Can children catch up in growth after living donor liver transplantation?†
The authors did not receive any grant or financial support to perform this retrospective study.
Abstract
Several studies have shown improved growth after liver transplantation, but long-term follow-up data have been lacking. This study was aimed at evaluating the ability of children to catch up in height after living donor liver transplantation (LDLT) and at clarifying factors affecting growth. Growth was assessed by serial height measurements performed during follow-up. Standardized height scores (z scores) were calculated for each patient preoperatively (at the baseline) and at 1, 2, 3, 5, 10, and 15 years after LDLT. The risk potential of several preoperative and postoperative variables was evaluated. A total of 237 patients, including 159 females (67.1%), met the inclusion criteria. The mean age at the time of transplant was 3.89 ± 0.28 years. The mean z score was −1.70 ± 0.09, whereas the baseline height deficit was −6.50 ± 0.39 cm. After LDLT, the z score improved significantly and reached −0.64 ± 0.14 by the end of the first year. The best height improvement was seen after 10 years (−0.33 ± 0.16). However, significant growth retardation remained at 15 years (−0.47 ± 0.17). Height showed 3 distinct phases after transplantation: a growth spurt, a plateau phase, and a late declining phase. Univariate and multivariate analyses showed that children under 2 years and those with the most growth retardation at the time of LDLT achieved the best height gain in the first year. Late growth retardation was related to the baseline z score, ABO-incompatible grafts, and graft dysfunction. In conclusion, children have the potential ability to catch up in growth to normal levels after LDLT; they can show impressive height gains in the first year followed by protracted improvement over 10 years and then late growth retardation. Young age is a determinant for early height gain, whereas ABO-incompatible grafts and graft dysfunction are determinants for late growth retardation. In contrast, the baseline z score is a determinant for both. Liver Transpl , 2010. © 2010 AASLD.
Liver transplantation has become a routine lifesaving therapeutic procedure for children with end-stage liver disease. The limited donor supply, especially for children, has led to technical advances in reduced size liver transplantation, split liver transplantation, and living donor liver transplantation (LDLT), which have been developed to achieve excellent outcomes.1 The current patient survival rate exceeds 82% at 5 years after LDLT.2 Consideration of the subsequent quality of life is thus important, and this includes fostering appropriate growth.3 Our initial report1 and studies from other centers4-6 have shown improved growth after liver transplantation, but data on long-term outcomes for height have been lacking. The present study evaluated the ability of children to catch up in height after LDLT and examined factors affecting growth.
Abbreviations:
GRWR, graft-recipient weight ratio; LDLT, living donor liver transplantation; re-Tx, retransplantation; SEM, standard error of the mean.
PATIENTS AND METHODS
Our study included pediatric patients (<18 years old) who underwent primary LDLT in Kyoto University Hospital between 1990 and 2007 for chronic end-stage liver disease and achieved 5-year survival with adequate follow-up. Cases of deceased donor liver transplantation, domino liver transplantation, and auxiliary partial orthotopic liver transplantation were excluded. Follow-up was considered adequate if the patient had height measurements at the time of transplant and at least 1 posttransplant record.

Mean values and standard deviations were obtained from standardized age-corrected and sex-corrected tables for growth in Japanese children (National Centers for Health Sciences, 2005).
z scores were calculated preoperatively (at the baseline) and at 1, 2, 3, 5, 10, and 15 years after LDLT. For each time point, height deficits were determined as the number of centimeters below the 50th percentile on growth curves standardized for age and sex. Growth retardation was defined as a z score < −2, and catch-up growth was defined as the achievement of a z score ≥ 0.
For time points up to 3 years after transplantation, height measurements were obtained if they were available within 1 month before or after each point. However, for records after this time, measurements were obtained if they were available within 3 months around each time point.
We evaluated the risk potential of several preoperative and postoperative variables. The examined preoperative factors were the age at the time of LDLT, gender, z scores, preoperative clinical status, underlying liver disease, total bilirubin, albumin, prothrombin time, creatinine, donor age and gender, graft type, graft-recipient weight ratio (GRWR), and ABO mismatching. Postoperative factors included graft dysfunction, cumulative doses of steroids and tacrolimus, retransplantation, and chronic rejection. Values used for analysis were obtained from the last records before LDLT. Graft dysfunction was defined as persistent abnormal liver function with serum aminotransferase levels at 2 to 3 times normal, with or without elevated bilirubin, and abnormal biopsy findings (eg, fibrosis or biliary strictures). Cumulative doses of tacrolimus were trichotomized into 3 equal groups of low, medium, and high doses at 1, 2, 3, 5, 10, and 15 years after LDLT, and cumulative doses of prednisone in the first year were trichotomized into 3 equal groups of low, medium, and high doses.
Operative Procedure
Techniques for donor and recipient operations have been described previously.8, 9 The left lateral segment was the primary choice. However, if the estimated GRWR was >4%, a monosegment graft was used.10 For larger recipients, graft selection was extended to the left lobe11 and right lobe12 according to the GRWR and residual liver volume in the donor after hepatectomy.
Immunosuppression Therapy
The immunosuppressive regimen consisted of tacrolimus and low-dose steroids.13 Tacrolimus was begun orally on the day before the operation (0.075 mg/kg twice daily). The target trough serum levels for tacrolimus were initially >10 ng/mL and decreased gradually to 6 to 8 ng/mL within a few months after LDLT. Methylprednisolone (10 mg/kg of body weight) was administered intraoperatively prior to reperfusion. Postoperatively, the same drug at a dose of 1 mg/kg was given for the first 3 postoperative days, and this was followed by 0.5 mg/kg for the next 3 days and 0.3 mg on postoperative day 7. This was changed to oral prednisolone at a dose of 0.3 mg/kg 8 days after transplantation. Prednisolone was reduced to 0.1 mg/kg/day by 4 weeks after transplantation if the postoperative course was free of liver dysfunction, and patients were routinely weaned off steroid therapy by 3 to 6 months after transplantation as long as graft function was maintained.
In cases of ABO-incompatible LDLT, additional immunosuppressants and preconditioning regimens were administered to inhibit humoral rejection; treatments included prostaglandin E1, cyclophosphamide, azathioprine, mycophenolate mofetil, and plasma exchange, as described elsewhere.
Statistical Analyses
Data are provided as means and standard errors of the mean. The Student t test for unpaired samples was used for the comparison of means, and the χ2 test was used to compare proportions. Statistical analyses between paired data were performed with the paired t test. For correlation testing, Pearson's correlation coefficient was used for continuous variables, and Spearman's correlation coefficient by rank was used for discrete variables. After factor analyses were performed, multivariate analysis using a general linear model was performed. Factors significant at P < 0.05 were included in the model; however, other factors that were thought to affect growth from the literature4 were forced into the model. SPSS version 16 software (SPSS, Chicago, IL) was used. Values of P < 0.05 were considered significant.
RESULTS
From June 1990 to November 2007, a total of 701 pediatric LDLT procedures were performed in 650 children (<18 years old) at Kyoto University Hospital. Only 237 patients (159 females, 67.1%) met the inclusion criteria with a mean follow-up of 11.36 ± 0.29 years. The mean age at the time of transplant was 3.89 ± 0.28 years (median, 1.58 years; range, 0.12-17.64 years). Indications for LDLT included biliary atresia (n = 190, 80.2%), Alagille's syndrome (n = 11, 4.6%), Byler's syndrome (n = 5, 2.1%), metabolic liver disease (n = 11, 4.6%), liver cirrhosis/hepatitis (n = 5, 2.1%), tumors (n = 7, 3%), and other (n = 8, 3.4%). The characteristics of the patients are shown in Table 1. The subjects included 155 patients at 1 year, 115 patients at 2 years, 106 patients at 3 years, 107 patients at 5 years, 65 patients at 10 years, and 42 patients at 15 years.
| Variable | Mean ± SEM | Median (Interquartile Range) |
|---|---|---|
| Age | ||
| <2 years (n = 133) | 0.97 ± 0.03 | 0.84 (0.65-1.23) |
| 2-10 years (n = 73) | 5.21 ± 0.25 | 5.04 (3.40-6.53) |
| >10 years (n = 31) | 13.33 ± 0.42 | 12.93 (11.14-15.39) |
| Gender (female:male) | 159:78 | |
| GRWR | 2.62 ± 0.08 | 2.55 (1.55-3.52) |
| ABO compatibility (identical:compatible:incompatible) | 169:44:24 | |
| Donor age (years) | 33.80 ± 0.44 | 33 (29-38) |
| Baseline z score | −1.70 ± 0.09 | −1.67 (−2.33 to −0.89) |
| Baseline height deficit (cm) | −6.50 ± 0.39 | −6.40 (−9.83 to −3.12) |
| Baseline weight standardized score | −1.28 ± 0.07 | −1.29 (−2.00 to −0.71) |
| Baseline weight deficit (kg) | −2.52 ± 0.32 | −1.98 (−3.52 to −0.73) |
| Total bilirubin (mg/dL) | 13.85 ± 0.74 | 12.4 (4.30-19.55) |
| Albumin (g/dL) | 3.54 ± 0.05 | 3.50 (3.00-4.00) |
| Creatinine (mg/dL) | 0.23 ± 0.01 | 0.20 (0.10-0.30) |
| Graft type (monosegment:left lateral segment:left lobe:right lobe) | 2:186:43:6 |
Growth was considerably retarded at the time of transplant, and marked changes in height were seen after LDLT. The mean z score was −1.70 ± 0.09, whereas the baseline height deficit was −6.50 ± 0.39 cm. At the time of LDLT, 79 patients (33.3%) showed growth retardation with a z score < −2.0. Children with retarded growth at the time of LDLT displayed significantly higher serum bilirubin levels than those with appropriate growth. Higher proportions of Alagille's syndrome and Byler's syndrome were seen in the growth-retarded group, whereas a higher proportion of biliary atresia was seen in normal-growth children. Age and gender did not differ significantly between groups (Table 2).
| Normal Growth (z score ≥ −2) | Retarded Growth (z score < −2) | P Value | |
|---|---|---|---|
| Patients [n (%)] | 158 (66.7) | 79 (33.3) | |
| Age (years) | 3.85 ± 0.36 | 3.97 ± 0.46 | 0.829 |
| Female gender [n (%)] | 112 (70.9) | 47 (59.5) | 0.078 |
| Biliary atresia [n (%)] | 135 (85.4) | 55 (69.6) | 0.004 |
| Alagille's syndrome [n (%)] | 3 (1.9) | 8 (10.1) | 0.005 |
| Byler's syndrome [n (%)] | 0 | 5 (6.3) | 0.001 |
| Total bilirubin (mg/dL) | 12.52 ± 0.79 | 16.47 ± 1.49 | 0.011 |
After LDLT, the z score improved significantly to reach −0.64 ± 0.14 by the end of the first year (P = 0.0001), and the height deficit dropped markedly to −3.82 ± 0.66 cm. After the first year, the z score showed no marked changes (−0.68 ± 0.14, −0.55 ± 0.15, and −0.48 ± 0.13 at 2, 3, and 5 years after LDLT, respectively). There was no significant change in the z score from 2 to 3 years (P = 0.539), from 3 to 5 years (0.357), or from 5 to 10 years (P = 0.298). Height improvement was optimal at 10 years and reached −0.33 ± 0.16. A significant growth decline to −0.47 ± 0.17 (P = 0.001) was then seen at 15 years; a total of 35 patients had complete recorded data at both 10 and 15 years, and 22 of them showed a decline in their z scores. The height deficit showed a slight drop to −3.65 ± 0.69, −2.51 ± 0.67, and −2.71 ± 0.64 cm at 2, 3, and 5 years after LDLT. The best improvement in the height deficit was achieved at 10 years (−1.65 ± 1.00 cm). After that time, a significant deterioration of height was seen, with −2.98 ± 0.93 cm reached at 15 years (P = 0.003).
Patterns of Growth After LDLT
Height showed 3 distinct phases after transplantation. Initially, a growth spurt was achieved by the end of the first year; the z score improved significantly from −1.70 to −0.64 (P = 0.0001) but did not reach the mean z score for normal children. Height subsequently improved slowly from −0.64 to −0.33 (P = 0.145) by the end of 10 years. After the second plateau phase, height was suppressed to −0.47 (P = 0.003) by the end of 15 years.
To clarify height trends according to the age at the time of LDLT, we divided patients into 3 groups: <2, 2 to 10, and >10 years. The mean height improved in all groups in the first year after LDLT. Although children < 2 years old showed marked growth retardation at the time of transplantation, their height improvement was the greatest in the first year (Fig. 1). Their z scores improved from −1.713 to −0.272 but did not reach the normal population mean. Older children showed poorer early growth. Children who received transplants at 2 to 10 years improved from −1.823 to −1.124, whereas children > 10 years old showed a slight improvement from −1.339 to −1.100 by the end of the first year.
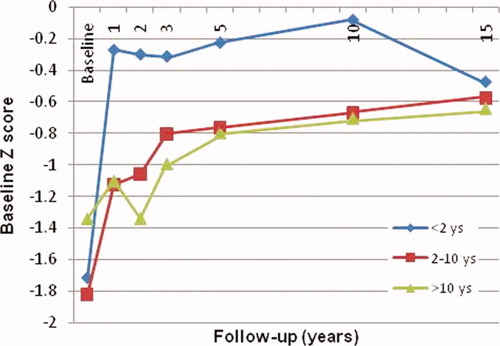
Changes in the z score according to the age at the time of LDLT. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Although all groups showed trends of gaining height with time, young children showed an unexpected height decline after 10 years. Conversely, children > 2 years showed a steady height gain. Finally, all groups achieved almost the same mean height, which was lower than that of their peers (Fig. 1).
Studying patients according to the z score at the time of LDLT, we again created 3 groups: <−2, −2 to 0, and >0 (Table 3). Children who had growth retardation at the time of transplantation (z score < −2) showed the best height gain in comparison with other groups, with the z score improving markedly from −3.164 at the time of transplantation to −1.519 and −1.271 after 1 and 15 years, respectively. Children with z scores between −2 and 0 improved significantly from −1.237 at the time of LDLT to −0.357 and −0.179 after 1 and 15 years, respectively. However, children with a mean z score > 0 showed minor changes over time. No change in height was seen for 3 years after LDLT, after which time a minor drop was seen from the baseline z score of 0.556 to 0.513 at 5 years, and this improved to 0.818 at 15 years. Although growth-retarded children showed a higher rate of growth, the mean height still did not reach normal values.
| Variable | Follow-Up (years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 | 2 | 3 | 5 | 10 | 15 | |||
| Age (years) | <2 | Mean | −1.713 | −0.272 | −0.301 | −0.313 | −0.227 | −0.082 | −0.474 |
| SEM | 0.089 | 0.173 | 0.151 | 0.172 | 0.144 | 0.194 | 0.145 | ||
| n | 133 | 87 | 63 | 59 | 57 | 38 | 18 | ||
| 2-10 | Mean | −1.823 | −1.124 | −1.057 | −0.803 | −0.764 | −0.670 | −0.576 | |
| SEM | 0.206 | 0.255 | 0.279 | 0.278 | 0.222 | 0.314 | 0.299 | ||
| n | 73 | 49 | 37 | 34 | 38 | 23 | 22 | ||
| >10 | Mean | −1.339 | −1.100 | −1.338 | −0.991 | −0.795 | −0.702 | −0.641 | |
| SEM | 0.315 | 0.322 | 0.435 | 0.535 | 0.599 | 0.506 | 0.537 | ||
| n | 31 | 19 | 15 | 13 | 12 | 4 | 2 | ||
| Baseline z score | ≥0 | Mean | 0.556 | 0.669 | 0.733 | 0.519 | 0.513 | 0.504 | 0.818 |
| SEM | 0.135 | 0.157 | 0.194 | 0.253 | 0.187 | 0.337 | 0.476 | ||
| n | 24 | 18 | 14 | 14 | 12 | 7 | 4 | ||
| 0 > z score ≥ −2 | Mean | −1.237 | −0.357 | −0.310 | −0.149 | −0.196 | −0.058 | −0.179 | |
| SEM | 0.049 | 0.171 | 0.128 | 0.133 | 0.134 | 0.172 | 0.176 | ||
| n | 134 | 83 | 62 | 57 | 67 | 38 | 23 | ||
| <−2 | Mean | −3.164 | −1.519 | −1.775 | −1.643 | −1.592 | −1.134 | −1.271 | |
| SEM | 0.119 | 0.231 | 0.262 | 0.298 | 0.271 | 0.343 | 0.261 | ||
| n | 79 | 54 | 39 | 35 | 28 | 20 | 15 | ||
| Gender | Female | Mean | −1.576 | −0.756 | −0.640 | −0.491 | −0.386 | −0.210 | −0.341 |
| SEM | 0.112 | 0.166 | 0.172 | 0.163 | 0.146 | 0.192 | 0.189 | ||
| n | 159 | 104 | 81 | 70 | 71 | 46 | 33 | ||
| Male | Mean | −1.946 | −0.411 | −0.775 | −0.675 | −0.671 | −0.615 | −0.962 | |
| SEM | 0.150 | 0.235 | 0.234 | 0.297 | 0.256 | 0.309 | 0.381 | ||
| n | 78 | 51 | 34 | 36 | 36 | 19 | 9 | ||
| Re-Tx | No | Mean | −1.707 | −0.629 | −0.650 | −0.532 | −0.501 | −0.346 | −0.521 |
| SEM | 0.094 | 0.139 | 0.144 | 0.151 | 0.133 | 0.165 | 0.180 | ||
| n | 228 | 149 | 110 | 102 | 104 | 64 | 39 | ||
| Yes | Mean | −1.457 | −0.629 | −1.326 | −0.117 | 0.190 | 0.825 | 0.124 | |
| SEM | 0.230 | 0.460 | 0.436 | 0.582 | 0.345 | − | 0.497 | ||
| n | 9 | 6 | 5 | 4 | 3 | 1 | 3 | ||
| Graft dysfunction | No | Mean | −1.795 | −0.520 | −0.717 | −0.361 | −0.273 | −0.265 | −0.291 |
| SEM | 0.120 | 0.168 | 0.172 | 0.147 | 0.141 | 0.242 | 0.172 | ||
| n | 135 | 94 | 73 | 64 | 57 | 32 | 25 | ||
| Yes | Mean | −1.607 | −0.876 | −0.692 | −0.773 | −0.753 | −0.465 | −0.836 | |
| SEM | 0.166 | 0.267 | 0.264 | 0.276 | 0.248 | 0.269 | 0.380 | ||
| n | 80 | 50 | 37 | 38 | 44 | 27 | 15 | ||
| ABO compatibility | Identical | Mean | −1.648 | −0.601 | −0.675 | −0.553 | −0.349 | −0.287 | −0.198 |
| SEM | 0.103 | 0.158 | 0.153 | 0.167 | 0.140 | 0.194 | 0.197 | ||
| n | 169 | 118 | 84 | 77 | 80 | 46 | 28 | ||
| Compatible | Mean | −2.024 | −0.864 | −0.671 | −0.453 | −0.671 | −0.471 | −1.053 | |
| SEM | 0.253 | 0.329 | 0.371 | 0.370 | 0.310 | 0.354 | 0.386 | ||
| n | 44 | 24 | 22 | 21 | 17 | 12 | 10 | ||
| Incompatible | Mean | −1.453 | −0.615 | −0.740 | −0.829 | −1.224 | −0.354 | −0.958 | |
| SEM | 0.234 | 0.460 | 0.607 | 0.604 | 0.605 | 0.636 | 0.399 | ||
| n | 24 | 13 | 9 | 8 | 10 | 7 | 4 | ||
Impact of Liver Disease
Biliary atresia was seen in 80.2% of cases, with much smaller numbers of patients showing other indications. Comparison was therefore difficult. Patients with biliary atresia had a mean z score of −1.542 before LDLT and were able to achieve good growth after transplantation. The z score improved markedly to −0.402 by the end of the first year, and a final z score of −0.213 was achieved at 10 years, with a decline in growth to −0.297 at 15 years. Patients with Byler's disease showed the greatest height retardation before LDLT, with a mean z score of −5.272, and this was followed by patients with Alagille's syndrome with a mean z score of −3.195. Patients with Alagille's syndrome gained height and showed improvements in the z score to −2.264 in the first year, and then they showed slow improvements after this time. Conversely, patients with Byler's disease showed almost no improvement until 3 years after LDLT, with a z score of −4.929, and this was followed by height gain to −2.673 after 5 years. As the numbers of followed patients with Alagille's disease and Byler's disease were getting smaller, it was difficult to describe their trends in growth for a long time after LDLT. Patients with metabolic liver disease had the best height at the time of LDLT, with a mean z score of −1.189; their height improved to −0.583 after 1 year and ultimately to −0.653 after 15 years. After 5 years, all liver diseases showed comparable z scores.
Impact of Gender
Of the 237 patients, 159 (67.1%) were females. No significant difference between girls and boys was seen with respect to the baseline z scores (Table 2), and after LDLT, they showed comparable growth rates at different time points (Table 3). Trends of growth for boys and girls differed slightly, with boys showing rapid growth in the first year and a stationary phase to 10 years followed by a decline in growth at 15 years, whereas girls showed substantial height gain in the first year followed by steady height improvement up to 10 years and then height suppression at 15 years.
Impact of Immunosuppression
With respect to steroids, all patients in our study group discontinued prednisolone within 6 months after LDLT. Within the first year of transplantation, a total of 66 of 155 patients (42.6%) received steroid pulse therapy, and 28 of 237 children (11.8%) restarted steroid therapy again at a later time during the follow-up period because of the development of acute rejection or de novo autoimmune hepatitis.
The mean value for cumulative steroid doses per kilogram of body weight in the first year of transplantation was 69.97 ± 4.06 mg/kg, and it did not show a significant correlation with the z score (P = 0.720).
For tacrolimus, there were variations in the trough levels and in the cumulative doses at different time points. The mean trough levels were 5.35 ± 0.22, 4.26 ± 0.21, 3.76 ± 0.21, 2.98 ± 0.19, and 3.30 ± 0.37 ng/mL at 1, 2, 3, 5, and 10 years after LDLT, respectively. The cumulative tacrolimus doses per kilogram of body weight were 44.22 ± 2.01, 31.12 ± 1.60, 26.21 ± 1.50, 18.73 ± 1.60, and 12.4 ± 1.78 mg/kg at 1, 2, 3, 5, and 10 years after LDLT, respectively. The z scores did not show any correlation with the tacrolimus trough levels or cumulative doses per kilogram of body weight at any time point.
Catch-Up Growth After LDLT
At the time of LDLT, 158 of 237 recipients (66.7%) showed a z score ≥ −2, but only 24 of 237 recipients (10.1%) were above the fifth percentile. After LDLT, 53 of 155 patients (34.2%) displayed acceleration of linear growth velocity and were above the fifth percentile by the end of the first year (P = 0.0001). However, by the end of the follow-up period, only 12 of 42 recipients (28.6%) showing normal height (Fig. 2). A total of 24 patients showed normal height at the time of LDLT. Follow-up of these cases showed retardation of growth (below the fifth percentile) in 9 recipients, with 3 displaying retarded growth by the end of the first year after transplantation, 2 by the end of the second year, 1 at 3 years, 2 at 10 years, and 1 at 15 years.
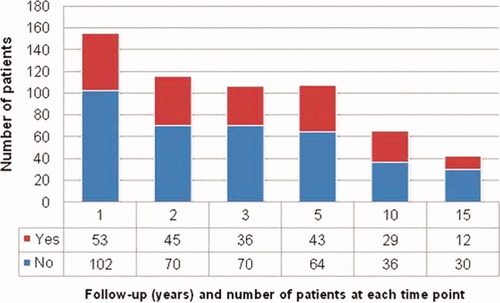
Ability of children to catch up in height after LDLT. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Univariate Analysis for Factors Affecting Growth After LDLT
We checked factors affecting changes in z scores at 1 and 15 years after LDLT. Correlation testing showed that age (P = 0.011) and growth retardation at the time of transplantation (P = 0.0001) were correlated with changes in z scores at 1 year. Gender, graft type, GRWR, clinical status, total bilirubin, albumin, prothrombin time, creatinine, ABO incompatibility, donor age, tacrolimus trough levels, cumulative doses per kilogram of body weight, and cumulative steroid doses per kilogram of body weight at 1 year did not show any significant correlations.
For changes in z scores after 15 years, changes in height could be checked only for a small number of patients, with complete data on height available at both 10 and 15 years for only 16 patients. Three factors showed correlations: growth retardation at the time of transplantation (P = 0.0001), graft dysfunction (Fig. 3; P = 0.046), and ABO incompatibility (P = 0.012). Age, gender, biliary atresia, graft type, GRWR, clinical status, total bilirubin, albumin, prothrombin time, creatinine, age of donor, graft dysfunction, retransplantation, and cumulative doses of tacrolimus did not show any significant correlation.
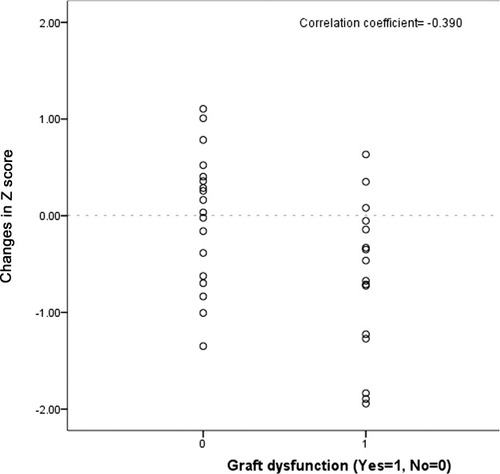
Correlation between changes in the z score (10-15 years after LDLT) and graft dysfunction.
Multivariate Analysis for Factors Affecting Growth After LDLT
Potential predictors were examined again with multivariate analysis. However, other factors (diagnosis of biliary atresia, diagnosis of metabolic liver disease, diagnosis of a tumor, and retransplantation) were forced into the model. Young age (<2 years; β coefficient = 0.980, 95% confidence interval = 0.297-1.663, P = 0.005) and growth retardation at the time of transplantation (>−2; β coefficient = 0.569, 95% confidence interval = 0.414-0.724, P = 0.0001) were predictors of higher height gain in the first year after LDLT.
DISCUSSION
Using data from 237 children who survived 5 years or more with a mean follow-up time greater than 11 years, the present study represents one of the largest investigations to date of growth after liver transplantation. The results show that children have the potential ability to catch up in growth and demonstrate 3 phases of growth after LDLT: an initial growth spurt, a protracted improvement phase, and a late decline in growth.
Previous growth studies have varied in quality, but many have been compromised by a small number of cases,3 insufficient follow-up,14 and heterogeneity of the immunosuppression regimens used.15
This report first examined growth retardation before LDLT and then investigated the factors affecting growth after transplantation. The mean height z score at the time of transplantation was −1.70, and 33.3% of the children had a height z score < −2. These results are comparable to those of other large series in which the baseline height z scores were −1.55 to −1.73.4, 5, 16 However, a difference was apparent with respect to the percentage of patients with biliary atresia. This pathology represented the indication for LDLT for about 80% of the patients in our series; this is higher than the rate in Western series, in which biliary atresia typically accounts for 43% to 54% of cases.4, 5, 14 Interestingly, age at the time of LDLR and biliary atresia as an indication for transplantation had no effect on growth retardation before LDLT, and this conflicts with the results described by Bartosh et al.5
In our series, children with biliary atresia had a baseline height z score of −1.5, which was better than that in other series with retarded growth scores of −1.8 to −2.2 at the time of transplantation.4, 5, 16 As expected, patients with Alagille's syndrome or Byler's disease showed higher growth retardation. Growth-retarded children had significantly higher bilirubin levels, which indicated that the severity of liver disease was an important contributing factor.
Previous studies have reported poor linear growth in the first year after liver transplantation.3, 4, 17 In our study, the most impressive growth was achieved in the first year after LDLT, with improvements of the z score from −1.63 to −0.63, and 58% gained more than 0.5 standard deviations. The initial delay in growth reported by other series has been attributed to high prednisone doses exceeding 5 mg/kg/day.18, 19 The lower steroid regimen and rapid tapering within 3 months may have spared our patients from the negative effects of steroids on growth reported by other authors.
Liver disease had no effect on long-term growth, with all patients showing comparable height after 5 years. However, a substantial difference was seen in patterns of growth. Patients with Byler's disease showed a slow rate of growth after LDLT. These patients were able to achieve height z scores comparable to those of other liver disease patients after 5 years, and this may be explained by the presence of chronic refractory diarrhea after LDLT and related malnutrition.20
In our study, multivariate analysis showed that young age and growth retardation at the time of transplant were associated with greater height gain in the first year after LDLT. Children under 2 years were more able to catch up in growth than older children and showed sustained growth over an extended period of time and a slight growth decline after 10 years; this contrasted with the findings of McDiarmid et al.4 and Renz et al.,21 who observed poor growth from an earlier time. Older children showed initially rapid growth (smaller in magnitude than that of younger children), which was followed by a steady height gain and final achievement of almost the same height scores as those of younger children after long-term follow-up.
We noticed that some patients who had normal growth before LDLT showed retardation of growth after LDLT. Retardation of growth for children who have normal height before LDLT seems to be related to graft dysfunction after LDLT rather than the underlying liver disease.
As described by other investigators,4 children with higher growth retardation at the time of transplantation showed greater ability to catch up in growth, and this was also responsible for the late decline in growth.
Slow growth, occurring late after transplantation, was related to graft dysfunction and ABO-incompatible grafts. Graft dysfunction was the only posttransplant factor that was confirmed to affect growth, whereas immunosuppression therapy and retransplantation did not affect growth. Other reports have shown graft dysfunction as one of the confounding factors that can affect growth after liver transplantation.4, 22 The liver is an important endocrine organ producing potent anabolic growth factors such as insulin-like growth factor I, which plays an essential role in growth.23 Because insulin-like growth factor I depends on liver function, this could be one of the main factors contributing to catch-up growth.24
ABO-incompatible grafts are associated with a higher incidence of biliary complications,25 and this may explain the association with growth retardation a long time after LDLT.
Several studies have shown that children < 2 years old are able to catch up in growth,4, 5, 21 and Viner et al.14 expected that those children would achieve normal height by the time of puberty. However, previous reports had shorter follow-up periods of 4 to 8 years after transplantation. The observation of young children for a longer time shows that most experience a slower growth rate by the time of puberty in comparison with their normal peers, with subsequent growth retardation and ultimately retarded height scores. Codoner-Franch et al.18 observed retardation of growth in children under 2 years of age, but this was encountered only 3 years after transplantation, which was too early in comparison with what we observed.
This study was the first to follow the growth of children up to 15 years after liver transplantation and to define pubertal growth for children who underwent LDLT at a young age. However, the key limitation of this study was that the number of patients was too small to adequately compare subgroups, particularly after 10 years.
In conclusion, children have the potential ability to catch up in growth to normal levels after LDLT. Children show impressive height gain in the first year, which is followed by protracted improvement over 10 years and then a late slowing of growth. Growth retardation at the time of transplantation is a determinant for height gain, whereas the age at the time of transplantation and liver disease have no effect.




