Neonatal hemochromatosis: Radiographical and histological signs
First described in 1957,1 neonatal hemochromatosis is an iron storage disorder that is among the most common indications for liver transplantation in neonates.2 Distinct from hereditary hemochromatosis, neonatal hemochromatosis may not be due to a single etiology but rather may be a common endpoint for several infectious, autoimmune, and/or genetic etiologies.3 Infants with neonatal hemochromatosis typically present with abdominal distention, jaundice and hepatomegaly.4 Typically, decreased albumin, elevated bilirubin, and normal alanine aminotransferase and aspartate aminotransferase values are present.3 Serum iron levels are often normal, and other markers of iron metabolism such as elevated ferritin are nonspecific.5 Despite intensive medical treatment and liver transplantation, mortality is as high as 70% in some series.5, 6 Treatment with an antioxidant/chelator cocktail (desferrioxamine, N-acetylcysteine, vitamin E, selenium, prostaglandin E1) has been beneficial when administered early to patients who present with mild disease.5 Liver transplantation is considered the treatment of choice for patients not responding to medical therapy.7, 8 Here we present two cases of patients that presented with neonatal hemochromatosis soon after birth.
Case 1
A 5-week-old female was admitted to our institution with abdominal distention, jaundice, hepatomegaly, and perineal edema. The pregnancy had been uncomplicated, and other than 2 days of phototherapy for ABO incompatibility, the remainder of the patient's perinatal history was unremarkable. No family history of metabolic disorders or liver disease was found. Laboratory studies on admission showed a serum glucose level of 38 mg/dL, a total serum bilirubin of 12.1 mg/dL, a serum albumin level of 1.9 g/dL, and an international normalized ratio of 3.4.
Studies for inherited metabolic disorders and infectious diseases were nondiagnostic. Neonatal hemochromatosis was suspected based on the clinical presentation. The patient had a normal iron level of 121 μg/dL (normal, 55-150 μg/dL), elevated ferritin level of 735 ng/mL (normal, 12-300 ng/mL), hypersaturation of transferrin (88%; normal, 13%-39%), and decreased absolute tranferrin concentration of 95 mg/mL (normal, 169-300 mg/mL). Minor salivary (lip) gland biopsy was performed; a Prussian blue stain demonstrated small cytoplasmic granules corresponding with grade 1 iron accumulation (Fig. 1). Magnetic resonance imaging of the abdomen revealed a relative decrease in signal intensity of the liver compared to other (Fig. 2), a finding suggestive of iron deposition.9
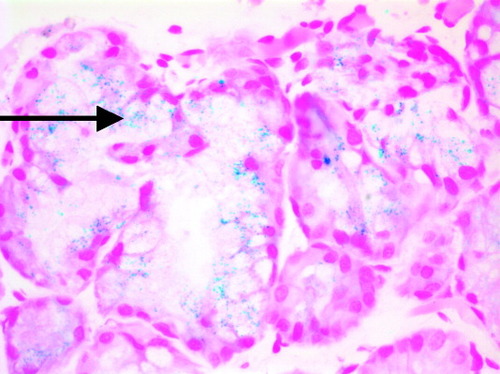
Histology from a minor salivary gland biopsy demonstrating multiple intracellular iron deposits (blue deposits indicated by arrow). Prussian blue with hematoxylin; original magnification, ×400.

Axial T2-weighted magnetic resonance imaging of the upper abdomen from Case 1 demonstrating diffuse liver parenchymal signal loss (solid arrow), compatible with iron deposition from hemochromatosis. The spleen is normal in appearance without signal loss to suggest excess iron deposition (dashed arrow). Normal signal intensity of the pancreas excludes hemosiderosis (dotted arrow).
Antioxidant/chelator cocktail therapy was initiated. The patient developed worsening severe liver failure and encephalopathy in spite of treatment; therefore, the decision was made to proceed with liver transplantation. A size-appropriate donor liver became available, so the patient underwent orthotopic liver transplantation 10 days after initiation of medical therapy. Gross appearance of the liver explant demonstrated a shrunken, grossly abnormal liver. Microscopic examination revealed abundant cholestasis and intracellular iron accumulation but to a much lesser extent than anticipated (Fig. 3). The patient's posttransplantation course has been unremarkable, and she now has good liver function with no evidence of recurrence of iron deposition.
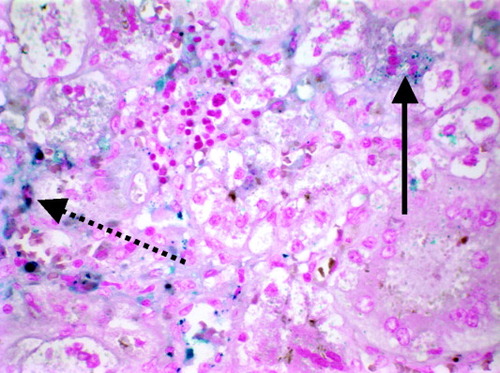
Histology from a liver biopsy demonstrating a multinucleated hepatocyte with iron deposits (solid arrow). Multiple intracellular iron deposits in Kupffer cells are also visible (blue deposits indicated by dashed arrow). Prussian blue with hematoxylin; original magnification, ×400.
Case 2
A 6-week-old female infant who was born full-term and had an uneventful perinatal course was admitted to our hospital with abdominal distention, jaundice, and hepatomegaly. Initial laboratory workup revealed a serum glucose level of 45 mg/dL, a serum bilirubin of 13.2 mg/dL, a serum albumin level of 2.0 g/dL, and an international normalized ratio of 6.1. Iron panel showed a serum iron level of 100 μg/dL (normal, 55-150 μg/dL), an elevated ferritin level of 713 mg/mL (normal, 12-300 ng/mL), hypersaturation of transferrin (>99%; normal, 15%-39%), and a decreased absolute transferrin concentration of 68 mg/mL (normal, 169-300 mg/mL).
Evaluation for inherited metabolic disorders, infectious diseases, and endocrine problems showed only mild hypothyroidism. As with Case 1, findings of both the minor salivary (lip) gland biopsy and magnetic resonance imaging of the abdomen showed findings suggestive of abnormal iron deposition (Fig. 4).
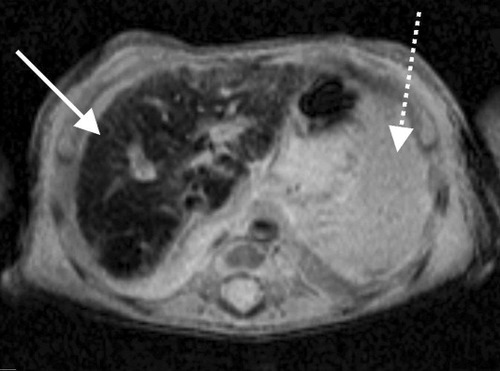
Axial T2-weighted magnetic resonance imaging of the upper abdomen from Case 2 demonstrating diffuse liver parenchymal signal loss (solid arrow), compatible with iron deposition from hemochromatosis. The spleen is normal in appearance without signal loss to suggest excess iron deposition (dashed arrow).
Antioxidant/chelation cocktail treatment was initiated, and a good response to therapy was seen. On the 44th day of life laboratory studies revealed total bilirubin of 5.5 mg/dL, albumin 2.3, international normalized ratio 1.7, ferritin 351 mg/mL, and transferrin 104 mg/mL. Although initially listed as a candidate for liver transplantation, the patient is now on inactive status. The patient continues to improve with ongoing inpatient therapy.




