7 integrin and immunoregulatory T cell functions.20
The immortalized HBEC line is known to express characteristics of differentiated human cholangiocytes, including the expression of CK-7, CK-19, and γ-glutamyl transpeptidase and the development of functional transmonolayer electrical resistance. In addition, they lack expression of desmin, which is present in stellate cells, and the hepatocyte-specific markers albumin and asialoglycoprotein.24 In this study, these cells were shown to express the epithelial markers E-cadherin (also expressed by hepatocytes) and ZO-1 within their junctional complexes; the unstimulated cells expressed only low levels of the mesenchymal markers S100A4, vimentin, and MMP9. Stimulation for 3 days with TGFβ1 induced proteomic features of EMT, including reduced cell surface expression of E-cadherin and ZO-1. The addition of T cells also reduced the expression of E-cadherin and ZO-1; these antigens became fragmented within the junctional complexes, and low levels of E-cadherin appeared within the cytoplasm. The expression of vimentin and S100A4 was also increased and reorganized, and they became associated with the cytoskeleton.
The IBEC line constitutively expressed a wide range of chemokines. Previous studies have demonstrated constitutive production of IL-8 by IBECs.28 The potential for chemokines secreted by these cells to promote T cell invasion was demonstrated in free-diffusion chemotaxis assays. The T cell line used for these assays expresses the chemokine receptors chemokine (C-C motif) receptor 1 (CCR1), chemokine (C-X-C motif) receptor 3 (CXCR3), and CXCR4, which allow chemotaxis toward IP-10 (CXCL10) and RANTES (CCL5) in the IBEC-conditioned medium.26 Chemokines have been implicated in promoting the infiltration of bile ducts during inflammatory liver diseases.29
Many epithelial cells form a 3D structure consistent with duct formation when they are cultured in laminin-rich and collagen IV-rich Matrigel.30 Epithelial cell differentiation is generally more complete in such structures with the formation of well-defined intercellular junctions and apicobasal polarity. Indeed, interactions between the ubiquitous epithelial α3β1 integrin and laminin can stabilize the differentiation of epithelial cells.31 This study has demonstrated that IBECs readily organize in Matrigel to form a branching, ductlike 3D structure in which E-cadherin is expressed at the cell junctions. However, these structures were broken down by stimulation with TGFβ1 to produce cystlike bodies surrounded by bipolar, fibroblast-like cells that invaded the matrix; this is consistent with the induction of EMT.8
The 3D cultures were rapidly penetrated by T cells, with optical sectioning demonstrating close contact between lymphocytes and E-cadherin on individual IBECs. This T cell infiltration is consistent with the expression of chemokines, which are also known to enhance the affinity of the αEβ7 integrin for its ligand, E-cadherin.26, 32 The infiltration of bile duct-like structures by T cells in vitro was similar to that observed in vivo during liver allograft rejection, during which CD3-expressing cells were observed around the bile ducts and between the viable epithelial cells; some of these epithelial cells expressed S100A4 and MMP-9 with reduced E-cadherin expression, and this indicated the induction of EMT.1, 10 Epithelial S100A4 expression was noted, in some instances, in the absence of adjacent inflammatory cells, and this indicated that the epithelial cells might have been responding to an alternative local source of active TGFβ.
The reduced invasion of Matrigel by IBECs that had been transfected with specific shRNA to reduce the expression of S100A4 protein is consistent with previous reports indicating that this protein plays a central role in regulating cell motility.33 Specific reduction of S100A4 also protected cholangiocytes from losing expression of the characteristic marker of differentiated epithelium, E-cadherin, after stimulation with TGFβ1. This suggests that TGFβ-induced expression of S100A4 plays a crucial regulatory role during the induction of EMT in the human bile duct; similar results have been reported for an immortalized murine renal epithelial cell line.11 These observations emphasize the importance of S100A4 as an early marker of the induction of biliary EMT in diagnostic human liver biopsy sections.10 The mechanism by which S100A4 expression is associated with reduced levels of cell-surface E-cadherin is unclear. However, it is thought that S100A4 can bind p53 and prevent ubiquitination and degradation, which may increase levels of cytoplasmic β-catenin and lead to redistribution and degradation of E-cadherin.4
Specific inhibition of S100A4 expression did not prevent biliary epithelial cells from producing MMP-2 and MMP-9 in response to stimulation in vitro by TGFβ1. This is consistent with the observation that S100A4 is induced by activation of the Smad signaling pathway, which results in the EMT phenotype,34 whereas MMPs may be separately induced by activation of the noncanonical TGFβ-signaling pathway, which includes the p38 mitogen-activated protein kinase. The combination of increased degradation of the basement membrane by these MMPs might provide further impetus for EMT in the portal tract by disruption of normal stimulation of the α3β1 integrin by laminin, which is known to protect some differentiated epithelia from TGFβ-induced EMT.31
We have demonstrated that intraepithelial T cells expressing TGFβ can damage biliary epithelial cells and result in increased expression of S100A4, MMP-2, and MMP-9 in conjunction with a reduction in expression of E-cadherin and specific CKs. This form of TGFβ-mediated injury in vivo is also consistent with the potential of TGFβ to inhibit the cytolytic activity of graft-infiltrating T cells35; in addition, it is clear that TGFβ can induce the mutually exclusive processes of epithelial apoptosis36 and replicative senescence.37 Although the results of this study suggest that EMT may contribute to the loss of biliary epithelium and the fibrosis observed in hepatic allograft biopsies demonstrating mild to moderate inflammation,38 proof of this process and its potential for reversibility will require the application of cell lineage tracing techniques.
Acknowledgements
The authors are grateful to Dr. T. A. Booth for his expert assistance with confocal microscopy.




