Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center†
See Editorial on Page 911
Abstract
The currently available indication criteria of living donor liver transplantation (LDLT) for patients with hepatocellular carcinoma (HCC) have high prognostic power but insufficient discriminatory power. On the basis of single-center results from 221 HCC patients undergoing LDLT, we modified the indication criteria for LDLT to expand recipient selection without increasing the posttransplant recurrence of HCC. Our expanded criteria, based on explant pathology, were largest tumor diameter ≤ 5 cm, HCC number ≤ 6, and no gross vascular invasion. One hundred eighty-six of the 221 HCC patients (84.2%) met our criteria, 10% and 5.5% more than those that met the Milan and University of California at San Francisco (UCSF) criteria, respectively. The overall 5-year patient survival rates were 76.0% and 44.5% within and beyond the Milan criteria, respectively; 75.9% and 36.4% within and beyond the UCSF criteria, respectively; and 76.3% and 18.9% within and beyond our expanded criteria, respectively. Although these 3 sets of criteria had similar prognostic power, our expanded criteria had the highest discriminatory power. Thus, these expanded criteria for LDLT eligibility of HCC patients broaden the indications for patient selection and can more accurately identify patients who will benefit from LDLT. Liver Transpl 14:935–945, 2008. © 2008 AASLD.
Liver transplantation is an effective treatment for unresectable hepatocellular carcinoma (HCC), but posttransplant HCC recurrence is associated with a dismal prognosis.1-6 Eligibility guidelines for transplantation, such as the Milan and University of California at San Francisco (UCSF) criteria, have been adopted to reduce the posttransplant recurrence of HCC and the wasting of donor organs.1, 3 The Milan criteria were originally established on the basis of pretransplant imaging findings but were reevaluated on the basis of explant liver pathology, whereas the UCSF criteria were based on explant pathology but validated by pretransplant imaging findings.1, 3 Each of these 2 sets of criteria is derived from the experience with deceased donor liver transplantation (DDLT) at a single center. Application of these criteria to living donor liver transplantation (LDLT) has resulted in patient survival outcome very similar to that following DDLT, as shown in large-volume multicenter cohorts from Japan and Korea.5, 6 Moreover, the prognostic powers of the Milan and UCSF criteria are the same in both DDLT and LDLT.
Discrepancies between the pretransplant radiological and explant pathologic stagings can occur, and candidates for DDLT should be selected after consideration of the extent of HCC at the time of listing and any further progression of HCC during the waiting period. LDLT, because of its shorter waiting time, is less affected by tumor progression, permitting more flexible selection of transplant candidates than DDLT.7
A substantial proportion of adult LDLT patients not fulfilling the Milan or UCSF criteria has been found to survive longer than expected after liver transplantation.5, 6, 8, 9 Therefore, it seems reasonable to attempt further reduction of unnecessary dropouts arising from the strict application of narrow selection criteria. Organ wastage has been an important issue in DDLT, but in LDLT, each liver graft is a private gift.10 However, because of the dedication of each living donor to the liver recipient, it is essential to improve the recipient survival rate by the exclusion of high-risk patients from LDLT. Therefore, we retrospectively analyzed our single-center experience to investigate the possibility of modifying the indication criteria of LDLT for HCC patients so that they would benefit the maximum number of recipients in an LDLT program while excluding high-risk patients.
Abbreviations
CI, confidence interval; CT, computed tomography; DDLT, deceased donor liver transplantation; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; MELD, Model for End-Stage Liver Disease; SD, standard deviation; UCSF, University of California at San Francisco; UNOS, United Network for Organ Sharing.
PATIENTS AND METHODS
Patients
From February 1997 to December 2004, 787 adult LDLTs were performed at the Asan Medical Center (Seoul, Korea).11 During pretransplant evaluation, 229 patients (29.1%) were diagnosed with HCC or were highly suspected of having HCC. Six of these patients had no evidence of viable HCC at explant pathology, whereas HCC was detected incidentally in the explant livers of 29 patients (11.6%). Two hundred fifty-two patients (32%) had pathologically confirmed HCC, but we confined our study group to 221 patients (28.1%), excluding those with incidental HCCs (n = 29) and mixed tumors containing HCC and cholangiocarcinoma components (n = 2). We retrospectively reviewed the medical records of these 221 HCC-associated LDLT recipients (31.8%) and performed follow-up until December 2006. Patient clinicopathologic profiles are summarized in Table 1.
| Parameter | Value |
|---|---|
| Age (years), mean ± SD | 51 ± 7 |
| Sex, n (%) | |
| Male | 175 (79.2%) |
| Female | 46 (20.8%) |
| Serology, n (%) | |
| Hepatitis B | 206 (93.2%) |
| Hepatitis C | 15 (6.8%) |
| Child-Pugh classification, n (%) | |
| Class A | 26 (11.8%) |
| Class B | 72 (32.6%) |
| Class C | 123 (55.6%) |
| UNOS status, n (%) | |
| Status 1 | 6 (2.7%) |
| Status 2A | 9 (4.1%) |
| Status 2B | 181 (80.9%) |
| Status 3 | 25 (11.3%) |
| MELD score, n (%) | |
| Median (range) | 16 (3–40) |
| <10 | 32 (14.5%) |
| 10–19 | 113 (51.1%) |
| 20–29 | 61 (27.6%) |
| ≥30 | 15 (6.8%) |
| Preoperative α-fetoprotein (ng/mL), median (range) | 22 (1–116,000) |
| Pathologic tumor stage, n (%) | |
| pT1 | 46 (20.8%) |
| pT2 | 94 (42.5%) |
| pT3 | 29 (13.1%) |
| pT4 | 52 (23.5%) |
| Living donor liver graft, n (%) | |
| Right liver graft | 125 (56.6%) |
| Left liver graft | 51 (23.1%) |
| Dual grafts | 44 (19.9%) |
| Graft-recipient weight ratio, n (%) | |
| Median (range) | 0.97 (0.56–1.53) |
| <0.8 | 42 (19%) |
| 0.8–1.0 | 95 (43%) |
| >1.0 | 84 (38%) |
| Perioperative mortality (<3 months), n (%) | 16 (6.4%) |
| Number of discharged patients, n (%) | 206 (93.2%) |
| Follow-up period (months), median (range) | 37 (0–119) |
- Abbreviations: MELD, Model for End-Stage Liver Disease; SD, standard deviation; UNOS, United Network for Organ Sharing.
Fifty-eight patients (26.2%) did not receive any antitumor treatment before transplantation, either because of poor liver function or because LDLT was imminently organized. The remaining 163 patients (73.8%) underwent transarterial chemoembolization (n = 101, 45.7%), radiofrequency ablation (n = 34, 15.4%), surgical resection (n = 13, 5.9%), or percutaneous ethanol injection (n = 9, 4.1%) for primary tumor control, but not for downstaging, before transplantation.12, 13 Because the outcome of salvage LDLT was very similar to that of primary LDLT in this series, we did not exclude the patients who underwent salvage LDLT following HCC recurrence.14, 15 For the patients undergoing salvage LDLT, only the extent of recurrent tumors was used for assessment of LDLT eligibility, without consideration of the extent of primary HCC at the time of prior resection.
Pretransplant HCC workup routinely included multidetector dynamic liver computed tomography (CT) with 3-dimensional reconstruction, positron emission tomography, radioisotope bone scan, and chest CT. Multidetector CT scanning was repeated 1 week before LDLT to confirm the suitability for LDLT with respect to HCC extent and hepatic vasculature.
All LDLT recipients were followed up periodically with α-fetoprotein measurements, chest X-rays, and dynamic liver CT scans. Patients who did not meet the Milan criteria underwent CT follow-up more frequently than those who met the criteria. When HCC recurrence was suspected, other diagnostic modalities were additionally performed. Recurrence was diagnosed when imaging studies revealed evidence of new tumors. Perioperative mortality was defined as patient death from any cause within 3 months of liver transplantation.
Study Design
Before LDLT, the patients were classified according to the Milan and UCSF criteria on the basis of the preoperative imaging study findings. The extent of HCC at the explant liver was classified according to the modified Tumor-Node-Metastasis classification proposed by the American Liver Tumor Study Group.16 After LDLT, the patients were again classified according to the Milan and UCSF criteria on the basis of explant pathology. After establishment of our new expanded criteria reported here (we will refer to them as the Asan criteria), the patients were also classified pathologically and radiologically. HCC lesions with borderline tumor sizes (for example, 3, 4.5, 5, and 6.5 cm) were prudently assessed after repeated size measurements. With the preoperative imaging study findings, the patients were classified according to the Milan, UCSF, and Asan criteria.
The patient population was divided into 2–3 subgroups as follows: for 2-arm comparison, within and beyond the Milan criteria, the accepted gold standard; for 3-arm comparison, within the Milan criteria, beyond the Milan criteria and within the UCSF criteria, and beyond the UCSF criteria; and for another 3-arm comparison, within the Milan criteria, beyond the Milan criteria and within the Asan criteria, and beyond the Asan criteria. To compare the efficacy of the various criteria for patient selection, we arbitrarily defined the prognostic power as recurrence or survival predictability for patients within the criteria, emphasizing eligibility for patient inclusion. Discriminatory power was defined as the difference in recurrence or survival predictability between patients within and beyond the criteria, emphasizing eligibility for patient exclusion.
In our institution, active multimodal treatments have been performed for recurrent HCC lesions after liver transplantation, but the responses to specific treatments have usually been unreliable, and rapid progression has been frequently observed.17, 18 To avoid bias due to the unpredictable responses to these treatments, we described the results for tumor recurrence and patient survival simultaneously.
All numeric data are reported as the mean and standard deviation or as the median and range. Survival curves were estimated with the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazard regression was used for multivariate analysis. Paired t tests and chi-square tests were used to compare the parameters on size and incidence, respectively. P values less than 0.05 were considered statistically significant.
RESULTS
Patient Survival
During the first 3 months after LDLT, 15 of the 221 HCC patients died from LDLT-related complications, and this resulted in a perioperative mortality rate of 6.8%. HCC recurred in 45 of the 206 surviving recipients (20.4%) during a median follow-up period of 43 months. Forty-four of these patients received various antitumor treatments, including systemic chemotherapy, transarterial chemoembolization, radiotherapy, and surgical resection. However, 37 died from HCC dissemination, and this resulted in a median survival period after detection of HCC recurrence of only 6 months. Another 12 recipients died from various causes other than HCC recurrence. The overall 1-year, 3-year, and 5-year patient survival rates were 84.6%, 74.2%, and 68.0%, respectively, and the 1-year, 3-year, and 5-year HCC recurrence–free survival rates were 80.1%, 68.6%, and 65.6%, respectively (Fig. 1).
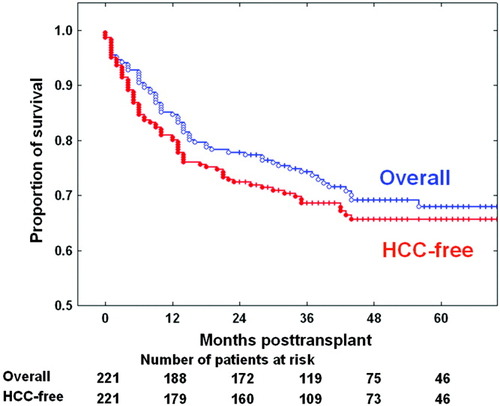
Overall patient survival curves and hepatocellular carcinoma (HCC) recurrence–free survival curves after living donor liver transplantation. Because of HCC recurrence in a small proportion of patients and a short survival period after HCC recurrence, these 2 curves did not appear significantly different (P = 0.338). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
HCC Recurrence and Risk Factor Analysis
All 45 HCC recurrences occurred within the first 44 months, and the 1-year, 2-year, 3-year, and 4-year cumulative recurrence rates were 11.2%, 18.3%, 22.0%, and 23.8%, respectively. Risk factor analyses using the clinicopathologic features of the 206 surviving recipients were performed to predict the overall 3-year patient survival rate and 3-year HCC recurrence rate. Results of the univariate analyses are summarized in Table 2.
| Parameter | Number | 3-Year Patient Survival Rate | 3-Year Recurrence Rate | ||
|---|---|---|---|---|---|
| % | P Value | % | P Value | ||
| Pretransplant α-fetoprotein | 0.003 | <0.001 | |||
| ≤20 ng/mL | 96 | 85.9 | 12.4 | ||
| >20 ng/mL, ≤200 ng/mL | 63 | 82.0 | 19.8 | ||
| >200 ng/mL, ≤1000 ng/mL | 23 | 73.9 | 22.3 | ||
| >1000 ng/mL, ≤2000 ng/mL | 6 | 83.3 | 25.0 | ||
| >2000 ng/mL, ≤3000 ng/mL | 8 | 62.5 | 28.6 | ||
| >3000 ng/mL | 12 | 25.0 | 100 | ||
| Largest tumor diameter | <0.001 | <0.001 | |||
| ≤2 cm | 73 | 89.0 | 9.9 | ||
| >2 cm, ≤5 cm | 119 | 78.5 | 22.4 | ||
| >5 cm, ≤6.5 cm | 8 | 18.8 | 75.0 | ||
| >6.5 | 6 | 50.0 | 83.3 | ||
| Tumor number | <0.001 | <0.001 | |||
| 1 | 110 | 82.8 | 18.4 | ||
| 2 | 43 | 81.2 | 21.2 | ||
| 3 | 25 | 86.9 | 16.0 | ||
| 4–6 | 10 | 88.9 | 11.1 | ||
| >6 | 18 | 26.7 | 73.7 | ||
| Total tumor diameter | <0.001 | <0.001 | |||
| ≤8 cm | 186 | 84.0 | 17.8 | ||
| >8 cm | 20 | 40.0 | 60.6 | ||
| Microvascular invasion | <0.001 | <0.001 | |||
| Absent | 190 | 82.7 | 18.5 | ||
| Present | 16 | 43.8 | 64.3 | ||
| Gross vascular invasion | <0.001 | <0.001 | |||
| Absent | 196 | 82.7 | 18.9 | ||
| Present | 10 | 20.0 | 85.0 | ||
| Tumor distribution | 0.002 | 0.002 | |||
| Unilobar | 161 | 83.9 | 18.0 | ||
| Bilobar | 45 | 64.4 | 36.3 | ||
| Edmondson-Steiner grade* | 0.087 | 0.051 | |||
| I | 14 | 92.6 | 7.1 | ||
| II | 59 | 80.8 | 18.0 | ||
| III | 65 | 74.7 | 31.7 | ||
| IV | 21 | 66.7 | 37.8 | ||
- * Tumor differentiation was not assessed in 47 explant livers.
Explant Pathology–Based Indication Criteria for HCC in LDLT
With the outcomes of the univariate analyses, multivariate analyses were performed with various cutoff values, especially for tumor size and number. We found that the independent pathologic risk factors for HCC recurrence were largest tumor diameter > 5 cm, HCC number > 6, and gross vascular invasion (Table 3). On the basis of these results, we set the eligibility criteria of LDLT for HCC patients as largest tumor diameter ≤ 5 cm, HCC number ≤ 6, and no gross vascular invasion.
| Risk Factor | Hepatocellular Carcinoma Recurrence | Patient Survival | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Largest tumor diameter > 5 cm | 6.08 | 2.72–13.59 | <0.001 | 4.63 | 2.11–10.19 | <0.001 |
| Tumor number > 6 | 6.65 | 3.02–14.63 | <0.001 | 6.22 | 2.96–13.08 | <0.001 |
| Gross vascular invasion present | 2.53 | 1.39–6.28 | 0.042 | 2.63 | 1.07–6.48 | 0.035 |
- Abbreviation: CI, confidence interval.
Of the 206 patients surviving after LDLT, 174 (84.5%) met these 3 conditions, 24 (11.7%) met 2, 6 (2.9%) met 1, and 2 (1%) met none. When 1 of these 3 conditions was not met, there was a significant increase in the HCC recurrence rate, and there was a definite decrease in the survival rate (Fig. 2).
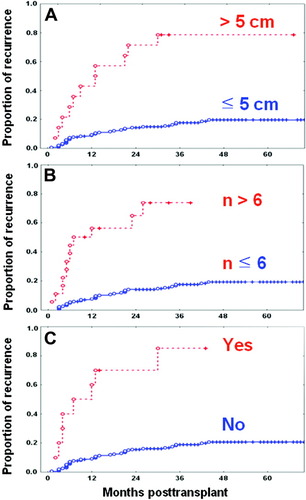
Explant pathology–based hepatocellular carcinoma recurrence curves of 206 surviving patients after living donor liver transplantation. (A) Patients with largest tumor size ≤ 5 cm and > 5 cm showed 3-year recurrence rates of 17.7% and 80.9%, respectively (P < 0.001). (B) Patients with tumor number ≤ 6 and > 6 showed 3-year recurrence rates of 17.5% and 76.4%, respectively (P < 0.001). (C) Patients with the absence and presence of macrovascular invasion showed 3-year recurrence rates of 18.9% and 86.8%, respectively (P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
There were significant differences in the rates of HCC recurrence and patient survival according to whether all of these 3 conditions were met at the same time or any of them was not met (Fig. 3). Because the prognostic and discriminatory powers of the combination of these 3 conditions appear to be sufficiently high, we consider them potential expanded criteria at our institution, which we have arbitrarily named the Asan criteria.
Hepatocellular carcinoma recurrence and patient survival curves for 206 surviving patients after application of the Asan criteria based on explant pathology. (A) Hepatocellular carcinoma recurrence curves showed 1-year, 3-year, and 5-year recurrence rates of 5.8%, 13%, and 15%, respectively, within the Asan criteria and 43.3%, 73.6%, and 73.6%, respectively, beyond the Asan criteria (P < 0.001). (B) Patient survival curves showed 1-year, 3-year, and 5-year survival rates of 94.3%, 87.5%, and 81.6%, respectively, within the Asan criteria and 71.9%, 37.2%, and 20.7%, respectively, beyond the Asan criteria (P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Comparison of Pretransplant Imaging and Explant Pathology
Because the newly defined Asan criteria were initially derived from the explant pathology, before the determination of their clinical applicability, it was essential to compare the major tumor features as determined by the pretransplant imaging and explant pathology. In the 133 patients with HCC lesions larger than 2 cm preoperatively, the mean diameter of the largest tumor was 3.5 ±1.5 cm by multidetector CT and/or magnetic resonance imaging and 3.7 ± 1.9 cm in the explant livers (P = 0.341). Six patients whose largest HCC was ≤5 cm preoperatively had tumors > 5 cm in the explant livers, whereas 4 patients whose largest HCC was >5 cm preoperatively had largest tumors ≤ 5 cm in the explant livers. Four of 20 patients with an HCC number > 6 in the explant livers were preoperatively diagnosed as having an HCC number ≤ 6. Two of these 4 patients had minute multifocal HCC lesions, some of which were not detected even by pretransplant hepatic arteriography and magnetic resonance imaging. There was no discrepancy between the pretransplant and explant determinations of gross vascular invasion because the former was determined by pretransplant imaging studies.
Consequently, of the 221 preoperatively diagnosed HCC patients, 189 (85.5%) met the Asan criteria by pretransplant imaging, whereas 186 (84.2%) met these criteria on the basis of explant pathologic analysis (P = 0.364, Table 4). Pretransplant assessment resulted in parameter underestimation in 9 patients (5 in tumor size, 3 in tumor number, and 1 in both) and parameter overestimation in 4 patients (4 in tumor size), with an incorrect estimation rate of 5.9%. Thus, the Asan criteria, using pretransplant imaging, correctly classified 94.1% of HCC patients.
| Pretransplant Imaging | Explant Pathology | |||
|---|---|---|---|---|
| Within [n (%)] | Beyond [n (%)] | Within [n (%)] | Beyond [n (%)] | |
| Milan criteria | 168 (76.0%) | 53 (24.0%) | 164 (74.2%) | 57 (25.8%) |
| UCSF criteria | 178 (80.5%) | 43 (19.5%) | 174 (78.7%) | 47 (21.3%) |
| Asan criteria | 189 (85.5%) | 32 (14.5%) | 186 (84.2%) | 35 (15.8%) |
- Abbreviation: UCSF, University of California at San Francisco.
Comparison of the HCC Recurrence Rates Based on the Milan, UCSF, and Asan Criteria
One hundred sixty-four of 221 patients (74.2%) met the Milan criteria on the basis of explant pathology. The UCSF criteria accepted 4.5% more HCC patients than the Milan criteria. The Asan criteria accepted 10% more HCC patients than the Milan criteria and 5.5% more patients than the UCSF criteria. Compared with the UCSF criteria, the Asan criteria would exclude patients with tumors larger than 5 cm but would include patients with 4 to 6 tumors. The proportions of the patient population meeting each set of criteria on the basis of explant pathology and pretransplant imaging are shown in Table 4.
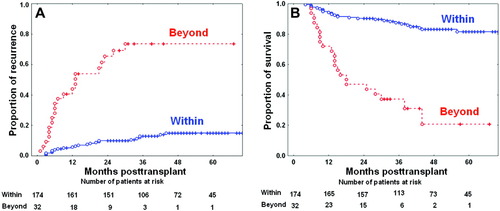
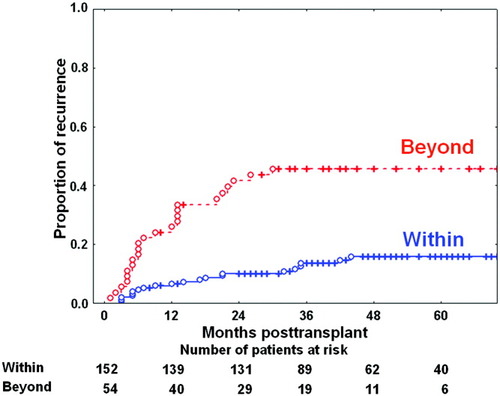
To assess the prognostic and discriminatory powers of these selection criteria, the HCC recurrence curves based on explant pathology were compared after exclusion of perioperative mortalities. The 3-year HCC recurrence rates in patients within and beyond the Milan criteria were 13.6% and 45.7%, respectively (Fig. 4, P < 0.001). The 3-year HCC recurrence rates in the patients within and beyond the UCSF criteria were 14.0% and 51.6%, respectively (Fig. 5, P < 0.001). Comparing the Milan and UCSF criteria, we found that the 3-year HCC recurrence rate was 13.6% in the patients within the Milan criteria, 20.0% in the patients beyond the Milan criteria but within the UCSF criteria, and 51.6% in the patients beyond the UCSF criteria (Fig. 6). Comparing the Milan and Asan criteria, we found that the 3-year HCC recurrence rate was 13.6% in the patients within the Milan criteria, 9.1% in the patients beyond the Milan criteria but within the Asan criteria, and 73.6% in the patients beyond the Asan criteria (Fig. 6). Thus, these 3 criteria have very similar prognostic power for HCC recurrence, but the Asan criteria have the highest discriminatory power.
Hepatocellular carcinoma recurrence curves for 206 surviving patients after application of the Milan criteria based on explant pathology. Hepatocellular carcinoma recurrence curves showed 1-year, 3-year, and 5-year recurrence rates of 6.6%, 13.6%, and 15.9%, respectively, within the Milan criteria and 26.0%, 45.7%, and 45.7%, respectively, beyond the Milan criteria (P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
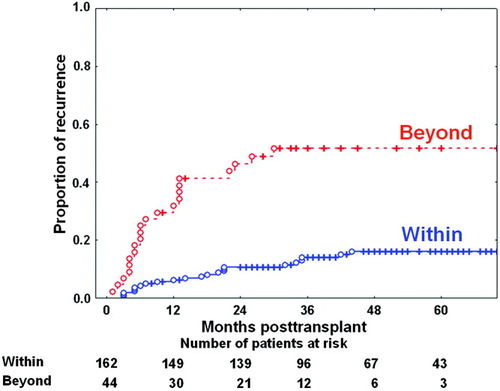
Hepatocellular carcinoma recurrence curves for 206 surviving patients after application of the University of California at San Francisco (UCSF) criteria based on explant pathology. Hepatocellular carcinoma recurrence curves showed 1-year, 3-year, and 5-year recurrence rates of 6.2%, 14.0%, and 16.1%, respectively, within the UCSF criteria and 31.9%, 51.6%, and 51.6%, respectively, beyond the UCSF criteria (P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
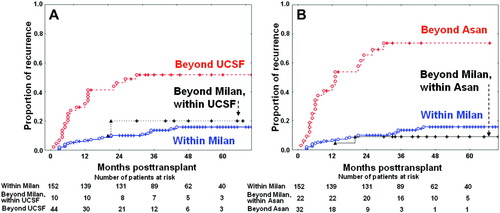
Comparison of the hepatocellular carcinoma (HCC) recurrence curves of 206 surviving patients based on explant pathology. (A) HCC recurrence curves of the patients beyond the Milan criteria but within the University of California at San Francisco (UCSF) criteria showed 1-year, 3-year, and 5-year recurrence rates of 0%, 20.0%, and 20.0%, respectively. There was no statistically significant difference in recurrence rates between the patient group beyond the Milan criteria but within the UCSF criteria and the patient group within the Milan criteria (P = 0.626). (B) HCC recurrence curves of the patients beyond the Milan criteria but within the Asan criteria showed 1-year, 3-year, and 5-year recurrence rates of 0%, 9.1%, and 9.1%, respectively. There was no statistically significant recurrence difference between the patient group beyond the Milan criteria but within the Asan criteria and that within the Milan criteria (P = 0.554). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Comparison of the Patient Survival Rates Based on the Milan, UCSF, and Asan Criteria
Comparing the Milan versus UCSF criteria and the Milan versus Asan criteria, we found that there were no significant differences in the patient survival rates (Fig. 7). Overall survival curves for the 221 patients are shown in Fig. 8. The overall 5-year survival rate including perioperative mortalities was 76.0% and 44.5% within and beyond the Milan criteria, respectively; 75.9% and 36.4% within and beyond the UCSF criteria, respectively; and 76.3% and 18.9% within and beyond the Asan criteria, respectively.
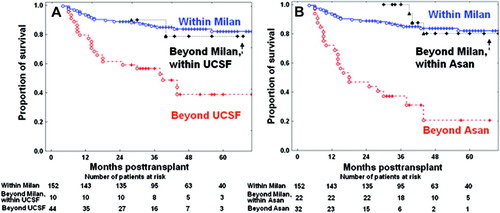
Comparison of the patient survival curves of 206 surviving patients based on explant pathology. (A) Patient survival curves of the patients beyond the Milan criteria but within the University of California at San Francisco (UCSF) criteria showed 1-year, 3-year, and 5-year survival rates of 100%, 90.0%, and 78.8%, respectively. There was no statistically significant difference in survival rates between the patient group beyond the Milan criteria but within the UCSF criteria and the patient group within the Milan criteria (P = 0.923). (B) Patient survival curves of patients beyond the Milan criteria but within the Asan criteria showed 1-year, 3-year, and 5-year recurrence rates of 100%, 88.9%, and 80.0%, respectively. There was no statistically significant survival difference between the patient group beyond the Milan criteria but within the Asan criteria and that within the Milan criteria (P = 0.334). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]

Overall survival curves of the 221 patients according to (A) the Milan criteria, (B) the University of California at San Francisco (UCSF) criteria, and (C) the Asan criteria based on the explant pathology. The overall 1-year, 3-year, and 5-year survival rates were 86.6%, 79.4%, and 76.0%, respectively, within the Milan criteria; 78.9%, 59.6%, and 44.5%, respectively, beyond the Milan criteria (P < 0.001); 87.4%, 80.0%, and 75.9%, respectively, within the UCSF criteria; 74.5%, 53.1%, and 36.4%, respectively, beyond the UCSF criteria (P < 0.001); 88.1%, 81.9%, and 76.3%, respectively, within the Asan criteria; and 65.7%, 34.1%, and 18.9%, respectively, beyond the Asan criteria (P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The gold standard for selection of HCC patients for DDLT and LDLT is the Milan criteria to date, and the UCSF criteria are regarded as acceptable guidelines. The prognostic power of these criteria appears sufficient, but their discriminatory power for HCC patients who do not meet these criteria is not sufficiently high, in that a moderate number of patients who do not meet these criteria survive longer than expected.1, 3, 5, 6
A living donor is not a public resource but is occasionally directed to a certain recipient with advanced HCC. Because of the unique features of LDLT, the indeterminate survival outcome, such as 5-year patient survival rate of 50%, can be rationalized without critical impairment of donor ethics.19 In contrast, early HCC recurrence can evoke serious psychosocial issues with respect to living donors.19, 20 Because not a negligible proportion of patients beyond the Milan or UCSF criteria survived for a long time, we could not decisively reject the patients with advanced HCC who earnestly wanted to receive LDLT in expectation of faint but prolonged survival.6, 19 Thus, there is a realistic need to modify the existing indication criteria in order to effectively exclude some high-risk HCC patients.
Although the Milan criteria have been accepted as the standard guidelines since 1996, they are the narrowest criteria.1, 3, 21 The UCSF criteria, which expand the maximal tumor size to 6.5 cm for a single HCC lesion, produce survival rates comparable to those of the Milan criteria. However, the study populations in the original studies evaluating these 2 criteria do not seem to be large enough, introducing the possibility of bias from small numbers of patients with recurrent tumors.1, 3
A recent report from an international registry of 1206 HCC patients revealed that those with 2 to 4 tumors each ≤ 5 cm in diameter or single lesions ≤ 6 cm in diameter had recurrence-free survival rates equivalent to those of patients with single tumors 3.1–5.0 cm in diameter or 2 to 3 lesions each ≤ 3 cm in diameter.22 In the current LDLT series, we observed a higher incidence of HCC recurrence when the tumor diameter exceeded 5 cm (Table 2). The 3-year HCC recurrence rate was 31.4% in the 7 patients with a single tumor between 4.1 and 5.0 cm in diameter but rose to 75% in the 6 patients with a single lesion between 5.1 and 6.5 cm in diameter (P = 0.312). Although these recurrence rates were not statistically different, it is necessary to reevaluate the significance of increased tumor size, even in patients with a single tumor.22-24
In contrast, we observed no clear difference in the recurrence rates according to the tumor number if the largest HCC diameter did not exceed 5 cm (Table 2). There was no significant difference in the recurrence rates between the 25 patients with 3 tumors and the 10 patients with 4 to 6 tumors (P = 0.189). Increasing the number of tumors up to 6 did not adversely affect HCC recurrence. Although the determination of a definite cutoff value for the tumor number requires scrupulous analyses with a much larger number of patients, the presence of numerous (>10) small HCC lesions was associated with a very high risk of recurrence. Interestingly, we have rarely encountered multiple large HCC lesions without gross vascular invasion in the shrunken cirrhotic liver. In contrast, total tumor size > 8 cm or the combination of tumor size and number was statistically significant in univariate analysis of recurrence but was not determined to be an independent risk factor in multivariate analysis of HCC recurrence and patient survival.
Macroscopic vascular invasion, which is usually defined as gross involvement of the lobar or segmental branches of the portal or hepatic veins, is a universal risk factor for poor prognosis in HCC patients who undergo resection or liver transplantation.23-25 Because gross vascular invasion into the portal or hepatic vein has a great impact on both HCC recurrence and patient survival, HCC patients highly suspected of having gross vascular invasion should not be selected for LDLT.25-28
Combining the 3 selection conditions in our expanded criteria resulted in a slight expansion of the eligible patient population in comparison with the Milan and UCSF criteria. However, the patient survival rates within our criteria remained unchanged, whereas those beyond our criteria decreased much. We found that the differences in 3-year HCC recurrence and overall 5-year survival rates between the patients within and beyond the criteria were 32.1% and 31.5%, respectively, with the Milan criteria; 37.6% and 39.5%, respectively, with the UCSF criteria; and 60.6% and 57.4%, respectively, with the Asan criteria. This indicates that our Asan criteria have the highest discriminatory power. The patient population sandwiched between the Milan and Asan criteria showed outcomes very similar to those for patients within the Milan criteria. In HCC patients beyond the Asan criteria, the 3-year recurrence rate was 73.6%, and the 5-year patient survival rate was only 18.9%. We think that such a dismal outcome can be a good reason to exclude such patients from receiving LDLT.
In this study, 93.2% of our patients had HCC associated with hepatitis B virus (HBV) infection. In Korea, HBV infection is prevalent in the general population and is the most common cause of end-stage liver disease. The clinical sequence of HCC in patients with HBV infection has been reported to be favorable compared to that in patients with hepatitis C virus infection.29 Furthermore, in our institution, the primary prophylactic regimen for HBV is lifelong administration of high-dose hepatitis B immunoglobulin (anti-surface antibody titer > 500 IU/L), which may suppress the occurrence of acute cellular rejection.30 These clinical features may have some beneficial effect on the sequence of posttransplant HCC recurrence. These homogeneous clinical features of our study population may be associated with more consistent outcomes regarding HCC recurrence and patient survival in comparison with the populations used to formulate the Milan and UCSF criteria.
In a concurrent study from our LDLT series, graft regeneration following partial liver graft implantation did not increase the risk of HCC recurrence when the tumor extent did not exceed the Milan or UCSF criteria.31 When we performed the same prognostic analysis using the Asan criteria, we obtained very similar outcomes. If HCC lesions do not exceed any eligibility criteria for liver transplantation, partial graft size and subsequent liver regeneration may not have a significant adverse effect on HCC recurrence, and this would make the indication criteria interchangeable for DDLT and LDLT.6 When we retrospectively applied our criteria to the 32 HCC patients who underwent DDLT at our institution during the study period, we found that the 3-year overall patient survival rate was 74.1% within the criteria (n = 25) and 34.3% beyond the criteria (n = 7, P = 0.045).
Similar to the UCSF criteria, the Asan criteria are primarily based on explant pathology. Because of the short waiting period between the pretransplant imaging study and LDLT operation, there was no need to account for tumor progression. However, pretransplant tumor staging and explant pathology do not always correlate.32 In this study, the largest tumor diameter determined by high-resolution imaging modalities was slightly smaller than that determined by explant liver pathology, whereas there was a more reliable correlation in tumor number. Because gross vascular invasion is usually diagnosed by imaging studies, pathologic evaluation has been usually used to confirm the presence of malignant tumor thrombi. Application of our criteria using pretransplant evaluation showed very high correlations in HCC recurrence and patient survival compared to explant pathologic assessments, and this indicates that pretransplant radiological assessment can be reliably used to select HCC patients, despite some potential risk of incorrect estimation.32-34
In addition to morphologic and pathologic assessment of HCC, the prognostic impact of the pretransplant serum α-fetoprotein level should be considered. A high serum α-fetoprotein concentration (>1000 ng/mL) is often a significant risk factor in univariate analysis but not in multivariate analysis. In this study, however, all patients with very high serum α-fetoprotein levels (>3000 ng/mL; range, 3320–116,000) experienced HCC recurrence within the first 3 years (Table 2). These findings indicate that pretransplant serum α-fetoprotein > 3000 ng/mL can be considered an important serological condition for excluding high-risk HCC patients. Applying this cutoff value for patient selection in addition to the Asan criteria, we estimated the 3-year recurrence rate to be 12.4% within the criteria and 80.3% beyond the criteria (P < 0.001).
In conclusion, use of our expanded criteria, consisting of pretransplant evaluation of tumor size, tumor number, and gross vascular invasion, reliably predicted tumor recurrence and patient survival in HCC patients undergoing LDLT and yielded higher discriminatory power than the Milan and UCSF criteria. Because our criteria were derived from a large series of patients undergoing LDLT and showed very close correlation between pretransplant radiological assessment and explant pathology, we propose their use as indication criteria for HCC patients who may undergo LDLT.




