Successful adult-to-adult living donor liver transplantation in a patient with moderate to severe portopulmonary hypertension
Abstract
Portopulmonary hypertension (PPHTN) is one of the most devastating consequences of end-stage liver cirrhosis. When a patient has moderate to severe PPHTN, his or her candidature for liver transplantation is denied. Here we report a successful adult-to-adult living donor liver transplantation (LDLT) in a patient with moderate to severe PPHTN. The patient was a 58-yr-old female who was diagnosed with end-stage liver cirrhosis due to chronic hepatitis C. Preoperative evaluation revealed that the patient had moderate to severe PPHTN. Her mean pulmonary artery pressure (mPAP) was 35-47 mmHg without treatment. Continuous epoprostenol therapy was introduced to lower the mPAP. She underwent LDLT using an extended right hepatic lobe graft which was donated by her daughter. Prolonged artificial ventilation was necessary until postoperative day (POD) 25, after which her general condition gradually improved. By POD 72, she was in good condition and was allowed to leave the hospital. Currently, 1 yr after the operation, she visits the outpatient clinic regularly and enjoys a normal life. It should be noted, however, that the PPHTN markedly improved but did not completely resolve, as assessed by right heart catheterization 1 yr after successful LDLT. Liver Transpl 12:481–484, 2006. © 2006 AASLD.
Portopulmonary hypertension (PPHTN) is defined as mean pulmonary artery pressure (mPAP) >25 mmHg in patients with a pulmonary capillary wedge pressure <15 mmHg (although this is not an absolute inclusion criterion) in association with portal hypertension, and without any intrinsic or underlying lung disease.1-5 PPHTN can be categorized into mild (mPAP of 25-35 mmHg) or moderate to severe (mPAP >35 mmHg).4, 5 Its occurrence is estimated at approximately 2 to 10% in patients with end-stage liver cirrhosis.6 The exact pathogenesis of this disorder remains to be elucidated. It is generally considered that moderate to severe PPHTN is a contraindication for liver transplantation because of increased risk of right-side cardiac failure and other cardiopulmonary complications.1-3 Krowka et al.2 reported that an mPAP of 50 mmHg or greater was associated with 100% cardiopulmonary mortality within 2.5 yr of liver transplantation and that all patients died of right heart/cardiopulmonary failure. Recently, continuous epoprostenol therapies for these patients were introduced to lower mPAP and a few successful cases have been reported.4, 7 Here we report 1 case of successful adult-to-adult living donor liver transplantation (LDLT) in a patient with moderate to severe PPHTN.
Abbreviations
PPHTN, portopulmonary hypertension; LDLT, living donor liver transplantation; POD, postoperative day; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance.
CASE PRESENTATION
A 58-yr-old female was diagnosed with end-stage liver cirrhosis due to chronic hepatitis C in 1995. She was further observed to have PPHTN in 1999 by chest X-ray. She visited our hospital on December 2000, hoping to undergo LDLT. The initial examination by right heart catheterization revealed that she had a high mPAP (Table 1). Chest X-ray examination showed remarkable dilatation of the pulmonary arteries. The electrocardiogram revealed right ventricular hypertrophy and right axis deviation. The ultrasonic echocardiograph examination showed increased right ventricle-right atrium gradient, right ventricular hypertrophy and tricuspid valve regurgitation. Other causes of pulmonary hypertension were ruled out and she was diagnosed with moderate to severe PPHTN (although pulmonary capillary wedge pressure was mildly elevated), and denied proceeding to LDLT. First, a calcium channel blocker (nifedipine) was given to decrease her mPAP, which was only partially successful (47 → 35 mmHg) but pulmonary vascular resistance (PVR) remained high (519 dynes · second/cm5). Nitric oxide inhalation was not effective. Continuous epoprostenol therapy was initiated to decrease her PVR. Epoprostenol was gradually increased to 20 ng/kg/minute, at which dose she experienced mild side effects, such as gastrointestinal symptoms, precluding further increases. Epoprostenol at this dose (20 ng/kg/minute) neither affected the platelet count adversely nor decreased the systemic blood pressure. Approximately 2 months after starting epoprostenol therapy, PVR was decreased and finally she was able to undergo LDLT in April 2004, using an extended right lobe graft donated by her daughter. Because the donor had a relatively large left hepatic lobe, the extended right lobe graft was selected. The graft volume was 600 gm and the graft volume/recipient's standard liver volume was 52.0%.
| Time | mPAP (mmHg) | PCWP (mmHg) | CO (L/minute) | PVR (dynes · second/cm5) | Epoprostenol dose (ng/kg/minute) |
|---|---|---|---|---|---|
| 4 months before Tx | 47 | 16 | NA | NA | 0 |
| 2 months before Tx | 35 | 4 | 4.77 | 519 | 0 |
| 1 week before Tx | 33 | 10 | 8.24 | 223 | 10 |
| On laparotomy | 33 | NA | 5.45 | NA | 20 |
| On anhepatic phase | 43 | NA | 9.23 | NA | 20 |
| Immediately after reperfusion | 31 | NA | 7.5 | NA | 20 |
| 2 months after Tx | 30 | 6 | 7.67 | 250 | 5 |
| 1 yr after Tx | 30 | 6 | 4.68 | 410 | 0 |
- Abbreviations: Tx, liver transplantation; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; PVR, pulmonary vascular resistance; NA, not applicable.
The operation was completed without any adverse event. Pump-driven venovenous bypass was used during the anhepatic phase to maintain hemodynamic stability. A piggyback technique was used. Because there was a large splenorenal shunt with partial portal vein thrombosis, renoportal anastomosis was performed to reconstruct the portal vein, using the gonadal vein as an interposition graft.8 The operative time was 15 hours 25 minutes and the estimated blood loss was 6348 mL. Immunosuppression was initiated with basiliximab, mycophenolate mofetil and with early withdrawal of steroid followed by maintenance immunosuppression with cyclosporin and mycophenolate mofetil. In the immediate postoperative period, her blood gas analyses showed that oxygen pressure continued to be low, so that a high inspired oxygen concentration of more than 0.5 and long-term management on artificial ventilation were imperative. On postoperative day (POD) 14, tracheostomy was done, and the next day sepsis occurred. Catheter infection seemed to be the cause of sepsis. By use of appropriate antibiotics and removal of catheters, this critical condition gradually improved. On POD 23, because elevation of aspartate transaminase was noted, a liver biopsy was taken, revealing not rejection but ischemic changes (centrilobular ballooning of the hepatocytes). According to the biopsy result, no specific treatment for the elevation of transaminase was given, and there was a gradual decrease of aspartate transaminase level (Fig. 1). On POD 25, artificial ventilation was withdrawn and a rehabilitation program initiated. The dose of continuous epoprostenol was gradually decreased. The fourth examination by right heart catheterization revealed that PVR remained low although the continuous epoprostenol dose was decreased to 5 ng/kg/minute, so that epoprostenol therapy was completely stopped. Finally, the patient was allowed to leave the hospital on POD 72.
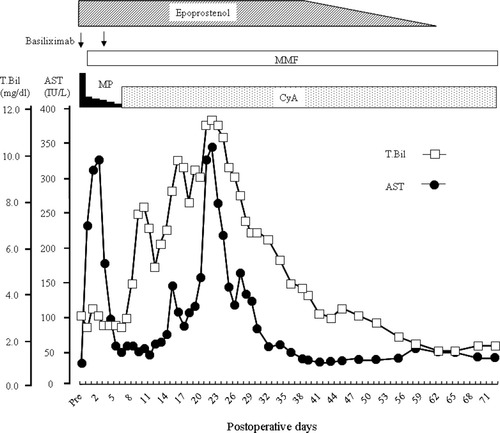
Postoperative course of total bilirubin and aspartate transaminase. Abbreviations: MMF, mycophenolate mofetil; MP, methylprednisolone; CyA, cyclosporine; T.Bil, total bilirubin; AST, aspartate transaminase.
One year after the operation, she has been visiting our outpatient clinic regularly and remains in a good condition. The final examination by right heart catheterization was performed on May 2005, which showed mPAP = 30 mmHg and PVR = 410 dynes · second/cm5. So far, there has not been any marked improvement in her cardiopulmonary profiles.
DISCUSSION
The precise mechanisms by which portal hypertension causes PPHTN remain unclear. Several factors, such as splanchnic volume overload and bowel congestion which result in release of endotoxins and various cytokines, are considered to lead to PPHTN. In the severe form, as reported by Krowka et al.,2 the mortality rate for liver transplant recipients is 100%. Some effective interventions are clearly needed to decrease the mortality rate of liver transplant recipients with moderate to severe PPHTN.
Continuous epoprostenol therapy has been shown to have some efficacy for PPHTN.4, 7 However, this therapeutic modality needs long-term venous access and uninterrupted drug infusion, so there are several serious problems in performing this therapy, such as catheter infection, line trouble, pump failure, and so on. Furthermore, there has been no randomized controlled trial on continuous epoprostenol therapy. Mair et al.9 reported that a liver transplant recipient with PPHTN died of right-sided heart failure 28 days after orthotopic liver transplantation although continuous epoprostenol therapy successfully decreased mPAP. Starkel et al.10 reported the feasibility of liver transplantation with good long-term outcome in patients with severe PPHTN without the need for pharmacological intervention. Although the patient's PVR decreased with the introduction of continuous epoprostenol therapy, whether it had favorable effects on the patient's outcome remains to be elucidated. However, it may be speculated that the decrease in mPAP and PVR associated with epoprostenol therapy results in a reduction in perioperative mortality in liver transplant recipients with PPHTN. Moreover, there is no effective therapeutic modality for PPHTN other than epoprostenol at the present time. Continuous epoprostenol therapy for liver transplant candidates with PPHTN must be investigated.
It has been speculated that nitric oxide may decrease PVR in patients with PPHTN. However, the efficacy of nitric oxide in PPHTN is controversial. Ramsay et al.11 demonstrated that nitric oxide did not reverse PPHTN associated with end-stage liver disease. In fact, we also tried to decrease mPAP by nitric oxide inhalation, but this was ineffective in our patient.
Other drugs such as iloprost12 and bosentan13 are used for the treatment of pulmonary arterial hypertension, showing some beneficial effects. Randomized controlled trials for these drugs in patients with PPHTN are warranted.
There have been several reports on the reversibility of PPHTN after liver transplantation.14-16 In contrast, Prager et al.17 reported that pulmonary hypertension associated with liver disease was not reversible after liver transplantation. In our experience, PPHTN was partially, but not completely reversed, 1 yr after successful LDLT. It can be considered that the more severe the PPHTN, the longer it takes to be reversed. How should recipients with PPHTN be monitored over the long term after liver transplantation? We will have this patient visit our outpatient clinic once every 3 months, undergo ultrasonic echocardiograph examination and blood gas analyses every 6 months, and undergo right heart catheterization every year to determine whether her cardiopulmonary profiles improve. If the latter fail to improve or even worsen in most recipients with PPHTN over the long term, even after successful liver transplantation, this approach by itself cannot represent an accepted indication for such patients (unlike hepatopulmonary syndrome). Further follow-ups are necessary for these patients with PPHTN.
A report of the Multicenter Liver Transplant Database demonstrated that transplant mortality was 36% (13/36) in patients with PPHTN.5 All deaths in such patients occurred within 18 days of liver transplantation; 5 of the 13 deaths occurred intraoperatively.5 Safer strategies for liver transplantation in patients with PPHTN are mandatory to reduce this high mortality rate. Our current strategies for LDLT in patients with moderate to severe PPHTN are as follows: 1) Try to decrease mPAP to less than 35 mmHg using epoprostenol, calcium channel blockers and other agents. If mPAP cannot be lowered by pharmacological interventions, liver transplantation is denied. 2) Use veno-venous bypass to maintain hemodynamic stability. 3) Prepare a heart assist device in case of cardiac arrest due to right-sided heart failure. 4) Never use a small-for-size graft to avoid small-for-size graft syndrome.18
To the best of our knowledge, this is the first report of a successful adult-to-adult LDLT in a patient with moderate to severe PPHTN. Because the number of recipients with PPHTN is relatively low, it is very difficult to decide optimal management of liver transplant candidates with PPHTN. Collaboration between major liver transplant centers regarding the management of PPHTN is therefore highly desirable.