A randomized, prospective, pharmacoeconomic trial of neoral 2-hour postdose concentration monitoring versus tacrolimus trough concentration monitoring in de novo liver transplant recipients
Abstract
Two-hour postdose cyclosporine (C2) monitoring is becoming an accepted method of therapeutic drug monitoring, although it is not known whether C2 monitoring is superior to tacrolimus (FK)-based immunosuppression. The purpose of this trial was to compare the safety, efficacy, and pharmacoeconomics of cyclosporine A (CsA) monitored by C2 levels versus FK monitored by trough levels in de novo liver transplant recipients. After informed consent, 60 de novo liver transplant recipients were randomized in a 1:1 fashion to receive either FK (trough, 6-10 ng/mL) or CsA (C2, 600-1200 ng/mL) and corticosteroids. The 2 groups were similar for gender, race, indication for liver disease, and age. At 1 year, patient survival was similar (93% for FK versus 90% for C2). One patient in the FK arm was retransplanted because of recurrent hepatitis C virus (HCV). Early acute rejection occurred in 27% of FK-treated patients and 23% of CsA-treated recipients [P = not significant (NS)]. Recurrent HCV occurred in 21% of FK-treated patients and 61% of CsA-treated patient (P = 0.04). The incidence of other infections, new onset diabetes mellitus, requirement for antihypertensives, and requirement for cholesterol medications were similar between the groups. Annual calcineurin inhibitor costs were lower in the C2 arm ($5432 ± 2091 for C2 versus $8291 ± 3948 for FK, P = 0.001). Annual pretransplant drug costs ($2292 ± 2331 for C2 versus $2831 ± 2358 for FK, P = NS) and 1-year posttransplant drug costs ($17,214 ± 16,600 for C2 versus $15,151 ± 11,699 for FK, P = NS) were similar. In conclusion, immunosuppression with CsA, monitored by C2 levels, is safe, effective, and economical in liver transplant recipients and provides immunosuppression at least equivalent to that of FK. Liver Transpl 14:173–180, 2008. © 2008 AASLD.
Therapeutic monitoring of cyclosporine A (CsA) is complicated by the narrow margin between adequate immunosuppression and toxicity. Sufficient CsA exposure during the first 4 hours after intake (AUC0-4) has been proven to correlate with freedom from rejection and toxicity.1 A 2-hour postdose cyclosporine (C2) level has been proposed as a more convenient yet reliable tool to monitor drug therapy. Recently, a multidisciplinary, international committee reviewed the pharmacokinetic and clinical data currently available regarding patient management by C2 monitoring.2 It was determined that C2 is the best single-timepoint predictor of the first 4-hour postdose CsA exposure (AUC0-4). CsA monitoring based on C2 has resulted in greater or similar clinical benefit in heart, kidney, and liver transplant recipients when compared to trough (C0) level monitoring.3-6
There is little information available that compares the pharmacoeconomics of C2-based immunosuppression and tacrolimus (FK)-based immunosuppression. One 12-month trial, which compared liver transplant recipients that received either CsA with C2 monitoring or FK, reported similar results in both treatment groups for patient and graft survival and the overall incidence of acute rejection.7 The safety profiles of patients on FK and C2 were similar, except that more diabetes occurred in patients treated with FK. Pharmacoeconomics were not evaluated.
C2 monitoring is becoming an accepted method of therapeutic drug monitoring, although it is not known whether C2 monitoring is superior to FK-based immunosuppression. Therefore, the purpose of this trial was to compare the safety, efficacy, and pharmacoeconomics of CsA monitored by C2 levels and FK monitored by trough levels in de novo liver transplant recipients.
Abbreviations
C2, 2-hour postdose cyclosporine; CMV, cytomegalovirus; CsA, cyclosporine A; D, donor; EtOH, alcoholic liver disease; FK, tacrolimus; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; NODM, new onset diabetes mellitus; NS, not significant; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; R, recipient
PATIENTS AND METHODS
This study was a single-center, prospective, randomized trial of adult liver transplant recipients at Washington University/Barnes-Jewish Hospital. The Human Studies Committee of the Washington University School of Medicine approved the protocol, and informed consent was obtained from all participants.
Patient Inclusion and Exclusion Criteria
All adult liver transplant recipients were considered for enrollment. Patients were excluded if they had a known allergy to CsA or documentation of malignancy within 2 years, with the exception of skin malignancies or hepatocellular carcinoma. Women of child-bearing potential who were not practicing a reliable form of birth control and patients with active infection were also excluded.
Immunosuppression
Methylprednisolone was administered intraoperatively (500 mg) and was followed by prednisone orally, which was tapered over 6 months to 5 mg/day and discontinued if possible by 12 months after transplantation. Patients in both groups received a calcineurin inhibitor orally twice daily as soon as a brisk diuresis ensued but not later than postoperative day 3. For C2-monitored patients (n = 30), Neoral (modified CsA capsules, USP, Novartis Pharmaceuticals, East Hanover, NJ), which was initiated at 4 mg/kg, was tapered with adjustments to maintain the C2 level by monoclonal fluorescent polarization assay between 800 and 1200 ng/mL during months 0-3 and between 600 and 1000 ng/mL thereafter (TDx, Abbott Laboratories, Abbott Park, IL). For FK patients (n = 30), FK was tapered with adjustments to maintain the 12-hour trough level by microparticle enzyme immunoassay (IMx, Abbott Laboratories, Abbott Park, IL) between 8 and 12 ng/mL for months 0-3 and between 5 and 10 ng/mL thereafter. Primary treatment of acute rejection episodes was corticosteroids. In the case of rejection, patients were switched to the alternate calcineurin inhibitor at the discretion of the prescribing physician. Mycophenolate mofetil was added to the immunosuppressive regimen, per the discretion of the transplant team, in cases of acute rejection or renal insufficiency.
Antimicrobial Prophylaxis
Patients received 500,000 units of nystatin 4 times daily for 3 months as fungal prophylaxis and single-strength sulfamethoxazole/trimethoprim daily for Pneumocystis jirovecii and bacterial prophylaxis. For patients allergic to sulfa, aerosolized pentamidine daily was substituted. For viral prophylaxis, when the donor was seropositive and the recipient was seronegative, oral ganciclovir or valganciclovir was given for 6 months. Other patients received 200 mg of acyclovir orally twice daily for 3 months post-transplant for herpes simplex prophylaxis. Hepatitis B immunoglobulin was given during the transplant procedure, daily for 6 days, and then at least monthly to maintain anti-HBs titers greater than 500 IU/L in hepatitis B patients.
Concomitant Medications
Antihypertensive treatment was prescribed at the discretion of the treating physician. The goal blood pressure was at least less than 140/90 mm Hg. Dyslipidemias were treated in accordance with the National Cholesterol Education Program III guidelines.8 Antihyperglycemics and insulin were prescribed at the discretion of the treating physician.
Observations and Follow-Up Care
Patients were observed at least every other week after discharge from the hospital, then at months 2, 3, 6, and 12, and as deemed clinically necessary. Laboratories that included a complete blood cell count, liver function tests, and a comprehensive metabolic panel were obtained daily while the patient was hospitalized, twice a week during the first month, weekly during the second month, every other week during month 3, and then monthly. Hemoglobin A1C and lipid panel were obtained quarterly in patients with diabetes or hyperlipidemia, respectively. Whole blood C2 or FK levels, which were monitored by the liver transplant nurse, transplant surgeon (primary investigator), and transplant pharmacist, were obtained daily while the patient was in the hospital and then with each blood draw as an outpatient. Protocol liver biopsies were performed at 12 months after transplant, and diagnostic biopsies were performed when deemed clinically necessary.
Definitions and Adverse Events
Rejection episodes were determined by the presence of clinical signs including but not limited to fever and rise in liver function tests. When medically possible, this was confirmed by histological evidence of rejection. Recurrence of hepatitis C virus (HCV) was defined by histological evidence of HCV after transplantation in conjunction with elevated liver function tests. Sustained oral hypoglycemic agents or an insulin requirement (>1 month), in a patient not already known to be diabetic, defined new onset diabetes mellitus (NODM). Hypertension and hyperlipidemia were defined as a need for medicinal treatment of the respective condition.
Outcomes and Endpoints
The primary endpoint of the study was the rate of acute rejection at 12 months. Incidence of infection, adverse events, and drug costs were secondary endpoints.
Pharmacoeconomics
Immunosuppressive medications were recorded weekly for 1 month and then monthly. Other medications were recorded at baseline and at 6 and 12 months after transplantation. Comparisons were made on the basis of changes in medication costs from baseline to 12 months; this controlled for interpatient variation in baseline drug utilization and costs, which may vary considerably. Subanalyses were performed for drug classes and individual drugs identified as important cost drivers. Medication costs were based on the average wholesale price in 2003.9
Statistics
The trial was designed as intent-to-treat analysis. Univariate analysis was performed by the Student t test for continuous variables and Fisher's exact test for categorical variables. All statistical tests were 2-tailed.
RESULTS
Between September 2002 and January 2004, 60 patients were enrolled in the prospective, randomized trial. Thirty patients were enrolled in each arm. The 2 groups were similar for gender (FK = 47%; CsA = 20% female), race (FK = 90%; CsA = 87% Caucasian), and age (FK = 54 ± 6; CsA = 52 ± 9 years; Table 1). Indications for transplant included viral hepatitis (FK = 50%; CsA = 70%), cholestatic liver disease (FK = 7%; CsA = 0%), and alcoholic liver disease (FK = 17%; CsA = 10%). Model for End-Stage Liver Disease scores were similar (25 ± 5 for FK versus 25 ± 7 for C2). Patients in the C2 arm were heavier at the time of transplantation (77 ± 17 kg for FK versus 89 ± 17 kg for C2, P < 0.01).
| FK (n = 30) | C2 (n = 30) | |
|---|---|---|
| Mean age (years) | 54 ± 6 | 52 ± 9 |
| Male | 16 (53%) | 24 (80%) |
| Female | 14 (47%) | 6 (20%) |
| Caucasian | 27 (90%) | 26 (87%) |
| African American | 3 (10%) | 1 (3%) |
| Weight (kg) | 77 ± 17 | 89 ± 17* |
| MELD | 25 ± 5 | 25 ± 7 |
| Disease leading to transplant | ||
| HCV | 6 (20%) | 6 (20%) |
| HCV/EtOH | 3 (10%) | 7 (23%) |
| HCV/HCC | 5 (17%) | 5 (17%) |
| EtOH | 5 (17%) | 3 (10%) |
| Autoimmune | 3 (10%) | 1 (3%) |
| Cryptogenic | 3 (10%) | 1 (3%) |
| PBC/PSC | 2 (7%) | 0 (0%) |
| HBV/HCC | 0 (0%) | 2 (7%) |
| HCC alone | 0 (0%) | 1 (3%) |
| HBV | 1 (3%) | 1 (3%) |
| Other | 2 (7%) | 3 (10%) |
| CMV sero-pairing | ||
| D+/R+ | 9 (30%) | 13 (44%) |
| D+/R− | 9 (30%) | 7 (23%) |
| D−/R+ | 7 (23%) | 6 (20%) |
| D−/R− | 5 (17%) | 4 (13%) |
- Abbreviations: C2, 2-hour postdose cyclosporine; CMV, cytomegalovirus; D, donor; EtOH, alcoholic liver disease; FK, tacrolimus; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; R, recipient.
- * P < 0.01.
Graft Outcomes
Twelve-month patient survival was similar in the 2 groups: 93% for FK and 90% for CsA (Table 2). In the FK group, 2 patients expired because of hepatic carcinoma and recurrent HCV. In the C2 arm, 3 patients expired because of multisystem organ failure, hepatic pseudoaneurysm, and recurrent HCV. One patient in the FK arm was retransplanted because of recurrent HCV. The incidence of acute rejection was similar between the FK and C2 groups [27% versus 23%, respectively, P = not significant (NS)]. The time to occurrence of acute rejection was shorter in the FK group than in the C2 group (11 ± 10 days for FK versus 136 ± 118 days for C2, P = 0.006). Recurrent HCV occurred in 21% of FK-treated patients and 61% of CsA-treated patients (P = 0.04). The grade and stage of the liver biopsies were similar between the groups. The time to recurrence of HCV was shorter in the FK group than in the C2 group (72 ± 42 days for FK versus 145 ± 43 days for C2, P = 0.006).
| FK (n = 30) | C2 (n = 30) | |
|---|---|---|
| Patient survival | 28 (93%) | 27 (90%) |
| Retransplant | 1 (3%) | 0 (0%) |
| Freedom from rejection | 22 (73%) | 20 (77%) |
| Early (<30 days) | 7 | 3 |
| Late | 1 | 7 |
| Biopsy-proven | 4 | 7 |
| Required hospital admission | 4 | 4 |
| Time to acute rejection (days) | 11 ± 10 | 136 ± 118* |
| HCV recurrence | 3/14 (21%) | 11/18 (61%)† |
| Time to recurrence (days) | 72 ± 42 | 145 ± 43‡ |
| Serum creatinine (mg/dL) | 1.2 ± 0.3 | 1.2 ± 0.3 |
| CMV infection | 0 (0%) | 2 (7%) |
| Length of stay, transplant admission (days) | 9 ± 6 | 13 ± 14 |
| Median (days) | 7 | 7 |
- Abbreviations: C2, 2-hour postdose cyclosporine; CMV, cytomegalovirus; FK, tacrolimus; HCV, hepatitis C virus.
- * P = 0.006.
- † P = 0.04.
- ‡ P = 0.02.
Safety
The average hospital stay for the initial transplant admission was shorter in the FK group than in the C2 group (9 ± 6 days versus 13 ± 14 days, respectively), whereas the median length of stay was 7 days in both groups. There were 2 cases of cytomegalovirus infection in the C2 group but no serious fungal infections or viral disease during the study period. The incidence of bacterial infections was similar between the groups. The mean serum creatinine at 12 months after transplantation was 1.2 ± 0.3 mg/dL in the FK group and 1.2 ± 0.3 mg/dL in the C2 group (P = NS), and calculated creatinine clearance was similar between the groups (Fig. 1). One patient in the FK group had recurrence of hepatic carcinoma, and 1 patient in the C2 group developed metastatic liver carcinoma. There were no occurrences of posttransplant lymphoproliferative disease.
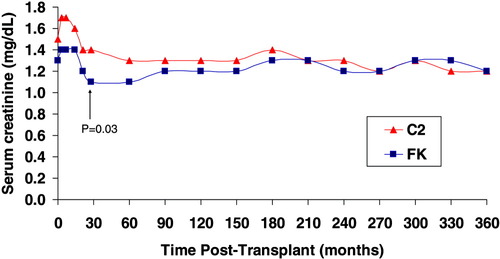
Serum creatinine. The mean serum creatinine was lower in the FK group at 30 days after transplant (1.1 ± 0.4 mg/dL for FK versus 1.4 ± 0.5 mg/dL for C2, P = 0.03) but similar at 12 months after transplantation (1.2 ± 0.3 mg/dL in the FK group and 1.2 ± 0.3 mg/dL in the C2 group, P = NS). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Adverse Events and Immunosuppression
The incidence of metabolic disorders was similar in the 2 arms at 12 months after transplant: NODM, 19% for FK versus 17% for C2; hypertension, 29% for FK versus 46% for C2; and hyperlipidemia, 3% for FK versus 3% for C2 (Table 3). Six C2 patients switched to FK for chronic rejection, acute rejection (n = 3), seizure, and nausea. Two C2 patients were switched to sirolimus for renal insufficiency, and 2 FK patients were switched to C2 for FK-induced hepatotoxicity and NODM. One FK patient was switched to sirolimus for renal insufficiency. Lastly, 2 patients in the FK arm experienced psychosis but were not converted to CsA.
| FK (n = 30) | C2 (n = 30) | |
|---|---|---|
| Postoperative infection | 0 (0%) | 2 (7%) |
| Postoperative technical | 2 (7%) | 2 (7%) |
| Readmission within 30 days of transplant | 9 (30%) | 7 (23%) |
| Adverse drug reaction | ||
| Seizure | 0 (0%) | 2 (7%) |
| Hepatotoxicity | 1 (3%) | 0(0%) |
| Psychosis | 2 (7%) | 0(0%) |
| Calcineurin inhibitor switch | 3 (10%) | 8 (27%) |
| Reason for switch | ||
| Nausea | 0 | 1 |
| Seizure | 0 | 1 |
| Rejection | 0 | 4 |
| Diabetes mellitus | 1 | 0 |
| Hepatotoxicity | 1 | 0 |
| Renal insufficiency: sirolimus | 1 | 2 |
| Mycophenolate mofetil: 6 months | 4 (13%) | 4 (13%) |
| Mycophenolate mofetil: 12 months | 7 (23%) | 4 (13%) |
| Metabolic complications | ||
| Pretransplant diabetes mellitus | 3/30 (10%) | 7/30 (23%) |
| NODM: 6 months | 5/27 (19%) | 5/23 (22%) |
| NODM: 12 months | 5/27 (19%) | 4/23 (17%) |
| Pretransplant hypertension | 2/30 (7%) | 2/30 (7%) |
| Hypertension: 6 months | 6/25 (24%) | 13/24 (54%)* |
| Hypertension: 12 months | 8/28 (29%) | 13/28 (46%) |
| Cholesterol medication: pretransplant | 0 (0%) | 1/30 (3%) |
| Cholesterol medication: 6 months | 0 (0%) | 0 (0%) |
| Cholesterol medication: 12 months | 1/30 (3%) | 1/29 (3%) |
- Abbreviations: C2, 2-hour postdose cyclosporine; FK, tacrolimus; NODM, new onset diabetes mellitus.
- * P = 0.04.
The mean C2 level was 649 ± 198 ng/mL at 6 months and 529 ± 193 ng/mL at 12 months after transplant (Fig. 2). The mean FK level was 6.9 ± 2.2 ng/mL at 6 months and 7.2 ± 2.3 ng/mL at 12 months after transplant (Fig. 3). The mean C2 dose was 228 ± 66 mg at 6 months and 200 ± 44 mg at 12 months after transplant. The mean FK dose was 6 ± 2 mg at 6 months and 6 ± 3 mg at 12 months after transplant. The average prednisone dose was similar after transplant, except at 7 months postoperatively, when the FK group had a lower mean prednisone dose (3.5 ± 1.5 mg/day for FK versus 5.0 ± 3.3 mg/day for C2, P = 0.03; Fig. 4). At 12 months, 37% of patients in the FK arm versus 61% of patients in the C2 arm remained on corticosteroids (P = NS). More patients in the FK arm were on mycophenolate (23% for FK versus 13% for C2) at 12 months postoperatively, although this was not statistically different. The use of medications that interact with calcineurin inhibitors was similar between the groups.
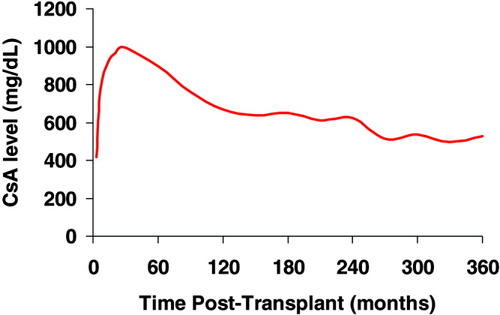
CsA concentration. The mean C2 level was 649 ± 198 ng/mL at 6 months and 529 ± 193 ng/mL at 12 months after transplant. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
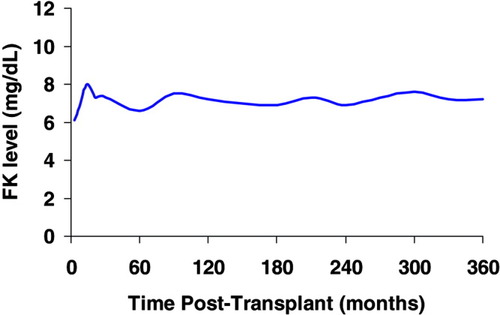
FK concentration. The mean FK level was 6.9 ± 2.2 ng/mL at 6 months and 7.2 ± 2.3 ng/mL at 12 months after transplant. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
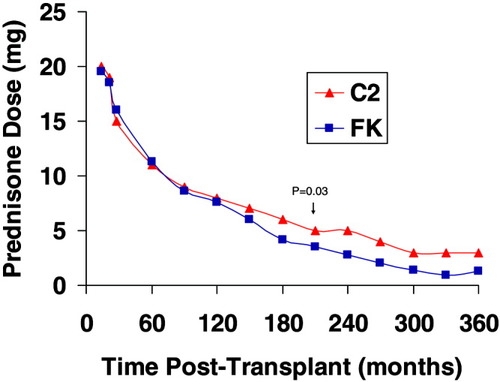
Prednisone dose. The mean prednisone dose was similar after transplant except at 7 months postoperatively when the FK group had a lower mean prednisone dose (3.5 ± 1.5 mg/day for FK versus 5.0 ± 3.3 mg/day for C2, P = 0.03).
Pharmacoeconomics
Annual calcineurin inhibitor costs were less expensive in the C2 arm ($5432 ± 2091 for C2 versus $8291 ± 3948 for FK, P = 0.001; Table 4). Annual pretransplant drug costs ($2292 ± 2331 for C2 versus $2831 ± 2358 for FK, P = NS) and 1-year posttransplant drug costs ($17,214 ± 16,600 for C2 versus $15,151 ± 11,699 for FK, P = NS) were similar. The use and cost of concomitant medications, including those to treat blood pressure, diabetes, and hyperlipidemia, were similar.
| FK | C2 | |||
|---|---|---|---|---|
| Pretransplant | Posttransplant | Pretransplant | Posttransplant | |
| Annual cost | ||||
| Total medications | $2831 ± 2,358 | $15,151 ± 11,699 | $2,292 ± 2,331 | $17,214 ± 16,600 |
| Calcineurin inhibitor | $8291 ± 3,948 | $5432 ± 2091* | ||
| Monthly cost | ||||
| Blood pressure | $36 ± 27 | $30 ± 28 | ||
| Diabetes mellitus | $34 ± 33 | $17 ± 12 | ||
- NOTE: The twelve-month medication cost was calculated from the actual immunosuppression cost and concomitant medication costs at months 6 and 12 after transplant.
- Abbreviations: C2, 2-hour postdose cyclosporine; FK, tacrolimus.
- * P = 0.001.
DISCUSSION
This single-center, prospective trial investigated the safety and efficacy of CsA monitored by C2 levels versus FK monitored by trough levels in de novo liver transplant recipients. Patient survival, graft survival, and acute rejection rates were comparable between the groups. Previous multicenter, prospective trials comparing FK and CsA monitored by trough concentrations in liver transplantation showed that an FK-based regimen reduced the incidence of acute rejection by approximately 10%-20% in comparison with a CsA-based regimen.10, 11 In the US FK506 Liver Study of FK-based immunosuppression (n = 263) versus CsA-based immunosuppression (n = 266), the number of patients with acute rejection episodes at 1 year was 154 (68%) in FK-treated patients versus 173 (76%) in CsA-treated patients (P < 0.01).10 Similar reductions were reported in the European FK506 Liver Study of 264 FK-treated patients and 265 CsA-treated patients, where acute rejection rates were 41% in FK-treated patients and 50% in CsA-treated patients (P = 0.04).11 Our present day results comparing C2 monitoring and FK trough monitoring contradict the pivotal trials, in that rejection rates were lower (23%-27%) and there was not a difference in the incidence of acute rejection between the groups. Although the rates of rejection were similar between the groups, there was more early rejection in the FK group and more late rejection in the C2 group. This may have occurred because many FK-treated patients did not achieve target FK levels early after transplantation.
HCV reinfection occurs in all patients post-transplant and can lead to recurrent hepatitis in greater than 50% of patients.12 Yet, the effects of varying immunosuppressive regimens on viral replication and disease recurrence remain unclear. Calcineurin inhibitors have not been reported to be associated directly with either increased viremia or severity of histological recurrence of HCV.13-16 In contrast, Gane and colleagues17 associated the use of steroids with transient increases in HCV RNA titers and an increased frequency for the subsequent development of acute viral hepatitis. Sheiner and colleagues18 also showed a correlation between exposure to steroids and histological recurrence of HCV. In our trial, recurrence of HCV was statistically higher in C2-treated patients, perhaps because of slightly higher doses of prednisone used in these patients. It is also possible that this study was not powered to detect a difference in HCV recurrence and that larger trials are necessary.
Although the incidence of hypertension, hyperlipidemia, and posttransplant diabetes mellitus was similar in both the FK and C2 arms of our trial, others have reported a blood pressure benefit with C2 monitoring.19 Perhaps the fact that patients in the C2 arm were heavier in our trial contributed to a higher rate of concomitant disease processes. The fact that the C2 group also tended to receive higher doses of prednisone after transplantation may have predisposed them to increases in blood pressure. Renal function tended to be similar between the groups throughout the study, although many FK patients did not achieve target levels in the first 90 days of the trial. In this trial, FK patients with actual mean blood levels between 6 and 8 ng/mL had similar renal function when compared to C2-treated patients with actual mean C2 levels between 550 and 1200 ng/mL. It is unclear whether or not higher or lower drug levels would have affected renal function.
Our trial is, to the best of our knowledge, the first to report pharmacoeconomic outcomes associated with C2 monitoring in a prospective fashion. C2-treated patients had lower calcineurin inhibitor costs than FK-treated liver transplant recipients, yet their overall drug cost was not statistically different from that of FK-treated patients. It is unclear whether or not the differences in weights among the groups may have affected drug cost. Furthermore, the results of this trial should be based on the actual drug levels achieved, not the target levels.
Although this trial was not designed to examine each individual drug class specifically, there were more patients in the C2 arm that received costly hepatitis B immunoglobulin for hepatitis B virus (HBV) prophylaxis, and this resulted in overall increased drug costs. When HBV patients were removed from the cost analysis, the total 1-year drug cost of C2-treated patients was lower without HBV patients versus with HBV patients ($11,920 ±6948 versus $17,214 ± 16,600), whereas the FK group remained similar ($13,512 ± 7820 versus $15,151± 11,699, respectively).
This study did not prospectively evaluate physician time, tests, investigations, assessments, and monitoring related to recurrent HCV. Therefore, the true economic impact of HCV recurrence cannot be fully defined between the C2 and FK arms of this study. Estimated costs associated with HCV treatment include roughly $1250 for a diagnostic biopsy,20 $1800 for monitoring of 48 weeks of treatment with interferon and ribavirin,20 and $1200 for medication costs associated with 48 weeks of pegylated interferon alfa and ribavirin.9 In this trial, more patients in the C2 arm had biopsies, but not all patients required treatment. Two patients in the C2 arm and 1 patient in the FK were prescribed antiviral medications, which were included in the cost analysis. The total number of biopsies performed, which was not included in this analysis, may lead to higher costs in the C2 arm. Overall, the most significant economic impact of recurrent HCV was seen as a result of retransplantation and death; in the FK arm, 1 patient required retransplantation, and 2 patients, one in each arm, died of HCV. Larger, adequately powered, economical trials are needed to specifically examine the influence of HCV and immunosuppressive regimens.
In summary, this study demonstrates that immunosuppression with CsA monitoring with C2 levels is economical, safe, and effective in liver transplant recipients. C2 monitoring provides immunosuppression comparable to that of FK and does not have an increased incidence of side effects. A larger study is needed to determine the long-term advantages and disadvantages between the 2 groups.




