Vitamin D supplementation in preventing the recurrence of benign paroxysmal positional vertigo
Tae Hoon Kong, and Su Young Jung contributed equally to this work.
Abstract
Objective
To evaluate the effect of vitamin D supplementation on the recurrence rate of benign paroxysmal positional vertigo (BPPV).
Methods
A single-center, prospective, double-blind, placebo-controlled, parallel-group randomized controlled trial was conducted between November 2018 and May 2020. After successful treatment with canalith repositioning maneuvers, patients diagnosed with BPPV were randomized to either the vitamin D (n = 20) or placebo (n = 18) group. Only patients with serum vitamin D levels <20 ng mL−1 were included. The vitamin D group received 7000 IU of vitamin D weekly for a year, while the placebo group received a matching placebo drug. The final endpoint was the BPPV recurrence rate and correlation with serum vitamin D levels after 6 and 12 months in both groups.
Results
Among 38 patients, 37 were followed up for 6 months and 30 for 12 months. Significantly higher serum vitamin D levels were observed in the vitamin D group compared to the placebo group at both the 6-month and 1-year follow-ups (p < .001 at each timepoint). The recurrence rate was lower in the vitamin D group than in the placebo group after 6 months (p = .008) and 1 year (p = .003).
Conclusion
Vitamin D supplementation, in the absence of calcium, may be beneficial for patients prone to recurrent BPPV episodes, particularly when serum vitamin D levels are suboptimal (PRE20181024-001, Clinical Research Information Service, South Korea).
Level of Evidence
1b.
1 INTRODUCTION
Benign paroxysmal positional vertigo (BPPV), the most common peripheral vestibulopathy, is characterized by recurrent rotational vertigo that occurs suddenly with head movements during daily activities, such as lying down or turning over in the supine position.1, 2 In BPPV, calcium carbonate crystals called otoconia, which should be attached to the utricular macula, get dislodged for various reasons and enter the semicircular canal or get stuck in the cupula, causing vertigo with head movements.1, 2 Fortunately, the resolution rate of BPPV is considerably high, although it varies according to the subtypes of BPPV.3 Many studies have shown that posterior canal BPPV (PC-BPPV) and horizontal canal BPPV (HC-BPPV) have resolution rates between 61% and 90% following the implementation of the Epley, Lempert, or Gufoni maneuvers.3, 4
Despite the high-resolution rate, BPPV has a 1-year incidence of 0.6% and a 1-year prevalence of 1.6%, which is prominent among vestibular disorders.3, 5 In addition, when patients with BPPV are followed up for 5–10 years, recurrence rates are reported to be as high as 22%–50%, with 70%–80% of patients facing recurrence within 1 year.3, 6, 7 Furthermore, the patients experiencing fear of recurrent episodes of severe dizziness is a major problem. Consequently, various studies have explored the risk factors for BPPV recurrence and have found them to be varied, including pre-existing otologic conditions (e.g., Meniere's disease), trauma, pre-existing medical conditions (e.g., hypertension and diabetes), vitamin D deficiency, and osteoporosis.7-14 Although a causative link between these risk factors and BPPV recurrence is yet unknown, few studies have conclusively shown that targeting these risk factors prevents BPPV recurrence. Recently, several studies have highlighted the potential of vitamin D supplementation in preventing BPPV recurrence.13-15 A notable multicenter prospective study observed a significant reduction in recurrence rates after vitamin D and calcium carbonate supplementation in vitamin D-deficient patients, compared to the control group. Here, however, vitamin D and calcium carbonate were administered concomitantly, and the control group was not administered any placebo. Therefore, to elucidate the specific effect of vitamin D monotherapy on BPPV recurrence, our study aimed to compare the outcomes of weekly supplementation with 7000 IU of vitamin D in these patients against a placebo group.
2 MATERIALS AND METHODS
2.1 Subjects
Individuals who were aged 20 years or older, diagnosed with BPPV, and who underwent treatment at the Department of Otolaryngology, Myongji Hospital, between November 2018 and May 2020 were eligible. The diagnosis of BPPV included the canalolithiasis type of PC-BPPV and the canalolithiasis type (“HC-BPPV can”) and cupulolithiasis type of HC-BPPV (“HC-BPPV cup”) in patients with vertigo induced by head movements, according to the BPPV diagnostic criteria proposed by the Committee for the Classification of Vestibular Disorders of the Bárány Society.2 Patients with the following conditions were excluded: (1) anterior canal BPPV, (2) multiple canal involvement in BPPV, (3) positional nystagmus attributable to other causes, such as vestibular migraine or central vestibulopathy, (4) vitamin D supplementation before study enrollment, and (5) a significant adverse medical reaction to the study product. A diagnosis of PC-BPPV was made when patients showed characteristic torsional upbeating nystagmus (duration <1 min), oriented toward the tested ear during the Dix–Hallpike maneuver. The diagnosis of HC-BPPV was based on the results of the supine roll test. “HC-BPPV can” was identified when the participants exhibited geotropic direction-changing horizontal nystagmus lasting <1 min. Conversely, “HC-BPPV cup” was identified in the presence of apogeotropic direction-changing horizontal nystagmus that persisted beyond 1 min. All positional testing procedures were performed using a video Frenzel goggles system (SLMED, Seoul, Korea).
The trial was registered at the Clinical Research Information Service (CRIS, PRE20181024-001) of South Korea, which is the International Clinical Trials Registry Platform approved by the World Health Organization. This study was approved by the Institutional Review Board of Myongji Hospital and performed in accordance with the ethical principles of the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines, and applicable regulatory requirements (MJH 2018–08-029). Written informed consent was obtained from all the study participants.
2.2 Study design
This was a single-center, randomized, 1:1 parallel-group trial designed to evaluate the efficacy of once-weekly oral administration of 7000 IU of vitamin D without calcium carbonate in preventing recurrent episodes of BPPV following successful canalith repositioning procedures (CRP). Successful treatment with CRP was defined as the absence of both positional vertigo and nystagmus after a follow-up period, during which the patient underwent reassessment positional tests and, if necessary, a single CRP treatment daily. Of the 360 patients screened for eligibility, 38 were randomized into either the vitamin D or the placebo groups (Figure 1).
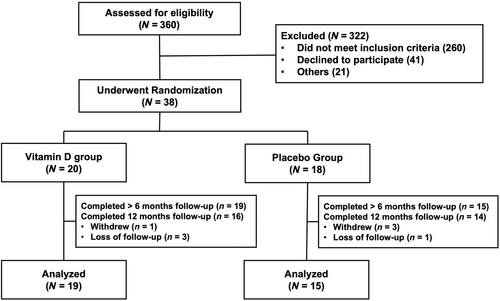
All participants, in both the vitamin D and placebo groups, were evaluated for serum 25-hydroxyvitamin D (25-OH vitamin D) levels. Only those with levels <20 ng/mL were enrolled and randomized into either of the two groups. Participants in the vitamin D group received a weekly dose of 7000 IU vitamin D (Tab, Dalim BioTech Co., Ltd., Korea). In contrast, those in the placebo group were administered placebo tablets, identical in color, size, and shape, and manufactured by the same company, once a week for 12 months. Adherence was meticulously tracked by tallying the remaining tablets during each patient visit or through weekly telephone interviews spanning the entire year. Any cessation of supplementation owing to adverse reactions or other considerations was noted and aligned with the primary outcome data. To monitor and assess any disparity in serum 25-OH vitamin D levels between the groups, participants from both groups were requested to return to a clinic and provide follow-up blood samples at the 6-month and 12-month timepoints.
Upon completion of eligibility screening, patients were assigned a unique, randomly generated number. After the initial step, the researcher shared these numbers with the clinical pharmaceutical research team. The clinical research pharmacist then referred to a manual containing unique numbers generated by an independent statistician before the commencement of the study. This manual was used to identify the interventions assigned to each patient. Medication was administered to these patients during the study period based on their allocated interventions. The treatment allocations were concealed from the principal investigators, participating researchers, and patients, as previously described. Randomization was performed using a concealed, computer-generated list of management assignments based on a predetermined simple randomization schedule provided by the statistical expert. The randomized patients were followed up for all clinical endpoints and serious adverse events, with the primary and multiple secondary endpoint events confirmed by a central adjudicator blinded to the study assignment.
2.3 Outcome measurements
The primary outcome of this study was the change in serum 25-OH vitamin D levels between the two groups over a year. The secondary outcome was the percentage of patients who faced recurrence during the follow-up. To ensure precision in capturing these outcomes, patients underwent weekly telephone interviews in which they were asked, “Have you experienced any recurrence of positional vertigo similar to your initial BPPV episode?” They were advised to return to the clinic immediately if they experienced analogous dizziness symptoms. In the instances of reported recurrence of dizziness, patients were re-evaluated in the clinic to confirm whether it was a genuine recurrence of BPPV. Regardless of whether they experienced dizziness, all participants were scheduled to revisit the clinic at the 6-month and 12-month periods. During these visits, they underwent positional tests for symptom recurrence and provided blood samples for assessing serum 25-OH vitamin D concentrations.
2.4 Sample size
Based on our calculations, a sample size of 40 participants would yield 80% power to detect a 20% reduction in the frequency of BPPV recurrence upon vitamin D supplementation over 12 months, with the significance level set at 0.05. This anticipated 20% relative risk reduction was determined by consensus among the investigators. Given the focus on vestibular disorder stewardship, the team collectively believed that advocating for increased use of vitamin D for this prevalent vestibular condition necessitated robust evidence. We assumed that BPPV recurrence would adhere to a Poisson distribution. Furthermore, it was hypothesized that, in the vitamin D group, an average of 1.09 new BPPV recurrences per individual would be observed, accounting for an anticipated non-adherence rate of up to 30%.
2.5 Statistical analysis
We used SPSS (version 25.0; IBM, Armonk, NY, USA), R version v3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria), and R studio v1.1.453 (Posit Software, Boston). We performed chi-square and Fisher's exact tests on categorical variables and the student's t-test on continuous variables to compare patients with and without recurrence. We also performed a Kaplan–Meier survival analysis with a log-rank test to evaluate the recurrence rate in both groups. Results were considered statistically significant when the p-value was <0.05. In case of multiple recurrences, each recurrence event was assumed to be independent, and patients who recovered after recurrence were considered different individuals.
3 RESULTS
3.1 Clinical and demographic characteristics
Among 38 participants who were randomized in a 1:1 ratio, 34 (89.5%)—19 in the vitamin D group and 15 in the placebo group—completed at least the first 6 months of follow-up (Figure 1). The median follow-up duration was comparable between the vitamin D and placebo groups (185 and 182 days, respectively). At the end of 1 year, 30 patients (88.2%) completed the study, with 84.2% and 93.3% in the vitamin D and placebo groups, respectively. Three participants in the vitamin D group (15.8%, 3/19) and one in the placebo group (6.7%, 1/15) were lost to follow-up before concluding the study. On average, they had been followed up for 5.7 months (5.7 months in the vitamin D group vs 5.8 months in the placebo group).
The average age among the 38 patients was 55.5 ± 14.5 years. The ratio of males to females was 11:27. BPPV was observed on the right and left sides in 16 and 22 patients, respectively. The breakdown of the BPPV subtypes was as follows: 71.1% PC-BPPV, 21.1% HC-BPPV can, and 7.9% HC-BPPV cup. Approximately 50% of the participants reported sun exposure of <30 min per day, and approximately 18.4% reported sun exposure exceeding 1 h (Table 1).
| Total (n = 38) | Placebo group (n = 18) | Vitamin D group (n = 20) | p-value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 55.5 ± 14.5 | 57.5 ± 13.7 | 53.8 ± 15.3 | 0.440 |
| Sex (male:female) | 11:27 | 4:14 | 7:13 | 0.485 |
| Affected side (right:left) | 16:22 | 8:10 | 8:12 | 1.000 |
| Subtype of BPPV | 0.413a | |||
| PC-BPPV | 27 (71.1%) | 14 (77.8%) | 13 (65.0%) | |
| HC-BPPV(Geo) | 8 (21.1%) | 2 (11.1%) | 6 (30.0%) | |
| HC-BPPV(Apo) | 3 (7.9%) | 2 (11.1%) | 1 (5.0%) | |
| Sun exposure | 0.911a | |||
| Less than 30 min | 19 (50%) | 10 (55.6) | 9 (45.0%) | |
| Over 30 min, less than 1 h | 12 (31.6%) | 5 (27.8%) | 7 (35.0%) | |
| Over an hour | 7 (18.4%) | 3 (16.7%) | 4 (20.0%) |
- Abbreviation: SD, standard deviation.
- a Fisher's exact test.
3.2 Primary outcomes: serum vitamin D levels
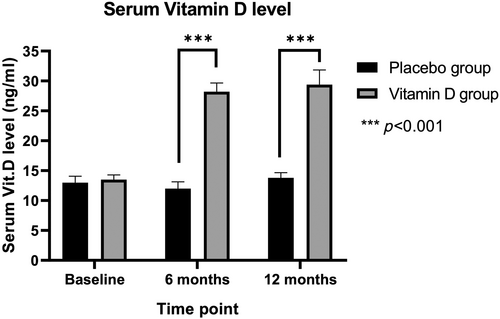
At baseline, the serum 25-OH vitamin D levels were comparable between the groups (p = .714, Figure 2). Following vitamin D supplementation, there was a significant increase from 13.3 ± 3.9 ng mL−1 at baseline to 28.2 ± 6.5 ng mL−1 at 6 months and 29.4 ± 10.9 ng mL−1 at 1 year (p < .001, Figure 2). At both the 6-month and 12-month follow-ups, the serum 25-OH vitamin D levels in the vitamin D group were significantly higher than those in the placebo group (p < .001; Table 2).
| Placebo group (n = 15) | Vitamin D group (n = 19) | p-value | Odds ratio (95% CI) | |
|---|---|---|---|---|
| 6 months | ||||
| Vitamin D level | 12.0 ± 4.8 | 28.2 ± 6.5 | <0.001* | |
| Recurrence rate | 4 (26.7%) | 1 (5.3%) | 0.146a | 6.20 (0.52–339.10) |
| 12 months | ||||
| Vitamin D level | 13.8 ± 3.7 | 29.4 ± 10.9 | <0.001* | |
| Recurrence rate | 6 (44.4%) | 2 (9.5%) | 0.101a | 5.37 (0.76–65.10) |
| 12-month cumulative number of recurrences | 0.80 ± 1.37 | 0.13 ± 0.34 | 0.041* |
- *p value < 0.05.
- a Fisher's exact test.
3.3 Secondary outcomes: recurrence rate of BPPV
Table 2 shows the number of patients with recurrence at the 6- and 12-month timepoints after resolution of BPPV. The placebo group consistently showed a higher estimated proportion of patients with BPPV recurrence than did the vitamin D group. Nonetheless, there was no significant difference in the distribution of patients with and without recurrence between the two groups at any timepoint.
The cumulative odds ratios for BPPV recurrence in the vitamin D group stood at 6.20 and 5.37 at 6 and 12 months, respectively. Despite these numbers indicating a trend toward reduced recurrence in the vitamin D group, the differences were not statistically significant (Table 2). Furthermore, we conducted an analysis comparing the cumulative number of BPPV recurrences over 12 months across all subjects. Within this period, among the six participants in the placebo group who experienced BPPV recurrences, three had one recurrence, two had two recurrences, and one had five recurrences. Conversely, in the vitamin D group, each of the two participants who experienced recurrences had one recurrence. The frequency of recurrence in the placebo group was significantly higher than in the vitamin D group, indicating a statistically significant difference (Table 2).
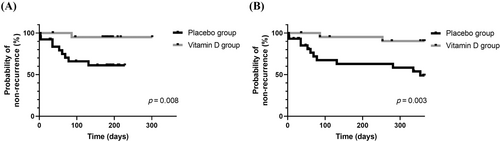
Further examination of BPPV recurrence was conducted via Kaplan–Meier survival analysis for each recurrent event. Figure 3 shows the first BPPV recurrences in each group at the 6- and 12-month timepoints. The vitamin D group showed a significant reduction in BPPV recurrence events compared to the placebo group at both the 6- (Figure 3A, p = .008) and 12-month follow-ups (Figure 3B, p = .003).
4 DISCUSSION
Our study assessed the recurrence rates of BPPV in correlation with serum 25-OH vitamin D levels following exclusive vitamin D supplementation, without the inclusion of calcium carbonate, in a randomized placebo-controlled trial with a 1-year follow-up. In the vitamin D group, the serum 25-OH vitamin D levels rose from an initial 13.3 ng mL−1 by an average of 11.1 ng mL−1 at the 6-month mark, demonstrating a statistically significant difference from the placebo group. Furthermore, although the recurrence of BPPV observed at both the 6- and 12-month timepoints was not statistically significant, the group receiving vitamin D supplementation exhibited a recurrence rate that was approximately 20%–30% lower than that of the placebo group. In the comparison of the 12-month cumulative recurrence numbers, the vitamin D supplement group showed significantly fewer recurrences. Moreover, Kaplan–Meier survival analysis, in which each recurrence was regarded as an independent occurrence, exhibited a significant variance in recurrences between the two groups.
BPPV is known to be affected by seasonal factors, specifically sunlight exposure, which in turn influences vitamin D levels.16 While sources other than vitamin D supplements, such as sun exposure, can impact an individual's vitamin D concentration, our study found no significant difference in sunlight exposure between the vitamin D group and the placebo group. Therefore, confounding factors related to vitamin D levels from sun exposure can likely be ruled out.
Studies presenting a model of normal otoconial formation have suggested that otoconial generation shares numerous similarities with bone matrix vesicle mineralization. This intricate process necessitates elaborate temporal and spatial control of developmental and biochemical events for normal formation.17 In addition, extreme hypercalcemia or hypocalcemia can alter ion content and cause abnormal mineralization and degeneration of otoconia. Vitamin D is a very important factor for calcium metabolism in the human body and is known to be deeply related to bone metabolism, as in osteoporosis.18, 19 In fact, vitamin deficiency in the elderly increases the risk of fractures, and vitamin D supplementation reduces the risk of fractures.19 Based on this physiology, many recent studies have reported that vitamin D is an important factor in the occurrence and recurrence of BPPV.10, 13, 14 One study comparing data from 100 idiopathic BPPV patients with 192 controls (individuals from the same community without prior issues of vertigo or imbalance) via the Korea National Health and Nutrition Examination Survey revealed lower serum vitamin D levels in the BPPV group. This discrepancy supported an association between reduced serum vitamin D levels and BPPV.10 Another retrospective analysis of 232 BPPV patients found that 41 patients (17.7%) who experienced a relapse during an average 10-month follow-up had lower serum 25-OH vitamin D concentrations when compared to those without any relapse. Notably, factors such as age, sex, follow-up duration, and BPPV type did not significantly influence the results.13
Based on these previous studies, some recent studies have also published results showing that supplementation of vitamin D in BPPV patients can prevent recurrence.15, 20, 21 The most recent meta-analysis study, in this regard, has shown a decrease in BPPV recurrence with vitamin D supplementation in patients with reduced vitamin D levels, although qualitative studies are still lacking.22 Consistently, our study also showed the same results. The serum 25-OH vitamin D levels had already increased to more than 24 ng mL−1 in the sixth month alone, and the vitamin D group had a recurrence rate of <5% until 6 and 12 months, which was lower than the placebo group that had a recurrence rate of more than 15%; the survival analysis results showed statistically significant differences.
To prevent BPPV recurrence, the roles of the concomitant administration of vitamin D and calcium versus vitamin D monotherapy have not yet been definitively established. Although animal experiments have demonstrated that vitamin D influences bone metabolism and also affects the metabolism of otoconia composed of calcium carbonate, thereby contributing to otoconial regeneration, the underlying mechanisms remain unclear.22 Our study successfully supplemented vitamin D deficiency through vitamin D monotherapy. This significantly reduced the recurrence rate of vertigo. Furthermore, in our study, neither the vitamin D group nor the placebo group reported any adverse effects. A previous study, which supplemented participants with calcium and vitamin D, noted two cases of adverse effects attributed to the calcium supplementation.21 Therefore, considering potential side effects, administering vitamin D alone appears to be preferable for preventing BPPV recurrence.
However, the specific effects and mechanisms by which vitamin D supplementation in vitamin D deficiency prevents BPPV recurrence are still unknown. A daily supplementation dose of 1000 IU of vitamin D is sufficient to replenish vitamin D deficiency.22 However, whether this dose is the minimum effective dose for preventing vertigo recurrence remains unknown. In non-randomized study designs, the reported minimum dosage of vitamin supplementation was 5000 IU.22
Our study had some limitations. First, the small sample size posed a constraint. Out of the 360 patients with BPPV evaluated at a single institution over 18 months, only those with subnormal serum 25-OH vitamin D levels among PC-BPPV and HC-BPPV patients were included. The rising global emphasis on the role of vitamin D in preventing various diseases has led many to take vitamin D supplementation, further limiting our sample size. Future endeavors should focus on multicenter prospective studies to overcome these challenges. Second, this study alone could not determine whether the vitamin D supplementation dose used was optimal for preventing BPPV recurrence. Our study supplemented patients with 7000 IU per week based on the recommended daily dose of 1000 IU for patients with subnormal serum vitamin levels. This was based on a study that showed no difference in average serum vitamin D levels of 13.0, 12.6, and 12.9 ng/mL after 3 months when patients with vitamin D deficiency were supplemented with 1000 IU per day, 7000 IU per week, and 30,000 IU per month.23 In our study, weekly supplementation with 7000 IU of vitamin D increased serum 25-OH vitamin D levels by 11.1 ng/mL at the 6-month timepoint compared to the placebo group, but no further increase was observed at the 12-month follow-up. Therefore, in future studies, it will be necessary to determine the target serum level that may have a better effect on the prevention of BPPV recurrence through measures, such as increasing vitamin D intake. However, to the best of our knowledge, this study is the first randomized, placebo-controlled trial to confirm the effect of vitamin D supplementation alone on idiopathic BPPV recurrence.
5 CONCLUSION
In this prospective randomized, double-blind, placebo-controlled clinical trial, vitamin D supplementation, in the absence of calcium, for treating vitamin D deficiency did not exhibit a significant difference in the recurrence rate of BPPV among the participants. However, there was a significant distinction in serum vitamin D levels between the vitamin D and placebo groups. Notably, vitamin D supplementation alone led to a marked reduction in the occurrence of BPPV recurrence events over a year when compared to placebo.
ACKNOWLEDGMENTS
This research (1801-06-06) was supported by Research Program for Clinical Professor of Myongji Hospital.




