The immunological monitoring of alloreactive responses in liver transplant recipients: A review
Abstract
The aim of this work is to review the current knowledge in the field of immunological monitoring of allogenic responsiveness in clinical liver transplantation. When compared to other solid-organ transplants, liver allografts are considered as immunologically privileged, and, accordingly, constitute a favorable setting to develop experimental as well as clinical strategies for minimization of immunosuppression and even induction of operational tolerance. The validation of simple, reliable, noninvasive assays exploring antidonor alloreactivity will constitute a crucial step toward implementing such approaches in the clinic. In contrast to research in rodents claiming the development of donor-specific tolerance in case of graft survivals of over 100 days without immunosuppression, it is impractical to confirm tolerance induction in this way in humans. Promising candidate assays include the detection of post-transplant immune deviation, of circulating precursors of dendritic cells subtypes, and of regulatory T cells. A conceptual framework for the development of tolerance assays in clinical liver transplantation is also proposed. Liver Transpl 12:373–383, 2006. © 2006 AASLD.
Since the first human liver transplant (LT) some 52 years ago, the vast majority of hepatic allograft recipients have remained under life-long maintenance immunosuppression (IS). Despite the dramatic improvement of early graft survival, late graft losses due to chronic rejection as well as the morbidity and mortality secondary to long-term IS remain major concerns in this field.1, 2 Induction of tolerance to the transplant would prevent these complications, and clinical LT probably constitutes an ideal setting to develop such strategies, considering its immunologically privileged status when compared to other types of allografts.3 However, despite the observation that tolerance has been relatively “easy” to induce in rodent models, the translation of these researches in humans has not yet been shown to reproducibly induce IS-free tolerant state in transplant recipients. In experimental models, tolerance can be assessed by graft survival beyond 100 days without IS, and by absence of rejection of a donor strain skin transplant; such an approach is of course impractical in humans, and the only currently available method to ascertain tolerance in a given patient is the ability to progressively withdraw all IS with preservation of graft function and histology. Unfortunately, although a small number of grafts are not rejected after removal of all medications used for the maintenance IS, trials involving the deliberate withdrawal of immunosuppressive drugs according to a protocol do not suggest that it is safe to do so for most patients: Such trials are associated with the inherent risk to induce acute or even chronic rejection with the need for higher levels of IS when compared to the stable situation before attempting at IS withdrawal.1, 4, 5
In this context, the development of tolerance assays and the implementation of clinical tolerance trials are intimately dependent upon each other: The introduction of tolerance induction protocols in solid-organ transplantation will require the identification of simple, robust, ideally noninvasive surrogate biomarkers reflecting the immune alloreactivity of the recipient toward his/her donor. After a brief introduction summarizing the terminology and overall mechanisms of allogenic tolerance, the candidate assays proposed for immunological monitoring in allograft recipients will be systematically reviewed, with particular respect to human LT. A tentative approach toward research and development in tolerance monitoring in the clinical setting will also be outlined.
ALLOGENIC TOLERANCE: TERMINOLOGY AND GENERIC MECHANISMS
A clear, widely accepted definition of the immune statuses underlying the term “tolerance” constitutes a minimal requirement for the study of immunological monitoring in solid-organ transplant recipients. The following concepts should be distinguished. (1) Transplantation tolerance refers to an actively acquired, permanent immunological unresponsiveness, as described in mice that had been injected in utero or neonatally with bone marrow–derived donor cells6; this “plain” tolerant state is characterized by an absence of donor-specific alloreactivity in vivo as well as in vitro, and it is believed to occur through central mechanisms in the thymus. (2) Operational tolerance is defined as the absence of acute and chronic rejection, and indefinite graft survival with normal function in an IS-free, fully immunocompetent host, usually as the end result of a successful attempt at IS withdrawal. In contrast with transplantation tolerance, operational tolerance does not necessarily mean complete unresponsiveness of the recipient immune system toward the donor cell (split tolerance), but rather refers to the lack of a destructive immune response toward the graft despite the presence of generalized immune competence.5 (3) Prope tolerance, as proposed by Calne, describes a state of “almost tolerance” in patients who maintain normal allograft function and histology under minimal IS, usually a monotherapy calcineurin inhibition with infratherapeutic blood levels.7-9 (4) Graft acceptance defines the common situation in which a transplant recipient has a normal immunosuppressive load, with absence of immune injury toward the graft; this condition is typically observed in the subgroup of recipients without acute rejection episode in the early and middle-term posttransplant period, under routine immunoprophylaxis, usually with combination of drugs including calcineurin inhibition.
Several review papers are available in the literature, providing in-depth discussion of the putative mechanisms of operational and transplantation tolerance.5, 9-16 Such detailed description is beyond the scope of this work. In brief, central and peripheral mechanisms have been described to be involved in the development of allogenic tolerance, as studied in various experimental models.17 (1) Central tolerance is operative through intrathymic clonal deletion of precursor T cells expressing T-cell receptor (TCR) with high affinity for self-antigens or alloantigens belonging to the major histocompatibility complex; the generation of natural T regulatory cells (Treg) has also been recently described as another possible mechanism to maintain central tolerance.12 (2) Various types of tolerogenic dendritic cells (DC) have been identified, including immature DC that, as antigen presenting cells (APC), fail to provide adequate costimulation for T cell activation and tend to promote tolerance induction.18 At the alloreactive T-cell level, the end result of an encounter with a tolerogenic DC may be deletion, anergy, or induction of Treg. (3) Peripheral clonal deletion of allogenic T cells may occur peripherally via passive death, or activation-induced cell death which constitutes an active, donor APC-induced process leading to apoptosis of alloreactive T cells.9, 14 (4) Anergy of allogenic T cells refers to a state of T-cell inactivation following antigenic stimulation, characterized by a functional inertia with inability to respond to subsequent antigenic stimuli that includes an impaired capacity to produce interleukin (IL)-2, in the absence of T-cell deletion.14, 19, 20 (5) Immune regulation through Th1/Th2 cytokine deviation is based on the subdivision of CD4+ cells into T helper (Th)1 and Th2 cells on the basis of their pattern of cytokine secretion: Th1 type cells produce essentially the proinflammatory, immunogenic cytokines IL-2, IL-3, interferon (IFN)-γ, tumor necrosis factor-α, and granulocyte-macrophage colony stimulating factor, and are involved in cellular immunity and allograft rejection; in contrast, Th2 type cells produce essentially the regulatory, tolerogenic cytokines IL-3, IL-4, IL-5, IL-6, IL-10, and transforming growth factor-β possibly involved in graft acceptance; however, this simplistic model of immune deviation leading to tolerance through preferential Th2 cytokine production has been seriously challenged in experimental models and is not yet fully validated in humans.19, 21-25 (5) Cellular regulation of T cells is emerging as a key mechanism for actively inducing and maintaining unresponsiveness to donor alloantigens.10, 12, 26, 27; several populations of Treg have been identified in tolerance models as well as in clinical settings, including CD4+CD25+Foxp3+, CD4−CD8−, and CD8+ cells.28-30
Abbreviations
LT, Liver transplantation; IS, Immunosuppression; TCR, T-cell receptor; Treg, regulatory T cell(s); DC, dendritic cell(s); APC, antigen presenting cell(s); IL, interleukin; Th, T helper cell(s); IFN, interferon; pDC, precursor(s) of dendritic cell(s); mRNA, messenger RNA; ELISPOT, enzyme-linked immunosorbent spot assay; HLA, human leukocyte antigens; DTH, delayed-type hypersensitivity; sCD30, soluble CD30.
CANDIDATE ASSAYS OF ALLOGENIC IMMUNE RESPONSE AND TOLERANCE
From a clinical perspective, the assessment of alloreactivity using immunological monitoring in transplant recipient may be directed at the following aims: (1) to allow early, noninvasive detection of acute or chronic allograft rejection, before effector mechanisms and organ destruction have been initiated; (2) to evaluate the level of IS required for a given patient (choice of immunosuppressor molecule, dosage adjustment, and target therapeutic window according to blood monitoring), these adjustments being currently determined only on an empiric basis; and (3) to determine the immunological phenotype related to operational tolerance and to allow proper identification of the allograft recipients in whom IS withdrawal could be safely conducted. This review essentially concentrates on the latter issue, with a deliberate emphasis on the tolerance assays evaluated (or to be evaluated) in the context of clinical LT (Table 1).31
| Detection of tolerogenic dendritic cell precursors |
| Ratio between pDC1 and pDC2* |
| Measuring T cell alloreactivity |
| Mixed lymphocyte reaction* |
| Cytokine production by PBMC stimulated by donor-specific alloantigen* |
| Frequencies of cytokine-producing donor-reactive cells (ELISPOT) |
| Trans vivo delayed-type hypersensitivity assay* |
| Detection of Th1/Th2 immune deviation |
| Cytokine gene polymorphism (pretransplant analysis in the recipient)* |
| Profiles of circulating cytokines* |
| Profiles of intragraft mRNA precursors of cytokines* |
| sCD30 determination |
| Profiling of alloreactive T cells (detection of regulation/deletion) |
| Detection of circulating regulatory T cells (CD4+CD25+, CD4−CD8−, CD8+)* |
| Detection of alloreactive T cell apoptosis (TUNEL assay) |
| Patterns of Vβ usage (indirect detection of clonal deletion) |
| Graft immunohistochemistry |
| Other assays |
| Alloantibody and autoantibody titers (humoral immune response) |
| Defining “tolerance genes” by gene chip microarray technology |
| Defining “tolerance proteins” by proteomics technology |
- Abbreviations: PBMC, peripheral blood mononuclear cells; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling.
- * Assays with data available in liver transplant recipients.
Detection of Microchimerism
Chimerism is defined as the existence of replicating cells from different genetic backgrounds in a single organism.11 Macrochimerism or mixed chimerism is defined by the persistence of more than 5% of circulating donor-derived cells, as observed following hematopoietic stem cell grafting, a condition classically associated with intrathymic deletion of donor-reactive T cells and central tolerance.32-35 In contrast, in microchimerism, donor-specific cells, usually DC, are present at low frequencies (≤1/104 to 105 cells) in the transplant recipient. This latter finding has been related to the migration of donor passenger leukocytes from the graft-to-recipient lymphoid organs early after transplantation, as shown in the rat LT allograft model with spontaneous tolerance induction.36 Despite much controversy, a correlation between the degree of donor microchimerism in transplant patients and alloreactivity or allograft acceptance/tolerance has not been clearly established22, 37-41; particularly in LT patients, high levels of early microchimerism did not abrogate the persistence of an alloreactive response at 1 year posttransplant, such microchimerism being now considered as a consequence of graft acceptance under maintenance IS rather than a marker of allogenic unresponsiveness.42, 43 Moreover, it was suggested recently that chimeric cells identified in transplantation studies may not necessarily be donor-derived, and could have been present in recipient tissues before transplantation.44 Consequently, microchimerism detection is no longer regarded as a promising tool for immunological monitoring.
Detection of Dendritic Cells Precursors
These critically important APC circulate as immature DC precursors (pDC), which exit the blood to reside as immature DC in tissues throughout the body, and traffic via lymph or blood to secondary lymphoid organs following antigen uptake. In humans, 2 major subpopulations of blood pDC have been described, monocytoid pDC and plasmacytoid pDC.45 Monocytoid DC (CD11c+) can be derived from circulating monocytes in response to granulocyte-macrophage colony-stimulating factor and IL-4, whereas plasmacytoid DC (CD123+) develop after stimulation with IL-3 and CD40L. Monocytoid DC, which induce Th1 cell differentiation in vitro, and plasmacytoid DC, which promote Th2 cell responses, have been designated DC1 and DC2, which may be specialized for the induction of immunity and tolerance, respectively.46 Mazariegos and colleagues, from the Pittsburgh group, hypothesized that the circulating levels of these pDC subsets, relative to one another, might reflect the status of liver graft recipients with respect to clinical tolerance and safety of IS withdrawal vs. dependence on antirejection therapy with a persisting risk of rejection.47 Accordingly, these authors found that circulating pDC2 were more prevalent, relative to pDC1, in stable LT patients off IS and in those undergoing successful IS withdrawal, compared to recipients on maintenance therapy.18 According to their results, the authors speculated that a pDC2/pDC1 subset ratio of 0.1 could serve as the threshold above which a patient might be considered for IS weaning. Interestingly, since pDC2 could be mobilized selectively in living donors in response to granulocyte-macrophage colony-stimulating factor, such immunoregulatory approach may be of potential therapeutic value for the induction of tolerance to solid-organ grafts.48
Measurement of T cell Alloreactivity
Beside classical in vitro analyses of T-cell alloreactivity using mixed lymphocyte cultures,3 several approaches have been proposed to more accurately assess the reactivity of recipient T cell toward donor antigens.
In Vitro Cytokine Production by Recipient Cells
Levels of IL-2, IL-4, IFN-γ, IL-10 and transforming growth factor-β produced in vitro by peripheral blood mononuclear cells from 15 LT patients at 5-7 months posttransplant were analyzed by the Vancouver group, after stimulation by donor-specific alloantigens (liquid nitrogen-stored donor spleen cells), by third-party antigens (from other donors spleen cells), or by nonspecific pokeweed mitogen.49 Mononuclear cell response to stimulation, cytokine levels and cytokine messenger RNA (mRNA) from the cell cultures were assayed, and cytokine production was correlated with the clinical condition of the patient, including biochemical data and graft histology. When compared to third-party stimulation, results showed a highly significant correlation between donor-specific-stimulated IL-4 and IL-10 production from circulating mononuclear cells of recipients with stable liver graft function when compared to acute rejection, this correlation being independent of the level of IS.49 Using a similar approach, Zhou and colleagues in Brussels group recently described a real-time polymerase chain reaction method based on IL-2 and IFN-γ mRNA quantification upon in vitro stimulation of recipient whole blood with allogenic T-cell-depleted peripheral blood mononuclear cells.50 As studied in a tolerant recipient of a liver and hematopoietic stem cell transplant from the same living donor, this technique may constitute a promising whole blood mixed lymphocyte reaction assay to monitor allogenic immune responsiveness, with the particular advantages to only require small volumes of patient's blood, to provide results within 48 hours, and to allow storage of mRNA samples for later analysis of other genes of interest.50 Flow cytometric measurement of the capacity of recipient T cells for intracellular cytokine production was also tested by several groups as a tool for immune monitoring; it was essentially assessed in renal transplant recipients, where the technique could not, however, differentiate long-term outcomes of the allograft.51-53
Enzyme-Linked Immunosorbent Spot (ELISPOT) Assay
The frequencies of IFN-γ- or IL-10-producing donor-reactive cells in recipient blood can be determined by means of the ELISPOT assay, and this technique has been proposed as a surrogate marker of allogenic responsiveness, essentially in renal transplantation.54-59 Pretransplant frequencies of donor-specific IFN-γ ELISPOT were recently significantly correlated with posttransplant outcomes in 2 series of kidney recipients, high frequencies of alloreactive cytokine-producing cells in the recipient being associated with an increased risk of severe acute rejection episodes as well as with lower long-term glomerular filtration rate;54, 55 accordingly, these authors suggested that such an approach may serve as a pretransplant “cellular cross-match,” in addition to the classical detection of preformed anti-donor antibodies. Similarly, in the posttransplant period, ELISPOT measurements of IFN-γ-producing recipients cells could be correlated with acute rejection and renal function at 6 and 12 months.56-58 Interestingly, using the ELISPOT technique to monitor the indirect alloreactivity, the Harvard group studied the frequencies of Th1 (IFN-γ-producing) and Th2 (IL-5- and IL-10-producing) peripheral blood lymphocytes reactive with a panel of synthetic peptides corresponding to sequences from donor (HLA)-DR molecules (instead of donor cells)59: Such approach allowed the separation of patients with stable or impaired renal function according to a cutoff value below or higher than 60 IFN-γ spots/106 cells, and constitutes a promising tool for long-term immunological monitoring. In LT recipients, the ELISPOT technique has been used to assess hepatitis C virus eradication and Epstein-Barr virus cytotoxic T cells, but not yet, to our knowledge, to explore allogenic responsiveness.60, 61 Finally, the limitations of the ELISPOT approach should be mentioned, including the relatively reduced number of human cytokines assessable so far through this technique, as well as its questionable reliability particularly for stored cells.
Trans Vivo Analysis of Delayed-Type Hypersensitivity
It may be of interest to evaluate in vivo cell-mediated allogenic immunity without exposing patients directly to the challenge antigens, in order to avoid the risk of sensitization.62 An alternative method for human delayed-type hypersensitivity (DTH) was described; it involves the transfer of peripheral blood mononuclear cells plus donor antigen in the footpads of naïve, severe combined immunodeficiency mice.63 Antigen-driven swelling is then measured after 24 hours, postinjection measurements being compared to preinjection measurements to obtain specific swelling and quantify DTH reactivity. Such trans vivo DTH assay not only allows the determination of nonresponsiveness or of the level of sensitization of the recipient toward donor-specific antigen(s), but also permits identification of the existence of regulatory mechanisms operative in the transplant recipient, particularly of tolerizing antigens64: In brief, the colocalization of donor and recall tetanus toxoid or Epstein-Barr virus antigens can induce donor-antigen-linked suppression of the response to these recall antigens, an active downregulation mechanism partially dependent upon the local activity of transforming growth factor -β and IL-10.63 In LT patients, the trans vivo DTH assay was shown to be a valuable method for identifying operationally-tolerant recipients, in vitro mixed lymphocyte cultures tests failing to do so63; furthermore, co-localized soluble HLA-A and -B (and not HLA-DR) antigens were shown to allow the detection of such DTH regulation phenomenon.65 However, the logistical limitations of this assay should be mentioned, including the need to maintain immunodeficient mice in costly facilities.
Cytokine Immune Deviation
Despite the limitations of the Th1/Th2 paradigm in human immunology and particularly of the interpretation of preferential Th2-type cytokine environment as a surrogate marker of operational tolerance, 3 different approaches have been developed to assess the cytokine genotype and phenotype in transplant recipients in the context of immunological monitoring.
Cytokine Gene Polymorphisms
The production of cytokines varies among individuals and these variations are determined by genetic polymorphisms, most commonly within the regulatory region of the relevant gene. Such polymorphisms in the cytokine genes may be associated with the magnitude of cytokine production, usually categorized as “low,” “intermediate,” or “high,” and, accordingly, with the intensity of alloimmune responses following allogenic stimulation. As performed in the recipient before transplantation, the rationale of this type of genetic analysis would consist in the ability to evaluate the production capacity of Th1/proinflammatory vs. Th2/tolerogenic cytokines in a given patient, in order to predict his/her overall alloreactivity.66 Consequently, the aims of such studies would be to determine if an individual patient's propensity to develop acute or chronic rejection is related to the presence of these genetic polymorphisms (either alone or in combination). Unfortunately, such an approach has several theoretical limitations, including the overly simplistic view of the Th1/Th2 immune deviation paradigm, as well as the lack of consideration for the allogenic disparity between the allogenic recipient and his/her organ donor. Nevertheless, data from the Pittsburgh group suggested that genetic predisposition toward low tumor necrosis factor-α and high/intermediate IL-10 production may facilitate IS withdrawal in pediatric LT.67 Considering the lack of statistical power to detect small or moderate gene effects in limited clinical series, a meta-analysis studying the impact of cytokine gene polymorphism on graft acceptance in clinical LT was recently published, combining the data available from 7 centers.68 In the overall analysis, the only genetic risk factor associated with acute liver graft rejection was IL-10 polymorphism at position 1082.A, an allele corresponding to low in vitro production of IL-10.69
Circulating Cytokines Levels
The analysis of published clinical studies correlating circulating cytokines levels to the immunological status after human LT (rejection vs. graft acceptance vs. operational tolerance) only provides confusing and contradictory results.70-76 The immunological and clinical relevance of circulating cytokine levels in LT is considerably limited by several drawbacks, including the presence of confounding factors (surgical stress, blood transfusions, ischemic-reperfusion injury, hepatic regeneration, infectious complications), and the lack of serial cytokine profiles in most published series.70 Moreover, circulating cytokines levels should only be regarded as an indirect evaluation which may not necessarily reflect the exposure to cytokine locally within the allograft or the lymphoid organs of the recipient. And indeed, these works, most of them published in the mid 1990s, did not result in standard guidelines for the immunological monitoring in clinical LT.
Detection of In Vivo mRNA Precursors of Cytokines
The analysis of cytokine mRNA precursors using the reverse transcriptase-polymerase chain reaction technology was proposed to palliate the limitations of analyzing the circulating cytokine levels as surrogate marker of tissue exposure. In the clinic, such analysis has been essentially limited to the allograft, with the requirement to obtain control biopsy samples before as well as after graft reperfusion. Unfortunately, the published studies providing data concerning cytokine precursors with respect to graft acceptance were again rather conflicting, particularly concerning IL-2, IL-4, IL-15, and IFN-γ.71-73, 77, 78 As already suggested for circulating cytokines, these contradictory results make very hazardous the use of isolated determinations of intragraft cytokines precursors to predict the level of allogenic responsiveness of a given patient in order to propose IS withdrawal.
Pretransplant and Posttransplant Determination of Soluble CD30 (sCD30)
Originally described on Reed-Sternberg cells of Hodgkin's disease, CD30 is a membrane glycoprotein that belongs to the tumor necrosis factor superfamily. It is expressed on activated T cells, preferentially those secreting Th2-type cytokines, although the CD30 molecule is not considered as a physiological marker of Th2 cells but rather as a costimulatory molecule regulating the balance between Th1/Th2 responses.79, 80 After activation of CD30+ cells, a soluble form of CD30 (sCD30) is released and can be measured in the serum. In kidney recipients, the pretransplant detection of high sCD30 level was shown to constitute a more accurate predictor of acute rejection, when compared to panel reactive antibodies.81, 82 Similarly, in the early posttransplant period, a significant decrement of sCD30 levels was observed, with lower levels measured in the patients without rejection.83, 84 To our knowledge, no published data on sCD30 is available in LT recipients; a preliminary study in a limited number of patients in Brussels did not find any statistically significant correlation between pre-LT and early post-LT sCD30 values and the occurrence of acute rejection (D.Q.T., unpublished data).
Regulatory T Cells
Downregulation of immune responses by Treg is emerging as a key mechanism for inducing and maintaining unresponsiveness to donor alloantigens, as well as in the context of self-tolerance and autoimmunity.12, 27, 85, 86 Studies of Treg in transplantation have identified multiple populations of cells with different cell-surface phenotypes and different mechanisms of action, as recently reviewed by Walsh and colleagues10 (1) Naturally occurring CD4+CD25+ Treg, which are selected centrally in the thymus, are also characterized by other cell-surface markers, such as CD45RB, CTLA-4 and glucocorticoid-induced tumor necrosis factor receptor family–related receptor, as well as by the expression of the forkhead/winged helix transcription factor Foxp3; CD4+CD25+ Treg inhibit effector T-cell proliferation apparently mainly through cell-contact-dependent mechanisms. (2) Inducible CD4+ Treg, formed in the periphery following antigenic stimulation, included Th3 cells, known to promote tolerance following the oral ingestion of antigens through secretion of transforming growth factor -β, and Tr1 cells, similar to Th3 cells but secreting large amounts of IL-10. (3) Other Treg types have also been described outside the CD4+ compartment, including CD3+CD4−CD8− (double negative) cells and CD8+CTLA4+Foxp3+ cells.27-30, 85, 87, 88 In vivo and in vitro approaches have been proposed to determine the potential contribution of Treg detection in the monitoring of allogenic responsiveness in transplant recipients.
In Vivo Detection of Treg
The Kyoto LT program has developed a clinical strategy that enables a substantial proportion (approximately 40%) of LT patients to be weaned from IS completely, either in a physician-controlled, elective IS withdrawal protocol or for the management of posttransplant complications including Epstein-Barr virus–related malignancy.4 However, a subgroup of recipients submitted to such therapy withdrawal (approximately 25%) encountered rejection during the process of weaning from tacrolimus and required the reintroduction of steroids.4 The peripheral blood mononuclear cells of these LT recipients with successful or unsuccessful IS withdrawal were systematically phenotyped with the aim to characterize the immunological differences between these 2 distinct populations of patients.89 Interestingly, an increase was observed in the frequency of circulating CD4+CD25high+ cells in operationally tolerant patients, when compared to age-matched volunteers or patients still on IS. Furthermore, an increase of the percentage of B cells and of Vδ1/Vδ2 γδ T-cell ratio as well as a decrease of the percentage of natural killer cells were also found in operationally tolerant cases. This study suggests a role for cellular regulation through a circulating CD4+CD25high+ subset acting as Treg in the maintenance of operational tolerance in LT recipients. Beside these measurements of Treg in the peripheral blood, it is of interest to mention the possibility of detecting CD8+and CD4−CD8−Treg among the graft infiltrating T cells, as observed in animal models of transplantation tolerance30, 88: Such an approach may promise to better characterize the phenotype and significance of chronic cellular infiltrates within long-standing allografts in humans.
In Vitro Detection of Treg
The frequency of cytokine-producing indirectly primed alloreactive T cells (see Regulatory T Cells) determined by ELISPOT analysis was further refined by the Harvard group using in vitro depletion of putative CD25+ Treg.27 In a series of renal transplant recipients, incubation of peripheral blood mononuclear cells with anti-CD25 monoclonal antibody resulted in the change in HLA-allopeptide-specific IFN-γ and IL-10 frequencies in 8/17 (47%) of the patients with low allopeptide responsiveness, whereas 0 of 8 with higher responses demonstrated such regulation (P < 0.05). The regulatory cells were present in the circulation as early as 3 months posttransplant and persisted for a number of years, despite conventional IS.27 To our knowledge, such approach has not been published for LT recipients, but it may be valuable for assessing the existence of cellular regulation in the context of indirect alloimmunity in the long-term.
Long-Term Alterations of T-Cell-Receptor Repertoires
T cells recognize antigens through their TCR that is a heterodimer of either αβ or γδ chains.90 During T-cell ontogeny, the β chain undergoes somatic rearrangement of 4 noncontiguous gene clusters, V, D, J, and C, resulting in a large repertoire of TCR molecules. The TCR antigen-binding site is formed by 3 complementary determining regions, regions 1 and 2 being encoded by sequences of C genes alone; region 3 consists of rearranged sequences of V, D, and J genes and represents the most variable TCR region that contacts the central residues of the bound peptide.91 Therefore, the analysis of TCR-Vβ usage, as measured by the polymorphism of complementary determining region 3, may provide valuable information on the composition of T-cell repertoire selected during an immune response.90, 92, 93 Accordingly, such analyses could also reflect the occurrence of peripheral deletion of alloreactive T cells to be correlated with a status of putative operational tolerance. Using a global method of T-cell repertoire analysis, particularly polymerase chain reaction technique to analyze mRNA precursors of TCR repertoires and analysis of complementary-determining region 3 length distribution, 2 groups almost simultaneously published comparative studies of TCR Vβ usage in long-term kidney transplant recipients with variable levels of graft acceptance from chronic rejection to operational tolerance, as well as in healthy individuals.90, 92 Soulillou's team in Nantes showed a unique blood TCR pattern characterized by a restricted complementary determining region 3 length distribution92; similarly, Opelz's group in Heidelberg evidenced strongly altered Vβ usage, including an increased frequency of oligoclonality and a decreased frequency of polyclonality.90 Whether such studies will help to identify a potential surrogate pattern of operational tolerance in liver recipients under life-long IS remains to be determined.
Alloantibodies
For many years, attention was focused on cellular mechanisms of allograft rejection, humoral mechanisms being considered mainly as inductors of hyperacute rejection in presence of antidonor HLA antibodies before grafting.94 Increasing evidence now suggests that humoral responses to alloantigens could play an important role in both acute and chronic alloimmunity, particularly following activation of the indirect pathway; consequently, the detection of alloantibodies should be mentioned in the list of candidate assays for the immunological monitoring of transplant recipients.95, 96 A positive correlation between anti-HLA antibodies and poor graft outcome was established in kidney, heart, lung and liver recipients, whether those antibodies were present before grafting or appear after transplantation.94, 97 Similarly, posttransplant appearance of non-HLA antiendothelial antibodies could be correlated with coronary artery disease and chronic rejection in heart and kidney allograft recipients, respectively.94 Moreover, particularly in LT recipients, de novo autoimmune hepatitis has been described as a pathogenic mechanism in the long-term, a condition associated with the presence of autoantibodies.98 The hypothesis was formulated that damage of the graft tissues through preservation or immunological injury may lead to the exposure or release of putative autoantigens and secondary induction of autoimmune responses.99 So far, nevertheless, the role of detection of alloantibodies and autoantibodies in allograft monitoring is not yet fully defined, particularly in LT.
Microarray Analysis of Gene Expression
The term DNA microarray refers to a high-density array of oligonucleotides or polymerase chain reaction -products immobilized onto a solid support such as glass slides; the immobilized DNA selectively retrieves genes or sequences of interest when the array is hybridized to a mixture of complementary sequences obtained from tissue or blood samples of clinical relevance.100 Since miniaturization currently allows more than 50,000 genes to be spotted on a single slide, such technology is capable of generating genome-wide profiles of mRNA expression, allowing the exploration of gene expression patterns and regulation, as well as the characterization of the genetic diversity underlying pathological conditions. Accordingly, global microarray profiling does not require prior knowledge of the gene pathways involved in a disease and brings the potential to unravel the structured and coordinated physiological processes, within hypotheses-generation studies that might provide unexpected pathogenic pathways, possibly far removed from the standard hypotheses-based gene analyses.100, 101 In the context of transplant recipients, gene chips hold great promise for discovering noninvasive biomarkers for monitoring of intragraft events, and for stratifying patients toward more individualized treatment regimes, particularly using comparative analyses of the peripheral blood, of the graft and its local environment (bile in LT).101 To date, only a few published studies in organ transplantation use microarrays, the limitations being the cost of the technique as well as some procedural restrictions, including the heterogeneity of the sampling source, the need for sample RNA amplification, the variability in expression measurements, and the complexity of data management.102-105 Despite the current lack of published studies using the microarray technique for immunological monitoring in clinical LT, this approach has the potential to create innovative knowledge by better defining the complex interactions of various genes orchestrating the immune system, which may result in a large spectrum of responses in LT recipients, varying from rejection to tolerance.
CONCEPTUAL APPROACH TOWARD MONITORING OF ALLOGENIC RESPONSES
Considering the growing number of candidate assays available to monitor allogenic responses, a strategy of investigation in this field is clearly required to avoid testing of proposed tolerance assays at random, as already pointed out by the Pittsburgh group.106 Accordingly, we wish to propose hereafter an approach that might contribute to rationalize the investigation of putative tolerance assays in clinical transplantation (Fig. 1).
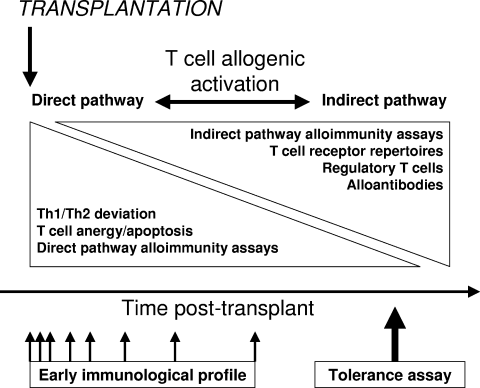
Conceptual framework for research and development of immunological monitoring and tolerance assays, according to the putative chronology of tolerance induction and maintenance mechanisms in the early and long-term posttransplant periods. Early immunological monitoring would rely on the study of early posttransplant profiles (Th1/Th2 immune deviation, direct allogenic pathway assays), whereas tolerance assays would rather rely on a “snapshot” evaluation of parameters related to T-cell regulation, to the indirect pathway of alloimmunity, alloantibodies, and T-cell receptor repertoire.
Early allogenic activation of T cells and early posttransplant acute rejection have been shown to be essentially mediated through the direct recognition pathway (presentation of allogenic stimulus by donor APC), whereas it is believed that the indirect recognition pathway (presentation of allogenic peptides in the context of self major histocompatibility complex by recipient APC) predominates with increasing duration of engraftment.11 Immunoregulation of direct-pathway T cell is thought to be realized through a combination of anergic and deletional mechanisms, in contrast to the indirect pathway, which is considered to be primarily regulated by Treg.27, 107 The combination of these observations led us to hypothesize that immunological monitoring should be approached in 2 different ways, with respect to the interval post-transplantation. (1) Early monitoring requires the analysis of posttransplant profiles of immunological parameters, mainly centered on cytokine immune deviation and concurrent cell anergy/deletion (in vivo/in vitro detection of cytokines, direct pathway ELISPOT assays); such profile studies should help to predict the immunological fate of the transplant, early rejection vs. graft acceptance vs. possibility of future operational tolerance. (2) Tolerance assay(s) should be used in the longer-term in stable transplant patients under maintenance IS as an immunological snapshot of the residual reactivity of the recipient against his/her allograft; according to this interpretation and the current knowledge, such tolerance assays should mainly focus on the detection of Treg (in vivo in the peripheral blood and within the allograft, as well as in vitro using the indirect ELISPOT assay after CD25+ depletion), of major histocompatibility complex and non–major histocompatibility complex alloantibodies (as markers of indirect alloimmunity), and on the alterations of TCR repertoires. In parallel, microarray technology would allow the development of hypothesis-generating studies to explore the mechanisms of graft acceptance, prope tolerance, and operational tolerance in long-standing allograft recipients. Such tolerance monitoring should probably be done regularly to assure the robustness of the tolerant state in patients constantly exposed to numerous environmental stimuli that could alter this putatively metastable condition.
CONCLUSION
The identification of a state of tolerance to an allograft requires that clinicians develop appropriate interactions with immunologists and other basic scientists to start the immunological evaluation of the patients of interest. This multidisciplinary approach should ideally be organized in a prospective fashion from the time of transplantation (evaluation of dynamic profiles), as well as in longer-term recipients having putatively developed operational tolerance (“snapshot” evaluation). Such clinical research will benefit from multicentric studies as well as from concerted projects as developed by the Immune Tolerance Network at the National Institutes of Health.108 Defining the clinical and laboratory phenotype of the tolerant patient should considerably facilitate the confident implementation of clinical trial of tolerance induction and IS withdrawal. A major impediment to the design and evaluation of new tools for the immunological monitoring frequently resides, however, in the complete lack or limited supply of donor cells available to be used in assays evaluating donor-specific alloreactivity in the recipients. Accordingly, prospective measures should be developed by transplant centers to appropriately retrieve, process, and store donor cells for later use in the immunological assays.109
Finally, the ethical aspects of immunological monitoring should not be underestimated. Which patients should be selected for immunological monitoring? What about the use of living donor blood in this assessment? On which basis could a patient be proposed for IS withdrawal? Accordingly, a new type of risk/benefit balance will have to be developed, taking into account the benefit of successful IS withdrawal in a subgroup of patients vs. the risk of acute/chronic rejection in case of failure in another subgroup. Such immunological monitoring protocols will require thorough discussion within the clinical teams, with the Institutional Review Board, as well as with the patients themselves.