Eighteen years of liver transplantation experience in patients with advanced Budd-Chiari syndrome†
See Editorial on Page 133
Telephone: 49-30-450-652363; FAX: 49-30-450-552900
Abstract
The long-term results of liver transplantation for Budd-Chiari syndrome (BCS) and timely indication for the procedure are still under debate. Innovations in interventional therapy and better understanding of underlying diseases have improved therapy strategies. The aim of this study was the analysis of patient and disease characteristics, outcome, and specific complications. Between September 1988 and December 2006 we performed 42 orthotopic liver transplantations (OLTs) in 39 patients with BCS. A total of 29 (74%) women and 10 men (26%) had a median age of 35 years; the median follow-up period was 96 months. Etiologically, 27 patients had a preoperative diagnosis of hematologic disease, including myeloproliferative disorders (MPD), followed by factor V Leiden mutation and antiphospholipid syndrome. The actuarial 5-year and 10-year survival rates were 89.4% and 83.5%, respectively, compared to 80.7% and 71.4%, respectively, for other indications (n = 1742). Retransplantation was necessary in 3 patients (7.1%) with portal vein thrombosis or recurrent BCS. Although the number of bleeding events was similar, incidence of vascular complications was significantly higher in patients with BCS. Thrombosis of the portal vein was observed in 4.8% versus 0.8% of the patients, whereas liver veins were affected in 7.1% versus 0.2%. Our data shows that severe acute or chronic forms of BCS with liver failure can be successfully treated by OLT. Despite higher rates of vascular complications, patient and graft survival are similar or even better compared to other indication groups. In conclusion, patients with reversible hepatic damage should be treated by combined strategies, including medical therapy and surgical or interventional shunting. Liver Transpl 14:144–150, 2008. © 2008 AASLD.
Budd-Chiari syndrome (BCS) is a rare disorder that is characterized by hepatic venous outflow obstruction, usually associated with a prothrombotic state. Potential predispositions to the development of BCS include congenital caval membranes in eastern countries.1 The western form is mainly caused by myeloproliferative disorders (MPD), dysfunction of the coagulation cascade, such as factor V Leiden mutation or protein C deficiency, and other hypercoagulable conditions (for example, oral contraceptive use or malignancy).2
Medical treatment alone is associated with a high mortality rate of more than 80%.3 Interventional therapy allows hepatic vein recanalization or the transjugular intrahepatic portosystemic shunt in an increasing number of patients.4 Lacking data comparing survival and complications prospectively should temper preference for intravascular stents,5 because shunt surgery is able to provide good long-term patency rates.6 For fulminant BCS with irreparable necrosis of liver parenchyma and chronic forms with established cirrhosis and progressive hepatic decompensation, orthotopic liver transplantation (OLT) is the treatment of choice.7 Several studies have reported survival rates ranging from a 45% 5-year survival8 to a 10-year survival rate of 78%.9
In the present study, we report on a consecutive series of 39 patients who underwent OLT for BCS, focusing on disease characteristics, specific risks, and long-term outcome. To clarify the impact of thrombotic or bleeding complications on patient survival, we compared our results with published data and other indication groups.
Abbreviations
BCS, Budd-Chiari syndrome; MPD, myeloproliferative disorders; OLT, orthotopic liver transplantation.
PATIENTS AND METHODS
Study Population and Follow-Up
Among 2007 OLTs performed in our institution between September 1988 and December 2006, the frequency of OLT for BCS was 2.1% (n = 42). These patients were listed for transplantation because of severe disease refractory to medical and interventional treatment, and due to continuous deterioration of their clinical condition. Data were collected retrospectively from a computerized database and analysis of their hospital charts. Demographic, clinical, and laboratory data were analyzed including the following parameters: age, gender, etiology, Child-Turcotte-Pugh classification, period from onset of symptoms to diagnosis and from diagnosis to OLT, interventions before OLT, preoperative occlusion type, histological findings, immunosuppression, rejection episodes, transfusion demand, postoperative complications, intensive care unit and hospital stay, patient and graft survival, cause of death, and retransplantation. Development of symptoms and disease was defined according to Rossle et al.10 To summarize, “acute disease” was defined as severe illness with pronounced congestion and necrosis developing within days to 2 months to a state requiring surgical therapy. Disease progress within a time frame of 2 to 6 months with little necrosis and absence of cirrhosis was defined as “subacute disease,” whereas “chronic disease” included patients with severe fibrosis or cirrhosis.
Diagnostic workup included duplex-ultrasound, computed tomography scan, magnetic resonance imaging, angiography, and liver biopsy. All patients received a hematological evaluation including a standard battery of labs supplemented with tests for protein C and S activity, factor V Leiden mutation, antiphospholipid antibodies, and so forth. Bone marrow biopsy was performed in patients with suspicion of MPD.
In the majority of cases OLT was performed using a standardized surgical technique with venovenous bypass. Aprotinin was administered intraoperatively as a bolus of 500,000 kIU and subsequently as continuous infusion at 100,000 kIU/hour to avoid reperfusion fibrinolysis and thereby reducing the need for perioperative blood transfusions. Liver grafts were reperfused after completion of portal and arterial anastomoses. Thereafter, the bile duct anastomosis was performed, preferably as side-to-side choledochocholedochostomy with insertion of a T-tube. In the event of regular liver function, the T-tube was closed after control cholangiography on postoperative day 5 and removed after 6 weeks.
Initial immunosuppressive regimes were based on calcineurin inhibitors and prednisone, supplemented with mycophenolate mofetil, azathioprine, rapamycin, antithymocyte globulin, basiliximab, or daclizumab according to the actual study protocol.
Statistical Analysis
Categorical variables were expressed as number (%) and compared using chi-square test or Fisher's exact test. Continuous data were presented as mean ± standard deviation or median (range) and analyzed using analysis of variance test or Mann-Whitney U test. Patient and graft survival after OLT were evaluated using Kaplan-Meier curves, and differences between groups were determined by the log-rank test. P values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS statistical software version 13.0.1 (SPSS Inc., Chicago, IL).
RESULTS
Patient Characteristics
The characteristics of 39 patients (29 women and 10 men) receiving 42 liver grafts for severe BCS are shown in Table 1. Patient age ranged from 14 to 66 years, with a median age of 35 years. The severity of liver insufficiency was classified as Child-Turcotte-Pugh B in 57% of the patients, followed by Child-Turcotte-Pugh C (31%) and A (12%). Etiologically, 27 (69%) patients had a preoperative diagnosis of hematologic disease, including MPD, factor V Leiden mutation, antiphospholipid syndrome, and heparin-associated thrombocytopenia. BCS-related interventions before transplantation were transjugular intrahepatic portosystemic shunt in 8 (19.1%) patients and surgical shunt in another 2 (4.8%) patients.
| Age (years)* | 35 (14-66) |
| Gender† | |
| Female | 29 (74) |
| Male | 10 (26) |
| Child-Turcotte-Pugh classification† | |
| A | 5 (11.9) |
| B | 24 (57.1) |
| C | 13 (31) |
| Etiology† | |
| Myeloproliferative disorders | 13 (33.3) |
| Factor V Leiden | 6 (15.4) |
| Antiphospholipid syndrome | 3 (7.7) |
| Heparin-induced thrombocytopenia | 2 (5.1) |
| Others | 3 (7.7) |
| Not determined | 12 (30.8) |
| Preoperative occlusion type† | |
| Hepatic veins | 39 (92.9) |
| Inferior vena cava | 7 (16.7) |
| Portal vein | 10 (23.8) |
| Hepatic artery | 2 (4.8) |
| Prevalent histopathological aspect† | |
| Cirrhosis | 11 (26.2) |
| Fibrosis | 5 (11.9) |
| Congestion | 2 (4.8) |
| Necrosis | 12 (28.6) |
| Nodular regenerative hyperplasia | 9 (21.4) |
| Hepatocellular carcinoma | 3 (7.1) |
- * Median (range).
- † Number of patients (%).
As to the course of BCS, chronic disease was apparent in 20 (51%) patients, whereas acute and subacute forms were observed in 8 (21%) and 11 (28%) patients, respectively (Table 2). The mean period from onset of symptoms to diagnosis ranged from 40.7 days in acute forms to 937.8 days in patients with chronic disease. Similar results were seen for the mean period from diagnosis to OLT. As expected, the preoperative occlusion type was characterized by occlusion of hepatic veins in 92.9% of the BCS subpopulation (Table 1). Additional or sole occlusion of inferior vena cava was noticed in 7 (16.7%) patients. The prevalent histopathological aspect for chronic disease was cirrhosis and fibrosis, whereas in acute forms necrosis was observed in most of the cases. Subacute disease mostly presented with congestion and nodular regenerative hyperplasia.
| Onset of disease* | |
| Acute | 8 (21) |
| Subacute | 11 (28) |
| Chronic | 20 (51) |
| Period from onset of symptoms to diagnosis (days)† | |
| Acute disease | 40.7 ± 23.2 |
| Subacute disease | 140.4 ± 119.2 |
| Chronic disease | 937.8 ± 1111.2 |
| Period from diagnosis to OLT (days)† | |
| Acute disease | 36.9 ± 31.5 |
| Subacute disease | 174.5 ± 107.4 |
| Chronic disease | 737.5 ± 809.8 |
- * Number of patients (%).
- † Mean ± standard deviation.
Perioperative Management and Surgical Technique
OLT was performed with replacement of the recipient's retrohepatic inferior vena cava and a venovenous bypass in 33 (78.6%) patients. In 3 patients (7.1%) a piggyback reconstruction was performed, whereas another 3 patients received living related right liver grafts. Retransplantation was necessary in 3 cases.
Postoperatively all patients received a dose of 5000 to 30,000 IU of heparin per day intravenously. Anticoagulation was initiated on day of transplantation in 93% of the patients and 2 patients with heparin-induced thrombocytopenia received lepirudin. After a median time period of 18 (7-141) days all patients were converted to phenprocoumon, aiming for a target international normalized ratio of 2.5-3.5. In case of thrombocytosis >400/nL (24% of all BCS-patients), hydroxyurea was added to the anticoagulation therapy.
Primary immunosuppression was tacrolimus-based in 33 (78.6%) patients, whereas 9 (21.4%) patients received cyclosporin as the main component. Compared to other indications, BCS patients had a mean transfusion demand of 6.1 ± 6.3 versus 6.5 ± 6.4 units of packed cells and 10.8 ± 6.3 versus 11 ± 8.2 units of fresh frozen plasma. Patients with BCS had a median intensive care unit stay of 10.5 (4-65) days and a median hospital stay of 34 (5-185) days, which was very similar to other patients requiring OLT. Differences did not reach statistical significance.
Patient and Graft Survival
Median follow-up was 96 (1-203) months. The Kaplan-Meier patient survival rates at 1, 5, and 10 years (Fig. 1) were 92.3%, 89.4%, and 83.5%, respectively, in the BCS group compared to 89.8%, 80.7%, and 71.4%, respectively, for other indications. There were no statistically significant differences (P = 0.271). Regarding graft survival (Fig. 2), the BCS population also showed slightly better results with 1-, 5-, and 10-year survival rates of 88.1% (versus 82.4%), 78.1% (versus 72.1%), and 68.0% (versus 63.2%), respectively. Again, analysis with the log-rank test showed no statistical significance.
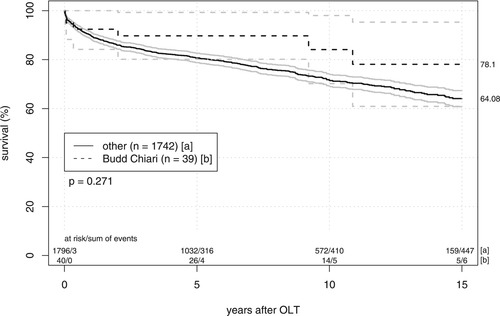
Patient survival after liver transplantation. Kaplan-Meier curves and 95% confidence intervals compare patient survival of 39 patients with BCS and 1742 patients with other underlying diseases. There were no significant differences between groups in the log-rank test.
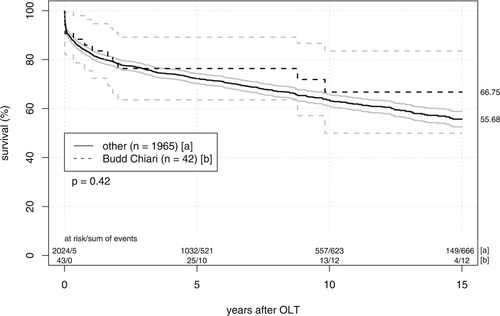
Graft survival after liver transplantation. Differences in the outcome of 42 livers transplanted for BCS and 1965 grafts allocated for other underlying diseases are displayed using Kaplan-Meier curves and 95% confidence intervals. There were no significant differences between groups in the log-rank test.
A total of 2 postoperative deaths, 10 and 23 days after OLT, were caused by early graft failure. The death of another patient was related to severe cytomegalovirus pneumonia and consecutive respiratory failure 4 months postoperatively. One patient died due to portal vein thrombosis and consecutive graft failure 2 years after transplantation. Recurrence of BCS after 9 years was responsible for the fifth death, whereas 2 patients died due to chronic graft failure 11 and 16 years after OLT.
Postoperative Complications
Patients with BCS had a higher rate of vascular complications after OLT (Table 3). Compared with other indications, the rate of portal and hepatic vein thrombosis (4.8% versus 0.8% and 7.1% versus 0.2%) was significantly higher. Postoperative stenosis of portal vein or inferior vena cava also occurred significantly more often in patients with BCS (2.4% versus 0.2% and 4.8% versus 0.5%). A total of 3 (7.1%) patients with BCS had intraperitoneal bleeding, which was almost the same percentage as seen in other patients. Bleeding events with the need for surgical reexploration were more frequent in other indication groups (4.8% versus 7.3%); however, the frequency of these events did not reach statistical significance.
| Budd-Chiari | Others | P* | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Thrombosis | |||||
| Hepatic artery | 3 | 7.1 | 76 | 3.9 | 0.28 |
| Portal vein | 2 | 4.8 | 16 | 0.8 | 0.0073 |
| Hepatic veins | 3 | 7.1 | 3 | 0.2 | <0.0001 |
| Inferior vena cava | — | — | 5 | 0.3 | 0.743 |
| Stenosis | |||||
| Hepatic artery | 1 | 2.4 | 25 | 1.3 | 0.53 |
| Portal vein | 1 | 2.4 | 3 | 0.2 | 0.0014 |
| Inferior vena cava | 2 | 4.8 | 9 | 0.5 | 0.0002 |
| Others | |||||
| Intraperitoneal bleeding | 3 | 7.1 | 149 | 7.6 | 0.915 |
| Leakage biliary anastomosis | 1 | 2.4 | 39 | 2 | 0.856 |
| T-tube–related leakage | 1 | 2.4 | 82 | 4.2 | 0.564 |
| Biliary stricture | 4 | 9.5 | 106 | 5.4 | 0.245 |
| Papillary stenosis | 3 | 7.1 | 140 | 7.1 | 0.996 |
| Ischemic-type biliary lesion | 4 | 9.5 | 88 | 4.5 | 0.122 |
- * Statistical significance was calculated with chi-squared test and Fisher's exact test.
Similar results in both groups were observed for biliary leakage and papillary stenosis, whereas the incidence of biliary stricture occurring during the follow-up was higher in the BCS group (9.5% versus 5.4%; P = 0.245). In addition, more BCS patients had ischemic-type biliary lesion (9.5% versus 4.5%), which was not significant in statistical testing.
Retransplantation had to be performed in 3 (7.7%) cases. A total of 2 patients developed recurrent BCS and received retransplantation 23 days and 21 months after primary OLT. Another patient developed graft failure caused by portal vein thrombosis and received a second liver graft 13 months after his first transplantation.
DISCUSSION
Growing understanding of the pathophysiology of BCS and innovations in interventional technique has changed therapeutic algorithms. Recently, expert panels have defined BCS as hepatic venous outflow obstruction between the hepatic veins and the right atrium.11 The authors emphasized that the cause of obstruction is not the determining factor and that veno-occlusive disease as nonthrombotic obstruction of sinusoids is excluded from this definition. Concerning therapeutic options, medical therapy alone is generally associated with a high mortality up to 90%.3, 12 In selected patients with mild symptoms and preserved liver function, satisfactory long-term results can be achieved.1, 13 Published data indicated that surgical portosystemic shunting provides good patency rates, showing a 5-year survival ranging from 69% to 94%.6, 14-17 Besides hepatic vein recanalization and stenting, transjugular intrahepatic portosystemic shunt has been established as a promising alternative to the various forms of surgical shunts.18 Experienced centers reported a 5-year survival of up to 87%.4, 10, 19 Considering the limited cadaveric organ pool, both types of shunting may bridge the time gap to OLT. In our series we present our single-center experience with OLT for severe acute or advanced chronic forms of BCS in 39 consecutive patients from 1988 to 2006.
MPD were the predominant etiologic factor in our series. In exactly one-third of our patients this diagnosis was confirmed. Several authors have reported similar results. Smalberg et al.20 reported that out of 40 patients with primary BCS, 33% had MPD. An analysis of the European Liver Transplantation Registry reports 111 (45%) BCS patients with MPD.21 Another common hematologic disease in our BCS population was factor V Leiden mutation, which was present in 15.4%. A published comparison of BCS patients and controls reported on a frequency of 20.3% versus 7.6%22 of mutant alleles. Deltenre et al.23 analyzed 20 patients with factor V Leiden mutation-related BCS and saw a significantly higher rate of combined prothrombotic states (70% versus 14%) and a higher frequency of inferior vena cava thrombosis and massive ischemic necrosis. Our rate of patients with antiphospholipid antibodies (7.7%) was comparable to other published data.20, 23, 24
Our series of 39 consecutive patients receiving OLT for BCS started in 1988. Better pathophysiologic background knowledge and constantly improving interventional techniques have changed therapeutic algorithms since this time. However, timely indication for severe acute and chronic forms of BCS has not changed, and present survival rates are comparable to former reports from our institution.25, 26 After the first report of a successful liver transplantation for BCS in 1976,27 2 series at the end of the 1980s observed limited 5-year survivals of up to 50% (Table 4).8, 28 In the 1990s, Shaked et al.29 and Ringe et al.30 published their series, clearly differentiating between shunt procedures performed in patients with portal hypertension and preserved hepatic function, and a subgroup suffering from progressive BCS and cirrhotic liver failure listed for transplantation. This algorithm resulted in an increased 3-year survival of 76% and 5-year survival of 69%. Because of advanced therapeutic strategies, results improved further during the last decade. In a series of 19 patients, Srinivasan et al.9 noticed an outstanding 5-year survival of 95%. Plessier et al.24 presented a standardized strategy with a stepwise introduction of procedures by increasing invasiveness. Finally, 11 of 51 patients with BCS required OLT and achieved a 3-year survival of 91%. Another single-center report observed a remarkably high incidence of BCS recurrence and other thrombotic events,31 leading to a 5-year survival of 65%. Compared to the data of the European Liver Transplantation Registry published in 2005,21 our own series shows an enhanced 5-year and 10-year survival of 89% versus 71% and 84% versus 68%, respectively. Although it is difficult to make speculations on the reasons for favorable results from Kings College, Clichy and our institution, we spotted 2 major goals. Besides a clear-cut therapeutic strategy and a timely indication for OLT, accurate monitoring of postoperative anticoagulation is crucial. Therefore, our liver transplantation outpatient clinic has guaranteed a standardized coordination of patient follow-up, ensuring periodical examination starting in weekly intervals.
| Author | Year | Number of Patients | 1-Year Survival (%) | 5-Year Survival (%) | 10-Year Survival (%) | Recurrent BCS (%) | Other Thrombotic Events (%) |
|---|---|---|---|---|---|---|---|
| Halff et al.8 | 1990 | 23 | 69 | 45 | NR | 9 | 13 |
| Jamieson et al.28 | 1991 | 26 | 69 | 50 | NR | 7 | 18 |
| Shaked et al.29 | 1992 | 14 | 86 | 76 (3-year) | NR | — | NR |
| Ringe et al.30 | 1995 | 43 | 69 | 69 | 69 | — | 14 |
| Srinivasan et al.9 | 2002 | 19 | 95 | 95 | 78 | 11 | 11 |
| Cruz et al.31 | 2005 | 11 | 81 | 65 | 65 | 27 | 27 |
| Plessier et al.24 | 2006 | 11 | 91 | 91 (3-year) | NR | 9 | 36 |
| Mentha et al.21 | 2006 | 248 | 76 | 71 | 68 | 2 | 14 |
| Present series | 2007 | 39 | 92 | 89 | 84 | 7 | 12 |
- Abbreviation: NR, not reported.
According to the literature and our own data, the rate of recurrent BCS usually ranges from 7% to 11% (Table 4). In contrast to these findings, Cruz et al.31 found a recurrence rate of 27%, whereas the European coumarin data retrospectively collected from 248 patients reported a surprisingly low rate of 2.4%.21 Having a look at the literature survey in Table 4, other thrombotic events after OLT have an incidence of 11 to 36%. Like in our own series, anticoagulation is based on intravenous heparin postoperatively and conversion to coumarin later on in the majority of cases. For patients with a hypercoagulable state corrected by OLT, antithrombotic therapy is probably not required. Melear et al.32 demonstrated that antiplatelet therapy using hydroxyurea and aspirin is a safe and effective alternative in MPD patients with BCS. Lately, 4 of 13 MPD patients in our series were converted from coumarin to aspirin and hydroxyurea or anagrelide. Patients are doing well, although follow-up is too short for meaningful results.
In conclusion, this single-center study in 39 BCS patients shows that liver transplantation can achieve excellent long-term patient and graft survival, although vascular and especially thrombotic complication rates are significantly higher compared with other indication groups. Individual tailoring of postoperative anticoagulation resulted in a low rate of bleeding complications and a satisfactory percentage of patients with recurrent BCS. Acute and subacute forms with reversible hepatic damage should first be treated by combination strategies including medical therapy, hepatic vein recanalization, and surgical or interventional shunting. Because disease progression after shunting can lead to liver cirrhosis in some patients, close follow-up of BCS patients is necessary. Liver transplantation remains the final therapy option for patients with severe acute or chronic forms with liver failure.
Acknowledgements
We thank Mrs. Sylvia Albrecht for excellent technical assistance in preparing the manuscript.




