Maxitrol as an antibiofilm agent with potential applications in Otolaryngology-Head and Neck Surgery
This work was presented as an oral presentation at the 29th Congress of the European Rhinologic Society in Sofia, Bulgaria, June 18–22, 2023.
Abstract
Objectives
Maxitrol (Novartis) is a topical ophthalmic medication that contains polymyxin B, neomycin, and dexamethasone. If it possesses antibiofilm activity, it may be useful for treating diseases of the head and neck in which biofilms are implicated, including chronic rhinosinusitis, chronic suppurative otitis media and osteoradionecrosis. We investigated the in vitro efficacy of Maxitrol against Staphylococcus aureus and Pseudomonas aeruginosa biofilms.
Methods
Minimum biofilm eradication concentration (MBEC) assays were performed using biofilms of P. aeruginosa ATCC 27853 and S. aureus ATCC 6538 type strains, grown on 96-pin lids and treated in Maxitrol for 30 min, 1 h, or 6 h. Isolates of both species were collected from the middle meatuses of patients with cystic fibrosis. Biofilms of clinical isolates were grown and treated in vitro for 6 h with Maxitrol, both undiluted at full concentration and at the identified MBEC, then cultured to identify bacterial survival.
Results
Neither type strain was eradicated at 30 min nor S. aureus at 1 h at any tested concentration. P. aeruginosa was eradicated by a median of 90% and 5.6% Maxitrol at 1 and 6 h, respectively, and S. aureus with 90% Maxitrol at 6 h. Undiluted Maxitrol reliably eradicated all clinical isolates of P. aeruginosa but only one of five S. aureus isolates.
Conclusions
Maxitrol reliably eradicates P. aeruginosa biofilm but not S. aureus biofilm in vitro. It may have a therapeutic role against biofilms in which P. aeruginosa is the dominant pathogen.
Level of Evidence
N/A.
1 INTRODUCTION
Bacterial biofilms are increasingly recognized as a feature of many chronic diseases of the head and neck, including chronic rhinosinusitis (CRS), cystic fibrosis (CF), chronic suppurative otitis media (CSOM), and osteoradionecrosis (ORN) following radiotherapy for head and neck malignancy.1-5 Biofilms act as a bacterial reservoir that is inaccessible to immune defenses and resistant to conventional antibiotics, leading to persistent infection.6 In CRS, Staphylococcus aureus biofilms are associated with more severe disease with greater rates of recalcitrance to usual medical therapy and functional endoscopic sinus surgery (FESS).7 Biofilms have been identified in nasopharyngeal and temporal ORN and are hypothesized to contribute to the chronicity of this difficult clinical problem.2, 3 Despite their importance, there are a small number of effective topical antibiofilm agents with which to eradicate biofilms in vivo. Of those available, few are suitable for otolaryngological application as such agents must also be non-ciliotoxic and non-ototoxic.
Maxitrol (Novartis Pharma AG, Basel, Switzerland) is an ophthalmic preparation containing polymyxin B 6000 international units per mL (IU/mL), equivalent to ~600 μg/mL; neomycin 3500 IU/mL (equivalent to ~4.6 mg/mL); dexamethasone 1 mg/mL; and benzalkonium chloride 0.04 mg/mL as a preservative. It is available as an ointment or as drops and has been used off-label for application in the external auditory canal or nose and paranasal sinuses. Similar otic preparations containing polymyxin B, neomycin, and a steroid such as Cortisporin (Pfizer Inc., New York, NY, USA) are available for the treatment of CSOM.8 Although polymyxin B and neomycin are ototoxic and caution may be advised with tympanic membrane perforation, these ototopical preparations have been safely used in this context and during tympanoplasty surgery without deleterious effects.8 Likewise, nasal sprays such as Polydexa (Rusfic LLC, Moscow, Russia) are available for use in the nose and paranasal sinuses. Neither polymyxin B, neomycin, nor dexamethasone are meaningfully ciliotoxic and may be safely applied in this setting.9, 10
Polymyxin B is recognized to be active against bacterial biofilms.7 It acts by physical disruption of cell membranes rather than interference with cellular metabolic processes which are reduced in the biofilm phenotype, though its mechanism of action remains incompletely understood.6, 11, 12 Benzalkonium chloride also acts by disruption of cell membranes.13 As a preparation containing both polymyxin B and a small amount of benzalkonium chloride, Maxitrol may demonstrate antibiofilm activity and therefore be useful in the management of biofilm-related disease in the head and neck. Further, as it contains a corticosteroid, it may be a useful combination product in the therapeutic armamentarium for managing recalcitrant CRS, in which the presence of biofilms is more likely.
We aimed to determine the in vitro efficacy of Maxitrol against biofilms of two important otolaryngological pathogens, S. aureus and Pseudomonas aeruginosa. If Maxitrol is effective against these biofilms in vitro, it may have potential as a treatment for the management of biofilms in the head and neck.
2 MATERIALS AND METHODS
2.1 Bacterial species
Planktonic testing (minimum inhibitory and bactericidal concentrations, MIC and MBC) and biofilm testing (minimum biofilm eradication concentration, MBEC) was undertaken using S. aureus ATCC 6538 and P. aeruginosa ATCC 27853. In addition, biofilm testing was undertaken using five clinical isolates each of S. aureus and non-mucoid P. aeruginosa, all collected from the middle meatuses of seven patients with CF. Isolates of these species were chosen because of the high rates of biofilm formation and multi-drug resistant strains in patients with this disease.14, 15 When more than one isolate was tested from a single patient, these were taken from samples collected at different time points. Antibiotic susceptibilities for each isolate, as reported by the hospital laboratory, are presented in Table 1.
| Isolate | Species | Susceptible, standard dosing regimen | Susceptible, increased exposure | Resistant |
|---|---|---|---|---|
| CF01-S08 | S. aureus | Erythromycin Flucloxacillin Cotrimoxazole Tetracycline |
Penicillin | |
| CF02-S02 | S. aureus | Flucloxacillin Cotrimoxazole Tetracycline |
Penicillin Erythromycin |
|
| CF04-S02 | S. aureus | Erythromycin Flucloxacillin Cotrimoxazole Tetracycline |
Penicillin | |
| CF09-S01 | S. aureus | Penicillin Flucloxacillin Cotrimoxazole Tetracycline |
Erythromycin | |
| CF10-S01 | S. aureus | Flucloxacillin Cotrimoxazole |
Penicillin Erythromycin Tetracycline |
|
| CF02-S04 | P. aeruginosa | Meropenem Tobramycin |
Ceftazidime | Ciprofloxacin |
| CF07-S01 | P. aeruginosa | Ceftazidime Ciprofloxacin Meropenem Tobramycin |
||
| CF07-S05 | P. aeruginosa | Meropenem Tobramycin |
Ceftazidime Ciprofloxacin |
|
| CF09-S04 | P. aeruginosa | Tobramycin | Ceftazidime Ciprofloxacin Meropenem |
|
| CF12-S04 | P. aeruginosa | Ceftazidime Ciprofloxacin Meropenem Tobramycin |
- Note: Isolates are named by patient (CF##) and sampling episode (S##). P. aeruginosa isolates with wild-type sensitivity to ceftazidime and ciprofloxacin are routinely reported as “susceptible, increased exposure” for these antibiotics.
Reference strains and clinical isolates were stored as previously described.15 Reference strains were subcultured from freezer stock onto tryptic soy agar (TSA) (BD Bacto Tryptic Soy Broth; BD Difco Agar, Bacteriological; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) using the streak plate method. Plates were incubated overnight at 37°C and subsequently stored at 4°C. Clinical isolates were collected following ethical approval (20/STH/24) from the New Zealand Health and Disability Ethics Committee and written informed consent from each participant, as previously reported.15 These were stored at −80°C in tryptic soy broth (TSB) (BD Bacto Tryptic Soy Broth; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with 50% glycerol. Overnight broth cultures were prepared by inoculating ~10 mL of media with a single colony of the reference strain or 10 μL of clinical isolate freezer stock thawed at room temperature. Mueller Hinton II broth (MHB) (BBL Mueller Hinton II Broth (Cation Adjusted); Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used for planktonic experiments, and TSB for biofilm experiments. These were then incubated overnight at 37°C, 200 revolutions per minute (RPM). All experiments were undertaken in biological duplicate.
2.2 Materials
Maxitrol stock solutions were prepared by mixing 9 parts Maxitrol with 1 part 10× MHB for planktonic experiments, and 9% sterile saline for biofilm experiments, producing a solution of 90% Maxitrol in MHB or 0.9% saline. This ensured that tonicity and nutrient concentrations remained similar across all tested concentrations of Maxitrol. Polymyxin B is known to bind to polystyrene, reducing its efficacy.16 Maxitrol was therefore handled entirely in polypropylene as previously described.15 Polypropylene centrifuge tubes (Falcon 15 mL conical centrifuge tube, part number 352096, Corning Inc., Corning, NY, USA) and pipette tips (Neptune Scientific, San Diego, CA, USA) were used, and testing was conducted in polypropylene 96-well plates (Nunc Microwell, part number 267334, Thermo Fisher Scientific, Waltham, MA, USA). Polystyrene 96-pin lids were used for biofilm growth (Nunc Immuno TSP lids, part number 445497, Thermo Fisher Scientific, Waltham, MA, USA); exposure to Maxitrol of the area of the pin not containing biofilm was kept to a minimum. Steps of the experiment where Maxitrol was not used were conducted in polystyrene 96-well plates (Nunc Microwell, part number 167008, Thermo Fisher Scientific, Waltham, MA, USA).
2.3 Planktonic experiments
Broth microdilution assays for MIC and MBC were performed according to previous protocols with minor modifications.17, 18 A stock solution of 90% Maxitrol in MHB was prepared, of which 200 μL was added to each well in the top row of polypropylene 96-well plates and a doubling dilution series in MHB was performed, leaving 100 μL remaining in each well. Overnight cultures of the reference strains were diluted to 1 × 108 colony forming units (CFU)/mL using optical density at 600 nm (OD600) (Libra S22, Biochrom US, Holliston, MA, USA). A total of 100 μL of this inoculum was added to each well, giving a final inoculum concentration of 5 × 107 CFU/mL and a total volume of 200 μL in each well. Plates were covered and incubated overnight at 37°C at 200 RPM.
After incubation, 95 μL from each well was transferred to a polystyrene 96-well plate for measurement of OD600 (EnSight multimode plate reader, PerkinElmer, Inc., Waltham, MA, USA), and an additional 10 μL was spot-plated on tryptic soy agar (TSA) (BD Bacto Tryptic Soy Broth; BD Difco Agar, Bacteriological; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and incubated at 37°C overnight. The lowest concentration of Maxitrol where OD600 did not increase during incubation was determined to be the MIC, and the lowest concentration where 25 or fewer colonies grew on TSA was determined to be the MBC (indicating an approximate 5 log10 reduction in viable bacteria).
2.4 Biofilm experiments
2.4.1 MBEC assays
S. aureus ATCC 6538 and P. aeruginosa ATCC 27853 biofilms were grown on 96-pin lids as previously described (Figure 1).15, 19 Briefly, wells of a polystyrene 96-well plate were inoculated with 150 μL of overnight cultures diluted to ~107 CFU/mL with TSB. A total of 150 μL TSB was added to 4 wells as a sterility control. The 96-pin lid was then positioned into the well plate and incubated for 24 h at 37°C (125 RPM for P. aeruginosa and 150 RPM for S. aureus).
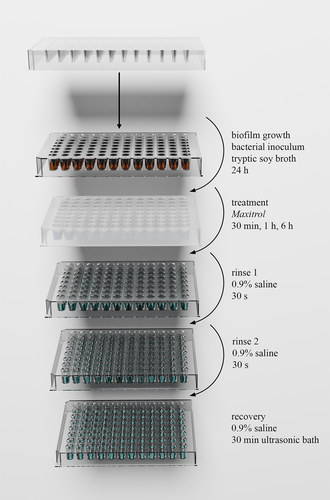
A 90% solution of Maxitrol in 0.9% saline was prepared and 400 μL was added to each well in the top row of a polypropylene 96-well plate. A doubling dilution series was performed with 200 μL treatment solution remaining in each well. The 96-pin lid was transferred to this plate for 30 min, 1 h, or 6 h at room temperature. Thirty minutes was chosen to reflect the potential transit time through the sinonasal tract that may be observed for antimicrobials applied as a spray or sinus rinse.20 Treatment times of 1 and 6 h were chosen to determine the antibiofilm activity of this preparation over longer time frames.
Following treatment, the pins were rinsed twice in sterile 0.9% saline and transferred to a 96-well plate containing 200 μL sterile 0.9% saline in each well. Biofilm cells were dispersed into suspension by placing this plate into a polypropylene tray in an ultrasonic bath for a total of 30 min at 37 kHz (ELP030H; Elma Schmidbauer GmbH, Singen, Germany). The bath was cooled using ice, which was replaced halfway through sonication to prevent heating of the samples. 10 μL from each well was cultured overnight at 37°C on TSA, and the MBEC was recorded as the lowest concentration of Maxitrol at which no colonies grew.
Additional biofilms for baseline CFU enumeration were grown by the same method, rinsed once, then recovered. A 10-fold dilution series in 0.9% saline was performed on the resulting suspension, 10 μL aliquots of which were cultured on TSA as above. Bacterial counts per pin were then determined by colony counting and back-calculation.
2.4.2 Clinical isolates
Biofilms of clinical isolates of each species were grown using the same methods. Inoculums for biofilm growth were prepared by adjusting the OD600 of the clinical isolate overnight culture to that of the reference strain inoculum, ensuring that inoculation was uniform across strains despite potential variation in growth of overnight cultures. After growth, biofilms were treated for 6 h in either undiluted Maxitrol or Maxitrol diluted to the median MBEC of the reference strain. Where the median MBEC of the reference strain was at the highest tested concentration, only undiluted Maxitrol was used for testing with clinical isolates. After treatment, pins were rinsed, recovered and cultured using the same methods as above. The proportion of pins from which no viable bacteria were recovered was recorded. Treatment solution was also cultured to determine whether any reduction in quantifiable cells from the pins was due to eradication in viable bacteria or dispersion into the treatment solution.
2.5 Scanning electron microscopy
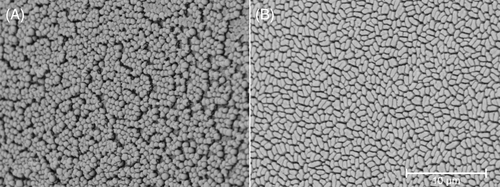
Pins were broken off the 96-pin lid following biofilm growth and fixed in 2.5% glutaraldehyde. These were prepared for scanning electron microscopy (SEM) by rinsing in phosphate-buffered saline (PBS) then dehydrating by immersion in increasing concentrations of ethanol, air drying, mounting on aluminum stubs and gold sputter coating (DSR1, Nanostructured Coatings Co., Tehran, Iran) before imaging (TM3030Plus, Hitachi Ltd., Tokyo, Japan).
2.6 Statistics
MIC, MBC, and MBEC are presented as median +/− range. Replicates were excluded when MBECs could not be determined with certainty. Statistical analyses were undertaken in R version 4.1.1.21 MIC, MBC, and MBEC data were analyzed using the Mann–Whitney U test, and comparison of susceptibility of clinical isolates to the laboratory reference strain was performed using Fisher's exact test.
3 RESULTS
3.1 Planktonic experiments
The minimum concentration of Maxitrol required to inhibit growth of planktonic S. aureus (i.e., MIC) was 0.044%, and the MBC was 0.044% (0.044%–0.088%). For planktonic P. aeruginosa, MIC was 0.18% (0.18%–0.35%) and MBC was 0.70%. Planktonic S. aureus was significantly more susceptible to Maxitrol than P. aeruginosa (p = .001).
3.2 Biofilm experiments
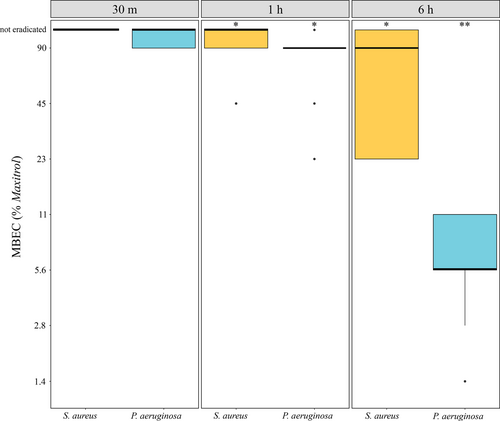
S. aureus ATCC 6538 biofilm was eradicated by a median of 90% (23%–>90%) Maxitrol at 6 h. No consistent antibiofilm activity was observed at shorter durations of exposure. P. aeruginosa ATCC 27853 was eradicated by 90% (23%–>90%) Maxitrol at 1 h and 5.6% (1.4%–11.3%) at 6 h, with no effect observed at 30 min (Figure 2).
3.2.1 Clinical isolates
In MBEC assays using S. aureus ATCC 6538, meaningful eradication at 6 h was observed only with 90% Maxitrol (the highest tested concentration). S. aureus clinical isolates were therefore tested only against undiluted Maxitrol. Three isolates were less susceptible to Maxitrol than the type strain (i.e., biofilm viability was completely or near-completely unaffected; p < .05), one was significantly more susceptible (i.e., entirely eradicated; p = .002), and one was not significantly different (i.e., equivalently susceptible; p > .05) (Table 2).
| Isolate | % eradicated | p-value |
|---|---|---|
| S. aureus ATCC 6538 | 50 | - |
| CF01-S08 | 0 | .002 |
| CF02-S02 | 6 | .02 |
| CF04-S02 | 0 | .002 |
| CF09-S01 | 75 | N.S. |
| CF10-S01 | 100 | .002 |
- Note: p-values denote differences in proportion of replicates eradicated compared to S. aureus ATCC 6538 type strain. Number of replicates per isolate n = 16. p > .05.
- Abbreviation: NS, not significant.
P. aeruginosa clinical isolates were tested using both the MBEC identified for the type strain and undiluted Maxitrol. At MBEC (i.e., 5.6% Maxitrol), one isolate was less susceptible (p = .04), two more susceptible (p < .005), and two not significantly different to the type strain (p > .05). All strains were entirely eradicated by undiluted Maxitrol (Table 3).
| Isolate | % eradicated (MBEC) | p-value (clinical isolate compared to type strain) | % eradicated (undiluted) | p-value (undiluted compared to MBEC) |
|---|---|---|---|---|
| P. aeruginosa ATCC 27853 | 31 | - | 100 | <.0005 |
| CF02-S04 | 13 | N.S. | 100 | <.0005 |
| CF07-S01 | 88 | .003 | 100 | N.S. |
| CF07-S05 | 0 | .04 | 100 | <.0005 |
| CF09-S04 | 100 | <.001 | 100 | N.S. |
| CF12-S04 | 63 | N.S. | 100 | .02 |
- Note: p-values for MBEC testing denote differences in proportion of replicates eradicated compared to type strain. p-values for testing using undiluted Maxitrol signify differences in proportion of replicates eradicated compared to that eradicated in MBEC testing. Number of replicates per isolate n = 16. N.S. = not significant, p > .05.
No bacterial growth was observed in any treatment solution wells for both type strains and in all clinical isolates.
3.3 Scanning electron microscopy
The presence of biofilm on 96-pin lids was confirmed for each type strain and clinical isolate by SEM (Figures 3, S1, and S2).
4 DISCUSSION
We have demonstrated time- and concentration-dependent biofilm eradication of P. aeruginosa using Maxitrol. P. aeruginosa ATCC 27853 was eradicated using a median of 90% Maxitrol at 1 h, with persistence in some replicates, and consistently eradicated at 5.6% Maxitrol at 6 h. S. aureus ATCC 6538 biofilms were eradicated only at the highest tested concentration at the longest duration of treatment, with persistent growth in a small number of replicates even at this maximum tested concentration. It is possible that with longer durations of treatment, greater efficacy might be observed.
Variation in susceptibility between clinical isolates was noted for both species, with some isolates more and some less susceptible to Maxitrol than the reference strains. Using undiluted product, P. aeruginosa biofilms of all isolates were consistently eradicated in contrast to S. aureus where complete eradication was observed with only one isolate. This suggests that if Maxitrol is to be used as an antibiofilm agent, it is best reserved for settings where P. aeruginosa is the dominant biofilm-forming pathogen, or where S. aureus with known antibiotic sensitivities can be treated with longer durations of exposure. The use of Maxitrol where S. aureus biofilm is present may increase the risk of treatment failure or generation of microbiological selection pressure favoring this organism. However, Maxitrol is highly effective against planktonic forms of both S. aureus ATCC 6538 and P. aeruginosa ATCC 27853. In this study, we did not evaluate the susceptibilities of clinical isolates in planktonic form as these are much more likely than biofilms to be predicted by routine culture and antimicrobial sensitivity testing.
We have recently reported on the efficacy of polymyxin B against S. aureus ATCC 6538. Although polymyxins are considered to be primarily active against Gram-negative species, we observed activity of polymyxin B against planktonic S. aureus with an MIC and MBC of 256 μg/mL and 512 μg/mL, respectively, in keeping with other reports.7, 11, 15 However, no clinically useful activity was observed against biofilms.15 In the present study, planktonic S. aureus was more sensitive to Maxitrol than P. aeruginosa, but in biofilm form, the opposite was true. It is likely that any anti-staphylococcal activity observed at the dilutions found to be effective here could be due to the neomycin component, with possibly some contribution from the benzalkonium chloride preservative. Like most antibiotics, aminoglycosides including neomycin are predictably less effective against biofilm.6 However, polymyxin B is active against both planktonic and biofilm cells, particularly those of Gram-negative organisms, and is therefore the likely source of anti-pseudomonal activity in the biofilm form.7 This forms the most likely explanation for these seemingly paradoxical findings. In addition, previous studies have demonstrated superiority of polymyxin-aminoglycoside combinations over monotherapy against P. aeruginosa biofilm. In the same in vitro model as used here, the combination of colistin sulfate and tobramycin led to significantly greater reductions in viable biofilm than either antibiotic alone at double the MIC (~5 log10 compared to ~3 log10 reductions in viable cells). A subsequent clinical pilot study demonstrated reductions in viable P. aeruginosa in expectorated sputum in patients with CF after 28 days of twice daily nebulized colistin and tobramycin.22
This study is limited by the testing of only one of several similar products used in this setting. Maxitrol was chosen as a product readily available and commonly used in New Zealand. Antimicrobial concentrations vary between products but others are likely to be at least as effective: Maxitrol contains lower concentrations of polymyxin B than Cortisporin otic drops and Polydexa nasal spray and a similar concentration of neomycin. Furthermore, biofilms in vivo are often polymicrobial but this study investigated single-species biofilms. Future testing of the other preparations, as well as testing with multi-species biofilms may be useful to further establish the role of these preparations in the management of biofilm-associated disease in ORL. Finally, neomycin can cause contact dermatitis, but the frequency of this adverse effect after intranasal application is unknown. A recent study in which neomycin was used intranasally for S. aureus decolonization prior to hip and knee arthroplasty reported no adverse events.23 More extensive safety and tolerability testing for the off-label use of this ophthalmic preparation should be completed prior to undertaking clinical trials.
We have employed well-established and repeatable methodologies to determine the antibiofilm efficacy of Maxitrol against reference strains of important otolaryngological pathogens. Further, we have tested Maxitrol against clinical isolates of both species to provide an indicator for the potential real-world applicability of our findings.
Overall, these findings suggest that this readily available combination product may be useful in managing difficult biofilm-associated otolaryngological conditions amenable to topical application of antimicrobials, particularly where P. aeruginosa is the dominant pathogen. A careful approach, directed by cultures where appropriate, may be beneficial to maximize the likelihood of treatment success, particularly when S. aureus is present.
Clinical trials are warranted to determine whether clinical benefit may be gained from the antibiofilm activity demonstrated here. In the context of CRS, this may hold the most promise in the management of CF where sinonasal biofilms of P. aeruginosa are common yet difficult to manage.14 For ORN, these preparations may be most useful for the temporal bone where ointment or drops could be applied regularly to the external auditory canal, allowing for prolonged durations of exposure.
5 CONCLUSION
Maxitrol is an effective antibiofilm agent against P. aeruginosa in vitro. It may be useful for the treatment of biofilm-associated disorders in ORL where P. aeruginosa is the dominant biofilm-forming pathogen, such as CRS in the context of CF. In vitro efficacy against S. aureus biofilms was found to be less, with more variability.
ACKNOWLEDGMENTS
The authors would like to thank Tilly Johnson, Gisselle Alarcon, and Melissa Zoing for their assistance with sample collection; LabPLUS laboratories at Auckland City Hospital, Auckland for their assistance in sample processing and isolation of clinical isolates; Associate Professor Joe Harrison for guidance in establishing biofilm growth; and Satya Amirapu and Yohanes Nursalim for their assistance with scanning electron microscopy. Open access publishing facilitated by The University of Auckland, as part of the Wiley - The University of Auckland agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
SH is the recipient of an Academic Surgeon-Scientist Research Scholarship, RK is the recipient of an Academic Surgeon-Scientist Fellowship, and KB is the recipient of a Mid-Career Fellowship, all generously funded by The Garnett Passe and Rodney Williams Memorial Foundation. BWM is generously funded by the Auckland Medical Research Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




