A Review of Functional Properties and Applications of Legume-Based Edible Coatings
Funding: The authors received no specific funding for this work.
ABSTRACT
Legumes are rich in starch, proteins, lipids, vitamins, and minerals, making them a better source for developing films with high nutritional value. They have become a sustainable and eco-friendly coating to prolong the shelf life of perishable products while preserving both quality and safety. Legume-based polysaccharides and proteins are cost-effective materials to use as an alternative to synthetic materials. Additionally, legume-based edible coatings are gluten free and acceptable to consumers with dietary restrictions for coatings derived from nonplant sources. Their use in edible coating further extends the economic significance of legumes. The physicochemical properties of these coatings may vary based on several factors including composition of the film, conditions of processing, and additives used. Legume-based edible coatings and films hold the ability to enhance the shelf life and quality of food items by acting as barriers against oxygen, moisture, and solute transfer. Also, they can aid in maintaining the nutritional value, flavor, and sensory qualities of food products. Incorporation of bioactive compounds, such as antioxidants and antimicrobials, into legume-based coatings further enhances their functionality in food preservation. This article provides an in-depth review of the current state of research on legume-based edible coatings, focusing on their development, physical and mechanical properties, applications in food preservation, and future scope.
1 Introduction
The food science and technology community has increasingly focused on exploring edible coatings because of widespread concerns about food waste, environmental sustainability, and the demand for inventive packaging options. Nowadays, there is a growing collaboration between packaging and food industries to minimize packaging waste and adopt biodegradable materials as a sustainable substitution for synthetic polymers (Murrieta-Martínez et al. 2018). Edible packaging is a kind of packaging that is safe for human consumption. It refers to a thin layer that can either be applied directly onto the food surface or made as a separate thin film to wrap the food (Jeya Jeevahan et al. 2020). Edible coatings and edible films can be seen in similar terms depending on the situation. Edible coatings are generally applied directly to the surface of food items, whereas edible films are designed to be used independently as packaging materials. Unlike edible coatings, edible films have enough structural integrity to be used independently as packaging (Iversen et al. 2022). Edible films are created in advance as separate entities and later applied to the surface of the food, whereas coatings are formed directly onto surfaces (Nayik, Majid, and Kumar 2015).
Edible films and coatings are made out of edible biopolymers and additives that are safe for consumption (Farshi et al. 2023). Different biological components, including polysaccharides, proteins, and lipids, can be employed in edible films or coatings, either alone or in combination (Al-Sahlany 2017). Each material on its own can create films or coatings with unique properties. However, combining materials and incorporating plasticizers and surfactants is commonly employed to improve their ultimate characteristics, including flexibility, barrier properties, and optical qualities (Guimarães et al. 2018). Proteins and polysaccharides tend to create stiff and brittle films. Therefore, plasticizers are often required in conjunction with these polymers to produce flexible and easier-to-handle edible films. Plasticizers have low molecular weight, and they can reduce the distance between polymer molecules, decreasing the ratio of crystalline to amorphous regions in protein- and polysaccharide-based edible films (Farshi et al. 2023).
Switching to edible food packaging provides different advantages. It eliminates typical waste cycles, and this packaging can be used not only for food packaging but also for food preservation. Natural edible packaging protects foods without leaving any residues. Also, it can retain the nutritious content and the taste. Furthermore, edible packaging enhances organoleptic properties such as sweetness and color (Prakash and Mishra 2023). Edible packaging can maintain the quality and can prolong the shelf life (L. Kumar et al. 2022). Edible coating has a positive effect on external appearance. It reduces weight loss and maintains firmness while preserving the fresh appearance (Rojas-Graü, Soliva-Fortuny, and Martín-Belloso 2009; N. Kumar and Neeraj 2019; N. Kumar, Ojha, and Singh 2019; N. Kumar, Upadhyay, Shukla, Bajpai, et al. 2023) and texture (Ch Momin et al. 2021). Furthermore, edible coatings act as a protective shield, guarding against chilling injuries and storage disorders (N. Kumar, Pratibha, Prasad, et al. 2023). Moreover, edible films and coatings serve as active packaging, extending the shelf life of various food products by preventing dehydration, surface browning, oxidative rancidity, microbial growth, and oil diffusion (Silva-Weiss et al. 2013). The edible coating forms a physical barrier to food, which delays respiration, enzymatic reactions, moisture loss, and microbial growth through the controlled release of bioactive compounds (Seididamyeh et al. 2024). Additionally, these films and coatings can contain bioactive components such as antioxidants, exhibit antimicrobial effects against bacterial reproduction and fungal contamination, incorporate healthy microorganisms such as probiotics that benefit the consumer, and are made from biodegradable natural materials (Díaz-Montes and Castro-Muñoz 2021). Also, they provide a barrier against mechanical damage, such as dents or cuts (Díaz-Montes and Castro-Muñoz 2021). Moreover, the usage of edible coating and films helps us save on taxes imposed on the shipment of packaging material in some countries (Dhall 2013; N. Kumar, Pratibha, Prasad, et al. 2023; N. Kumar, Upadhyay, and Shukla 2023; N. Kumar, Pratibha, Upadhyay, et al. 2023).
These coatings and films are used as blockers of oxygen, moisture, and solute movement for food without making any changes to the original ingredients (B. Hassan et al. 2018; N. Kumar et al. 2020). Edible films and coatings can maintain the gas exchange between fresh food and its surroundings, thereby reducing ethylene production and respiration rates, preserving natural volatile flavor compounds, and enhancing the appearance of the food (Dhall 2013; N. Kumar, Pratibha, Neeraj, et al. 2021; N. Kumar, Pratibha, Trajkovska Petkoska, et al. 2021). Furthermore, it delays the surface dehydration, prevents loss of aroma, reduces oxidation of ingredients, and absorbs frying oil (N. Kumar, Neeraj, et al. 2021; N. Kumar, Ojha, et al. 2021; Farshi et al. 2023). The incorporation of active ingredients such as antioxidants and antibacterial agents into film formulation inhibits microbial spoilage and browning. Moreover, edible film and coating materials have the capability to form nanocarriers for encapsulating additives and regulating their release, providing a controlled delivery system for additives within the films and coatings (Wu, Wu, and Hu 2023).
Edible films and coatings have become increasingly popular in food protection and preservation because of their numerous advantages over synthetic materials, including cost-effectiveness (Paidari et al. 2021), biodegradability, and environmental friendliness (Ponce et al. 2008). These are consumable layers designed to act as barriers against moisture, gases, and solute movement in food products. Arnon-Rips and Poverenov (2018) reported that incorporation of water-insoluble/poorly soluble components into edible coating formulations decreases their water vapor permeability (WVP) and helps prevent weight loss. These films serve as excellent alternatives to traditional packaging materials because of their remarkable properties, including biocompatibility and edibility, and their wide range of applications (Maan et al. 2021). This literature review aims to comprehensively explore the status, properties, effect on different food quality, novel applications, and future scope of the legume-based edible coating and films. This review aims to enhance the comprehension of how legume-based coatings contribute to the advancement of sustainable packaging solutions and stimulate innovation within the food industry (Figure 1).
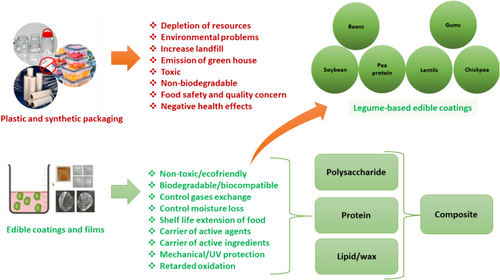
2 Legumes as Edible Coating Materials
Legumes are rich in starch, proteins, lipids, vitamins, and minerals, making them a better source for developing films with high nutritional value (Gupta, Biswas, and Roy 2022). They are highly significant in the human diet as the second most important crop, primarily because of their high protein content (17%–40%) (Saremnezhad et al. 2011). Legumes are cheap protein source with considerable nutritional value, making them excellent material for producing protein films (Saremnezhad et al. 2011).
Legume grains contain a higher amount of complex carbohydrates (including dietary fiber and resistant starch) and proteins, while generally having a low fat content (less than 7%) (Huamaní-Perales et al. 2024). Their high fiber content makes them a promising option for reducing the glycemic impact of staple foods and may also enhance feelings of fullness (Amoah et al. 2023). Legumes provide health benefits because of their biologically active compounds, including polyphenols and both soluble and insoluble fibers (nondigestible oligosaccharides). Polyphenols, such as phenolic acids and flavonoids, have antioxidant effects that safeguard tissues against oxidative stress (Aguiló-Aguayo et al. 2021; Perović, Pajin, and Antov 2022). Soluble fiber enhances the viscosity of intestinal contents and slows the absorption of monosaccharides, thereby reducing spikes in blood glucose levels (Amoah et al. 2023). Studies have shown that their antioxidant properties and ability to lower blood glucose and cholesterol levels can significantly help in preventing cardiovascular diseases, diabetes, and obesity. Additionally, the dietary fiber in legumes enhances the health of the intestine and reduces the risk of colon and digestive tract cancers (Huamaní-Perales et al. 2024). Moreover, legumes possess a range of nutritional qualities that make them ideal candidates for use as primary components in bio-based films (Moghadam et al. 2020).
Legume flours are a valuable material for film formation because they are rich in starch and protein, with some varieties also containing significant amounts of lipids (Díaz et al. 2019). Additionally, the characteristics of leguminous proteins have the potential to produce edible films (Linares-Castañeda et al. 2023). Most leguminous starch is characterized by high amylose content (Saberi et al. 2016). Generally, an increase in the pH of leguminous protein film–forming solutions leads to higher tensile strength and more excellent elongation of the resulting films (Kowalczyk et al. 2014).
2.1 Soybean
The protein content of soybeans (Glycine max) is around 38%–44%. Soy proteins consist of two significant fractions, 7S (conglycinin) and 11S (glycinin). Both fractions contain cysteine residues crucial for disulfide bridge formation, akin to gluten, thus exhibiting similar mechanical properties (Dhall 2013). Soy protein has the potential to develop as an edible and biodegradable film because of its characteristics, such as being abundant, inexpensive, biodegradable, and nutritious (Wittaya 2012).
Soybean-based films are traditionally made from soy milk, which is a water extract of soybeans. Soy protein films are typically produced using soy protein isolates (SPIs). The structure of SPI films is created through disulfide, hydrophobic, and hydrogen bonds. Research indicates that SPI films are effective barriers against oxygen and lipids. However, their ability to prevent water passage is limited because of their hydrophilic nature (Park et al. 2013). SPI is mainly used to make film-forming solutions (Prakash and Mishra 2023). SPI is considered the most common plant protein, derived as a refined form from soybean oil extraction. It is a blend of albumins and globulins (F. Hassan, Mu, and Yang 2024). Films that are made from SPIs are applied to precooked meat products to control the lipid oxidation and moisture loss from the surface. Furthermore, it can be applied to fruits, vegetables, and cheese coatings as well (Prakash and Mishra 2023). Moreover, they have the potential to carry flavors, antimicrobial compounds, and antioxidants (Prakash and Mishra 2023). Research indicates that the presence of covalent disulfide bonds within SPI is essential in determining the structural integrity of SPI films than the influence of hydrophobic forces. Furthermore, SPI exhibits notable stability under high temperatures and humidity conditions (F. Hassan, Mu, and Yang 2024). The ability to form a gel is valuable for producing appropriate structures for composite films containing lipids and bioactive substances like antioxidants and antimicrobial agents (A. Kumar, Hasan, et al. 2022). SPIs can generate flexible and transparent edible films with outstanding barriers against oxygen and lipids, whereas films derived from natural soy proteins tend to be brittle and exhibit inadequate WVP (Nandane and Jain 2018).
SPI films exhibit limited resistance to water vapor transfer, primarily because of their inherent protein hydrophilicity (Nandane and Jain 2015). SPI films show different properties, including flexibility, transparency, and low oxygen permeability compared with low-density polyethylene films. Additionally, they are easily available and cost-effective (A. Kumar, Hasan, et al. 2022). Because of their hydrophilic nature and the considerable use of hydrophilic plasticizers in their preparation, soy protein films exhibit poor moisture barrier properties (Kokoszka et al. 2010).
2.2 Pea
Pea proteins and starch can be used to develop edible packages (Van Soest et al. 2002). Pea proteins are primarily composed of globulins, with a small portion of albumins. Globulins make up about 80% of the total proteins in peas and are storage proteins found in the cotyledons (Choi and Han 2001). Pea starch (PS) is composed of two types of homopolymers. They are linear amylose and highly branched amylopectin. These polymers are made up of D-glucose units connected by two types of linkages, α-(1 → 4) for the main chain and α-(1 → 6) for the branch chains (Q. Liu 2005). The starch of peas contains a higher amount of amylose, and it can be developed as an edible film (Van Soest et al. 2002).
Pea protein concentrate films exhibited similar physical and mechanical properties at a lower cost and had excellent commercial potential (Choi and Han 2001). The structural properties depended on the PS source, content of the plasticizer, and processing conditions (Van Soest et al. 2002). The strength, stiffness, and elongation depended on the amylose content. High-amylose PS films possess outstanding oxygen barrier properties along with exceptional stretchability (Mehyar and Han 2004). Hopkins et al. (2019) found that pea protein is promising for future film applications because of its high tensile strength and elongation, as well as its low WVP and low opacity.
2.3 Lentils
The primary component of lentils (Lens culinaris) is carbohydrates, with starch making up a significant portion and accounting for almost 60% of their weight (Aydogdu et al. 2018). The protein content in lentil seeds typically ranges from 20% to 25% on average (Bamdad, Goli, and Kadivar 2006). Approximately 70% of the protein content in lentils consists of globulins, specifically 7S vicilin and 11S legumin. All lentil protein fractions are glycosylated, with vicilin being particularly rich in glycosylation (Joehnke et al. 2021).
The concentrations of glycerol and processing temperature directly affected the physical properties of lentil flour based biodegradable edible films (Aydogdu et al. 2018). The findings suggested that lentil flour is a promising material for the production of edible films, as it produced films with poor water solubility, high transparency, and good thermal stability (Aydogdu et al. 2018). By adjusting the film formulations and production methods, the researchers demonstrated that adding glycerol improved flexibility and hydrophilicity. Furthermore, using a higher processing temperature of 90°C led to films that were more mechanically robust and had lower WVP (Aydogdu et al. 2018).
Lentil starch demonstrates much stronger gel formation characteristics (Joshi et al. 2013). The promising aspect of lentil protein films is that they showed good mechanical properties and were effective in blocking moisture, which bodes well for lentil flour films (Aydogdu et al. 2018). Additionally, the purity of proteins, molecular weight, structure, and charge could affect protein–protein interactions, thus modifying the mechanical properties of films, such as tensile strength (Hopkins et al. 2019).
2.4 Chickpea
Chickpea (Cicer arietinum L.) is high in proteins, carbohydrates, and dietary fiber. The primary proteins in chickpeas are globulins, although albumin, as well as minor amounts of glutelin and prolamin, are also present. These proteins exhibit low solubility near their isoelectric point (pH 4–6) but become more soluble in alkaline conditions. Thermal denaturation usually starts at around 65°C for the most heat-sensitive globulins (Díaz et al. 2019). Starch is the main carbohydrate in chickpeas, making up 40%–44% of the dry weight. It comprises 30%–35% total amylose, with a midpoint gelatinization temperature between 64°C and 73°C in flours (Meares et al. 2004). Both chickpea starch and proteins exhibit advantageous properties for emulsifying, foaming, and pasting. Additionally, chickpeas contain bioactive substances like peptides and phenolic compounds, which have antioxidant properties. These attributes make chickpea flour a promising candidate for producing edible films (Díaz et al. 2019).
2.5 Faba Beans
Faba bean (Vicia faba L.) holds significant promise as a high-quality protein source, potentially containing up to 30% protein content (Montalvo-Paquini et al. 2014). The proteins in faba beans are predominantly globulins, comprising 80% of the total, which include 11S legumin and 7S vicilin/convicilin (Warsame et al. 2020). Films made from faba bean proteins tend to have reduced thickness, but the addition of glycerol improves their tensile strength and water vapor barrier properties (Linares-Castañeda et al. 2023). Moreover, films made from faba bean protein isolate and carrageenan exhibited improved compatibility between the macromolecules. This was achieved by adjusting the pH and protein denaturation temperature, which enabled its reactive sites to interact with carrageenan, leading to form stable structures (Castaño et al. 2017).
2.6 Mung Bean
Mung bean (Vigna radiata L.) contains approximately 25%–28% protein and is a source of bioactive compounds such as flavonoids, phenolic acids, organic acids, and polysaccharides (Moghadam et al. 2020). It is a starch-rich food, and mung bean starch (MBS) has a high amylose content ranging from 30% to 45% (Deshmukh et al. 2022). MBS forms dense and cohesive film networks because of its high amylose content. This high amylose content contributes to forming a transparent dried product, distinguishing it from other starches.
The linear structure of the amylose promotes the bonding of interchain hydrogen more effectively compared with branched structure of amylopectin. A relatively linear and long backbone of amylose chains, combined with the close proximity of starch chains with high amylose content, supports the development of cohesive film networks during the drying process. As a result, films made from amylose are denser and stronger compared with films made from amylopectin (Rompothi et al. 2017). Films containing higher levels of amylose typically exhibit higher values of glass transition temperature (Tg), tensile strength (TS), and modulus of elasticity (EM), while showing lower elongation (%E) compared with films with lower amylose content (Muscat et al. 2012). Gelatinized MBS forms a clear gel when cooled, leading to the production of transparent dried products (Rompothi et al. 2017).
2.7 Beans
The protein content of beans (Phaseolus vulgaris L.) ranges from 22% to 32% based on the variety. Around 75% of this protein content consists of globulins, specifically phaseolins, which serve as both the main storage proteins in seeds (Montalvo-Paquini et al. 2018). Beans are a cost-effective protein source with a high nutritional value because of their higher levels of essential amino acids such as lysine, tyrosine, and phenylalanine (Graham and Vance 2003). Therefore, beans are considered an alternative raw material source for producing protein-based edible films (Montalvo-Paquini et al. 2018).
2.8 Kidney Beans
Kidney bean, which contains 20%–30% protein, is an important bean among different beans (Shevkani and Singh 2015). On a dry weight basis, kidney beans usually contain protein ranging from 20% to 30% (Sathe 2002). Recently, there has been considerable interest in the storage proteins of this bean, because of their exceptional properties, such as emulsification properties, protein solubility, and their ability to form gels when exposed to heat (Ma et al. 2013). Tang et al. (2009) studied the film-forming properties of kidney bean protein isolates (KPIs) at a neutral pH. According to the results, the KPI films had significantly lower tensile strength and elongation at break compared with the soy protein films.
2.9 Guar Gum (GG)
GG (Cyamopsis tetragonoloba) is a linear galactomannan extracted from the endosperm of the guar bean (Prajapat and Gogate 2015). It is a polysaccharide with high molecular weight, and it possesses significant water absorption and cross-linking abilities (Rao et al. 2010). GG is known for its exceptional surface, interfacial, and emulsification activities (Prabaharan 2011). GG is a promising biopolymer for the development of food coatings. It has a good capacity to form films, biocompatibility, and biodegradability, making it an attractive option for these applications (Jiang et al. 2023).
According to Prajapat and Gogate (2015), GG has a mannose:galactose ration of 2:1. It has a molecular structure consisting of a backbone of β(1 → 4)-linked mannopyranose units. It features multiple branch points originating from the C-6 position of mannopyranose, which is linked by α(1 → 6) bonds to single D-galactopyranose sugars (Saberi et al. 2018). The presence of D-galactose units in the side branches of GG is crucial for its solubility and properties. These galactose units engage with water molecules, creating strong hydrogen bonds. These bonds play a crucial role in the high viscosity and thickening characteristics of GG solutions (Sharma et al. 2018). It can function as an edible coating similar to carrageenan, alginate, xanthan gum (XG), and gum Arabic; however, it is economically important (Ruelas-Chacon et al. 2017).
GG has become a promising material for the development of edible coatings because of its desirable properties. In the evaluation of GG-based films, attention is paid to both their physical and functional characteristics. Physical characteristics of GG-based films include thickness, mechanical strength, optical properties, color, gas permeability, and structure. These properties are essential for understanding the film's structure and behavior (Jiang et al. 2023). Efforts have been made to improve various attributes, including mechanical properties (tensile strength, elongation at break, and Young's modulus), barrier properties (ultraviolet-shielding capacity, moisture barrier property, and oxygen barrier property), and thermal stability. Strategies for improving these properties include modifying GG, blending it with other biopolymers, incorporating nanoparticles, and adding phytochemicals (Jiang et al. 2023).
2.10 Gum Arabic
Gum arabic is a dry, sticky substance extracted from the stems and branches of Acacia species. Gum arabic exhibits lower viscosity and higher solubility compared with other hydrocolloids (Ali et al. 2010). Elmanan et al. (2008) found that the commercially used gum is derived from Acacia senegal because of its excellent emulsification properties. Its widespread utilization in various industries is due to its capabilities in emulsification, film formation, and encapsulation. It is composed of a diverse blend of macromolecules with varying sizes and compositions. Gum arabic is primarily made up of carbohydrates (about 97%), mainly consisting of D-galactose and L-arabinose units, with a small amount of proteins (less than 3%) (Daisy et al. 2020). Furthermore, gum arabic has an ability to prolong the shelf life (Patel and Goyal 2015).
2.11 Locust Bean Gums (LBGs)
LBG is a white powder derived from milling the seed endosperm of the carob tree (Ceratonia siliqua L.) (Barak and Mudgil 2014). It is a polysaccharide extensively employed in the food industry for its functions as a stabilizer, viscosity modifier (Rojas-Argudo, del Río, and Pérez-Gago 2009), and dispersion agent (Aydinli and Tutas 2000). LBGs contain a linear chain of β-D-mannopyranosyl units linked to the main chain through 1,4 bonds, with α-D-galactopyranosyl units linked to the main chain via 1,6 bonds at side branches.
The most significant characteristic of this polysaccharide polymer is its ability to exhibit high viscosity in aqueous solutions across a wide range of pH levels and temperatures (Aydinli and Tutas 2000). The exceptional biodegradable nature, rheological attributes, and film-forming capabilities of LBG make it well suited for packaging film development (Yuan et al. 2020). It possesses the capability to create viscous solutions even at low concentrations and to generate compact and dense films and coatings exhibiting favorable mechanical strength and water vapor barrier characteristics (Mostafavi et al. 2016).
Kurt, Toker, and Tornuk 2017 found that a combination of LBG and XG is a potential polysaccharide source for producing films with preferred qualities. There is a synergistic interaction between LBG and κ-carrageenan that can enhance the appearance, structural integrity, mechanical strength, and barrier properties of the film (Martins et al. 2012) (Table 1).
| Legume | Introduced compounds/additive | Film/coating production conditions | Property enhancements | Reference |
|---|---|---|---|---|
| Soy protein isolate (SPI) | Glycerol | SPI was mixed with distilled water. Glycerol was used as the plasticizer, and the pH was adjusted with 1 N of sodium hydroxide. The solution was heated at 85 ± 5°C for 15 ± 5 min and then allowed to sit for 5 min to dissipate bubbles before pouring. | Increasing the SPI concentration led to higher film thickness and tensile strength. Young's modulus and elongation at break were decreased. Conversely, higher plasticizer concentrations resulted in lower thickness and tensile strength. | Nandane and Jain (2015) |
| Glycerol | SPI (6%–9% w/w) dissolved in distilled water with 700-rpm stirring. Glycerol (40%–70% w/w SPI) was incorporated as the plasticizer. Sodium hydroxide (1 M) was used to adjust the pH to 7.0 ± 0.1 and then heated at 70°C for 20 min at 200 rpm. | Changing the SPI and glycerol proportions affected the WVP, wetting, and thermal properties. Higher SPI content slightly increased WVP but did not alter surface properties. | Kokoszka et al. (2010) | |
| Soy protein isolate (SPI) | Rapeseed oil | SPI powder was dissolved in distilled water at a concentration of 10% (w/w) while being stirred at 250 rpm. The pH was adjusted to 10 ± 0.1 using 1 M of sodium hydroxide. The solution was heated to 70 ± 1°C for 20 min and subsequently cooled to 23 ± 1°C. Glycerol, at a concentration of 50% (w/w), was added as a plasticizer. Rapeseed oil, at concentrations of 0%, 1%, 2%, and 3%, was then homogenized into the soy protein isolate solution using an Ultra-Turrax homogenizer at 13,500 rpm for 5 min. | Decrease in WVP and tensile properties | Galus (2018) |
| Guar gum (GG) | Carboxymethyl cellulose (CMC), halloysite nanotubes (HNTs), litchi shell extracts (LSE) | HNTs (0.25 g) were dispersed in 100 mL of water. Then, GG (0.50 g) and CMC (0.25) were added, and the mixture was stirred for 4 h at 27°C. Glycerol (0.75 g) was added as the plasticizer, followed by LSE (1%–20% v/v). The mixture was stirred at 50°C, sonicated for 20 min, cast into Petri dishes, and dried at 50°C for 16 h. | Improved mechanical properties such as film thickness, elongation at break, ultraviolet barrier property, and opacity | Deshmukh et al. (2022) |
| Polylactic acid (PLA), thyme essential oil | GG (0.5% w/v) was dissolved in distilled water at 25°C, whereas PLA (15% w/v) was created in a 1:1 v/v ethyl alcohol and acetic acid solution. The PLA and GG solutions were combined in ratios of 95:5, 90:10, and 85:15 and mixed for 30 min. Thyme essential oil, amounting to 10% and 30% of the total polymer weight, was then encapsulated in the mixture. | Improved thermal stability and elongation at break | Yavari Maroufi et al. (2021) | |
|
Sago starch (SS) Carvacrol and citral |
A colloidal dispersion of 2.5% SS was formed by heating 2.5 g of SS in 97.5 mL of water at 90°C for 30 min. Similarly, a 0.20% GG dispersion was prepared. These dispersions were combined in equal parts by weight and stirred for 60 min at 500 rpm, after which glycerol (40% w/w of the biopolymer) was incorporated. The mixture was then sonicated for 10 min. Films containing essential oils, specifically carvacrol (0.75%) and citral (1.0%), were created by homogenizing the blend at 10,000 rpm for 10 min. The emulsions were cast into Petri dishes and dried at 26°C and 50% relative humidity for 72 h before analysis. |
Enhanced thickness, opacity, elongation at break, and puncture strength Reduced solubility and WVP |
Dhumal et al. (2019) | |
| Tamarind seed polysaccharide (TSP) and old corrugated box (OCB)–based cellulose nanofiber (OCB-NF) | A 0.5% TSP solution and a 0.5% GG solution were each prepared in distilled water. These solutions were combined and mixed for 3 h to form a blend. After sonication, varying concentrations of OCB-NF (0.1%–1% w/v) were added. Glycerol (0.5% w/w) and citric acid (0.1–0.3 M and 1.5–3.5% v/v) were then incorporated into the mixture. The mixture underwent polycondensation by being heated to 80°C for 30 min, then cooled, and poured into Teflon molds. The resulting films were dried at 65°C for 48 h. |
Improved Young's modulus, tensile strength, and thermal stability Reduced WVP |
Lal and Mhaske (2021) | |
| Pea protein isolate (PPI) | Sorbitol | Films were created using 10% (w/w) PPI solutions, with plasticizers comprising 3%–7% glycerol or 4%–8% sorbitol. The pH was adjusted to 7.0, 9.0, or 11.0, and the mixtures were degassed under vacuum. Eleven grams of the solution was poured onto polystyrene Petri dishes for casting and dried at room temperature for 12 h. | Sorbitol-plasticized films showed significantly higher tensile strength, elastic modulus, puncture strength, and elongation at break. | Kowalczyk et al. (2014) |
| Denatured pea protein concentrate (PPC) | Glycerol | PPC films were prepared from 10% aqueous solutions with glycerol (80/20 to 50/50 PPC/Gly). Solutions were vacuum degassed, heat-treated at 90°C for 25 min, and cooled. |
Exhibited strength, elasticity, and a favorable moisture barrier property, maintaining its physical integrity. Films with high strength and stretchability were achieved using a PPC/glycerol ratio of 70:30 and 60:40, respectively. WVP value was maintained across a glycerol concentration range of 20%–40% in the dry film. |
Choi and Han (2001) |
| Pea starch (PS) | Glycerol | A 3% (w/w) PS dispersion was prepared in water, with glycerol added in a 1:2 (w/w) ratio relative to the starch. The solutions were continuously stirred and heated until boiling and then maintained at boiling for 15 min to allow full gelatinization of the starch granules. Subsequently, the film-forming solutions were allowed to cool to approximately 60°C, then poured into Petri dishes, and left to solidify at room temperature. | The vapor permeability ranged from 130 to 150 g mm/m2/d/kPa. Oxygen permeability was notably low, less than 0.5 cm3/μm/m2/d/kPa at relative humidity levels below 40%, and slightly increased (1.2–1.4) at 45% RH. These films exhibited reduced water solubility, approximately 32.0%. | Mehyar and Han (2004) |
| Field pea protein isolate (FPPI) | Glycerol | Films were produced from a 10% (w/w) protein isolate solution combined with 4% (w/w) glycerol. The mixtures were stirred for 10 min, and then the pH was adjusted to 7.0, 8.0, or 9.0, followed by an additional 10 min of stirring. The solutions were then degassed, poured into 100 × 100-mm Teflon molds (each containing 9.5 g of the mixture), and incubated at 40°C for 24 h. | Demonstrated a light color, higher solubility, and higher surface charge; furthermore, resulted in higher tensile strength and transparency | Shevkani and Singh (2015) |
| Green pea pod extract (GPPE) | Chitosan | Two grams of chitosan powder was dissolved in 100 mL of 1% glacial acetic acid. Glycerol was added at 30% w/w, as a plasticizer. The mixture was then heated in a shaking water bath at 60°C and 100 rpm for 30 min and subsequently filtered to eliminate any undissolved impurities. After cooling to room temperature, EPPE was added to achieve concentrations of 0%, 1%, 3%, and 5% (w/v). The solutions were homogenized and degassed using a sonicator to eliminate air bubbles. Each solution was cast onto a ceramic plate and left to dry and then conditioned for 48 h at 25°C and 50% relative humidity before testing. | The incorporation of GPPE increased the film thickness from 0.132 ± 0.08 to 0.216 ± 0.08 mm and the density from 1.13 ± 0.02 to 1.94 ± 0.02 g/cm3. It also enhanced the opacity from 0.71 ± 0.02 to 1.23 ± 0.04. Barrier properties were notably improved, with a reduction in WVP from 2.34 to 1.08 × 10−10 g−1 s−1 Pa−1, a decrease in water solubility from 29.40 ± 1.23% to 18.75 ± 1.94%, a decrease in oil absorption ratio from 0.31 ± 0.006% to 0.08 ± 0.001%, and a reduction in the whiteness index from 88.10 ± 0.43 to 77.53 ± 0.48. Films exhibited enhanced tensile strength reaching a maximum of 26.87 ± 1.38 MPa and improved flexibility with an elongation percentage of 58.64 ± 3.00%. Biodegradability also increased. | Elsebaie et al. (2023) |
| Lentil flour | Glycerol | Aqueous solutions with 5% w/v lentil flour were stirred for 10 min at 500 rpm. Pure glycerol was added to reach final concentrations of 1.0%, 1.5%, and 2.0% w/v, with further stirring for 10 min. The pH was adjusted to 10.7 with 0.1 M of NaOH. Solutions were heated at either 70°C or 90°C for 30 min and then degassed via ultrasonication at 40 kHz for 10 min. Ten milliliters of the solution was poured onto LDPE Petri plates and dried at 40°C for 16 h until films could be easily peeled off. | Lentil flour films were highly transparent with low water solubility and WVPs and mechanical properties similar to other biodegradable films. Higher concentrations of glycerol produced more flexible, hydrophilic films. Films heated at 90°C were yellower, more transparent, and stiffer than those heated at 70°C. | Aydogdu et al. (2018) |
| Lentil protein concentrate (LPC) | Glycerol | A film-forming solution was prepared by dissolving 5 g of LPC in 100 mL of water with continuous stirring. Glycerin, at 50% w/w relative to LPC, was incorporated as a plasticizer. The pH was then adjusted to 11.0 ± 0.1 using 1 N of NaOH. The mixture was heated up to 70°C for a set period, filtered through cheesecloth, and then cast onto Teflon-coated glass. The film was allowed to dry at 60°C for 7 h before being peeled off. |
Resulted in a strong and elastic film. It has good moisture barrier properties and maintains good physical integrity, with tensile strength (TS) comparable with that of other edible films and an elongation at break (%E) that is higher than that of cellophane. Enhanced puncture suggests that it is cohesive but has a higher WVP. |
Bamdad, Goli, and Kadivar (2006) |
| Glycerol | Four percent (w/w) protein concentrate was dissolved in Milli-Q water to prepare the film-forming solution. Glycerol was added at varying levels of 50%, 75%, or 100% (w/w) relative to the protein. The mixture was stirred at 500 rpm for 1 h. To adjust the pH to 9.0, 0.5 M of NaOH was used. Then, solutions were degassed in an ultrasonic bath for 10 min and then heated to 90°C while stirring continuously. After heating, the solutions were stirred for an additional 5 min before being cast into PTFE molds and left to air-dry overnight at room temperature. | Increased thickness with higher plasticizer concentrations and reduced tensile strength | Hopkins et al. (2019) | |
| Lentil protein isolate (LPI) | Corn zein | With continuous stirring for 25 min, 1.4 g of zein was dissolved in 8.2 mL of 96% ethanol. Glycerol (0.4 mL) was added as a plasticizer, and the solution was brought to a boil for 5 min to denature the protein. After cooling, LPI at concentrations of either 130 mg g−1 of zein was added. Finally, the solution was homogenized at 10,000 rpm for 4 min. | Reduced tensile strength values and Young's modulus | Boyacı and Yemenicioğlu (2018) |
|
Cassava starch Glycerol |
Dissolved in distilled water were 4.5 wt% starch, 1.5 wt% glycerol, and proteins in 94 wt%, and the mixture was stirred for 40 min at room temperature. Then, the solution was heated to 80°C for 20 min to achieve gelatinization, followed by degassing for 7 min. The dispersions were poured into Petri dishes and dried at 50°C for 24 h. | Reduced moisture level, water solubility, WVP, Young's modulus, and strength at break | Ochoa-Yepes et al. (2019) | |
| Chickpea flour | Glycerol | Film-forming solutions were prepared by stirring chickpea flour in water at a concentration of 6 g/100 mL for 30 min at 20°C. Glycerol was added at concentrations of 3% and 1% (w/v). The pH was then adjusted to either 7.0 or 10.0, and the dispersions were stirred for an additional 30 min before being heated to 80°C for 20 min. The solutions (0.24 g/cm2) were poured onto silicone plates and dried at 35°C for 20 h. The films were conditioned at 20°C and 50% relative humidity for 48 h and then peeled off and stored in desiccators at 50% relative humidity for testing. | Films containing 1% glycerol showed reduced WVP, thickness, radical scavenging capacity, and elongation at break. However, increased dry matter content, swelling, opacity, elastic modulus, and tensile strength when compared with films with 3% glycerol. | Díaz et al. (2019) |
|
Glycerol Gallic acid |
A chickpea flour film-forming solution (7.5% w/v in 100 mL of water) was prepared, with an initial moisture content of 9.01%. Three grams of chickpea flour was dried at 105°C. The solution was stirred at 1000 rpm for 20 min, the pH was adjusted to either 9 or 11, and the mixture was heated in a water bath at 90°C for 30 min. After cooling to 45°C–50°C, gallic acid (at 5% and 10% w/w) and glycerol (at 1% and 3% w/v) were added. The solution was homogenized at 10,000 rpm for 3 min. An ultrasonic water bath was set at 40 kHz for 30 min, and it was used to remove the air bubbles. Six grams of the solution was cast in Petri plates, dried at 30°C for 18 h, and conditioned at 52% relative humidity for 24 h. | Demonstrated reduced water vapor permeability, increased antioxidant capacity, and strong mechanical characteristics | Kocakulak, Sumnu, and Sahin (2019) | |
| Chickpea protein isolate (CPI) |
Pullulan Glycerol |
A 2% (w/v) CPI solution was made by dissolving CPI in distilled water and stirring the mixture for 12 h at ambient temperature. Then the solution was heated up to 90°C for 30 min at pH 8.0 before being cooled to 25°C. CPI was combined with native and modified pullulan solutions (DS 0.02 and 0.06) using an Ultra-Turrax at 15,000 rpm for 2 min. Glycerol, constituting 30% of the total dry weight, was added, and the mixture was subjected to ultrasound treatment at 100 W for 10 min. The pH was adjusted to 7.0 using 1 M of HCl. The resulting mixtures were cast onto polystyrene plates and allowed to dry at 35°C for 24 h. Subsequently, they were conditioned in a desiccator with saturated MgCl2 at room temperature for 2 days. |
Increased tensile strength Reduced solubility, moisture content, and WVP |
Maedeh et al. (2021) |
| Kidney pea protein isolate (KPI) | Chitosan | Films were produced by solution casting at pH 3.0. Chitosan (1%) was dissolved in 0.1 M of lactic acid and left to disperse overnight with gentle stirring, whereas KPI (5%) was mixed in deionized water and stirred at room temperature. Glycerol was added to both solutions at a 2:10 w/w ratio. After adjusting to pH 3.0 with 6 M of lactic acid, chitosan and KPI solutions were mixed to achieve various CH ratios (0–40 wt%). The mixtures were heated at 80°C for 10 min, cooled, degassed, and cast onto polyethylene-coated glass plates. Films were air-dried at 25°C and 50% RH for 48 h and then peeled. | Resulted in less rigid and flexible films with low elastic modulus and glass transition temperature | Ma et al. (2013) |
| Faba bean protein isolate (FPI) | Glycerol | Five grams of FPI was dissolved in 100 mL of distilled water with constant stirring. The pH was adjusted to 7, 9, or 12 using 1 M of NaOH. Glycerol was added at 40%, 50%, and 60% w/w of FPI. The mixture was strained through cheesecloth and poured for casting on Teflon-coated glass plates and then dried at 25°C and 50% RH for 12 h. Films were conditioned at 25°C and 50% RH for 48 h before testing. |
Improved tensile strength, elongation, and solubility of the films with the increasing pH. Decreased WVP and lightness of the films. The lowest WVP and the highest tensile strength were achieved at a pH of 12 with a glycerol concentration of 40% (w/w of FPI). |
Saremnezhad et al. (2011) |
| Faba bean protein concentrate (FPC) | Glycerol | Three percent (w/v) FPC was dissolved in distilled water with continuous stirring. To adjust pH to 7.0, 8.5, and 10.0, 1 N of NaOH was used. Glycerol at 1.5% w/v was added as a plasticizer. After homogenizing the solutions at 9000 rpm for 1 min, they were degassed under vacuum, heat-treated at 80°C for 20 min, and then cooled to 37°C. A 4-mL portion of each solution was spread onto silica trays and allowed to dry at 40°C for 18 h. The films were then kept in desiccators at 50% RH for 48 h. |
Showed better physical integrity compared with other legumes' protein-based films Decreased WVP and increased puncture strength and elongation in alkaline conditions |
Montalvo-Paquini et al. (2014) |
| Faba bean protein isolate (FPI) |
Cellulose nanocrystals (CNCs) Glycerol |
FBP films were produced through solution casting. For the control films, 5% (w/v) FBP and 50% (w/w of FBP) glycerol were dissolved in distilled water. To adjust the pH to 10.5, 1 M NaOH was used, which was stirred for 1 h. The solution was then heat-denatured at 85°C for 30 min, allowed to cool to room temperature, and poured into polystyrene Petri dishes. For the CNC-reinforced films, CNCs (1, 3, 5, and 7 wt% of FBP) were incorporated after the denaturation step and stirred for 30 min. All films were dried at 40°C for 24 h, peeled out from the dishes, and conditioned at 52% relative humidity and 25°C for 48 h before analysis. |
Tensile strength was magnified with the increasing CNC concentration. WVP was reduced with the lowering of CNC content. Application of CNC enhanced the thermal stability of FBP films. |
Rojas-Lema et al. (2021) |
| Mung bean proteins (MBP) | Sorbitol, glycerol, and polyethylene glycol-400 | Freeze-dried mung bean proteins were dissolved in distilled water (3 g/100 mL) to prepare film solutions. pH was adjusted to 9.5. Then, plasticizers (sorbitol, glycerol, polyethylene glycol-400) were added at 30%, 40%, 50%, and 60% of protein. Components were homogenized at 10,000 rpm for 2 min and heated at 75°C for 30 min. The mixture was cooled to ambient temperature, vacuumed to remove air, and poured onto nonstick trays to set. Trays were kept overnight at 55°C, cooled to room temperature, and films were peeled off. | The increasing plasticizer concentration decreased tensile strength and increased elongation at break and WVP. | Wittaya (2013) |
| Sorbitol | Three grams of freeze-dried mung bean proteins (93.52%) was dissolved in distilled water (100 mL) to prepare the film solution. The pH was adjusted to 8.0, 9.0, or 10.0, and sorbitol was added at a protein-to-sorbitol ratio of 2:1. Components were homogenized at 10,000 rpm for 2 min and heated at 60°C, 70°C, or 80°C for 10, 20, or 30 min, respectively. The solution was cooled to room temperature, vacuumed to remove air, and poured onto nonstick trays to set. Trays were held overnight at 55°C and then cooled to ambient temperature, and films were peeled off. Films were stored in plastic bags in a desiccator at 60% RH for testing. | At pH 9.5 and 75°C, tensile strength was highest but low in elongation at break. Also, WVP, film solubility, and protein solubility were at their lowest. | Bourtoom (2008) | |
|
Glycerol Pomegranate peels |
Mung bean protein solution (4% w/v) was prepared in distilled water, pH adjusted to 8.0 with NaOH, and stirred overnight. The solution was heated at 85°C for 30 min to denature the protein and then cooled to 25°C. Pomegranate peel powder (0, 2.5, 12.5, and 25% w/w of dry protein) was added and stirred for 2 h. The mixture underwent ultrasound (100 W, 5 min) for solubility and then centrifuged (4000 rpm, 10 min) to remove large particles. The supernatant was mixed with glycerol (50% w/w of protein) as a plasticizer, stirred, and degassed. Fifteen milliliters of the solution was cast on Petri dishes and dried at 40°C for 12 h. Dried films were conditioned at 25°C and 53% RH for 24 h. | Increased the thickness, WVP, water contact angle, tensile strength, and flexibility | Moghadam et al. (2020) | |
| Mung bean proteins (MBS) | Glycerol | Mung bean starch (3.5%–5.0% w/w) was dispersed in distilled water. Glycerol (0%–30% w/w) was added as a plasticizer. The mixture was stirred at 750 rpm and heated at 85°C for 30 min to gelatinize the starch. It was then cooled to 50°C and sonicated for 20 min to remove bubbles. The 40 mL of casting solution was poured onto an acrylic plate and dried at 35°C for 20 h. The films were peeled and stored in a desiccator at 48.23% RH and 28 ± 2°C for 48 h before testing. | Increasing the concentration of plasticizer led to a decrease in tensile strength, elastic modulus, and oxygen permeability, while increasing elongation, solubility, WVP, and seal strength. | Rompothi et al. (2017) |
|
Guar gum Sunflower seed oil (SSO) |
A 4% (w/w) MBS suspension was heated to 90°C–95°C for 30 min and then allowed to cool to room temperature (22 ± 3°C) with continuous stirring. A 1.5% (w/w) GG dispersion was homogenized at 15,000 rpm until fully dispersed, then mixed with the MBS suspension, and stirred for 45 min. Glycerol, comprising 30% of the total weight of the GG/MBS mixture, was added and stirred for 15 min. SSO was incorporated at 0%, 0.5%, 1%, and 2% (w/w), followed by homogenization at 15,000 rpm for 3 min. The resulting emulsions were degassed in a vacuum chamber at room temperature for 30 min. The emulsions were then cast onto Petri dishes and dried at 40°C with 25% relative humidity for 24 h. Once dried, films were peeled off and conditioned at 25°C and 50% relative humidity for 48 h before being analyzed. |
Enhanced their water resistance properties Higher SSO content generally reduced tensile strength, crystallinity, elongation at break, WVP, and water solubility. |
Lee, Lee, and Han (2020) | |
|
Glycerol Citric acid |
A mixture of 1 g of MBS, 0.3 g of glycerol, and 20 mL of distilled water was prepared and heated at 85°C for 1 h with continuous stirring. Different quantities of citric acid (ranging from 0 to 150 mg, corresponding to 0%–15% w/w of MBS) were then added to the solution and heated at 100°C for 5 min. After cooling to 50°C, the solution was poured into plastic Petri dishes and allowed to dry at 30°C for 16 h. | Compared with MBS films, tensile strength and heat seal strength were increased while decreasing WVP and oxygen permeability | Yao, Wang, and Ming-Weng (2022) | |
| Navy bean |
Corn starch Glycerol Sorbitol |
Suspensions of navy bean and corn starch were prepared at concentrations of 35 and 40 g/L, respectively, and heated to 60°C for 1 h. The starches were then gelatinized at 85°C with vigorous stirring at 700 rpm for 30 min to break down the starch granules. Glycerol and sorbitol, at 10 g/L each, were added as plasticizers to each film-forming solution and then subjected to 30 min of stirring and subsequent homogenization at 10,000 rpm for 5 min. Forty-two milliliters of each dispersion was poured into a 10-cm-diameter Petri dish and left to dry at room temperature (21°C) for 24 h. | Better mechanical (high tensile strength and elongation at break) and water barrier properties | Y. Zhang and Li (2021) |
| Locust bean gum (LBG) | K-carrageenan | Mixtures of k-carrageenan and LBG were prepared to achieve a final polysaccharide concentration of 1% (w/w). The film-forming solutions were degassed under vacuum to eliminate air bubbles and dissolved gases. Then, 28 mL of the solution was poured into polystyrene Petri dishes and dried at 35°C for 16 h. After drying, the films were conditioned at 54 ± 1% relative humidity (RH) and 20 ± 1°C by storing them in a desiccator with a saturated Mg (NO3)2·6H2O solution. | Enhanced the barrier properties, tensile strength, and elongation at break while reducing water vapor permeability | Barak and Mudgil (2014) |
| Gum tragacanth | Edible films were made by dissolving 1% (w/w) gum powder in deionized water. LBG was heated to 80°C for 1 h, and the mixtures were kept overnight at 4°C. Binary gum solutions were mixed at room temperature for 10 min, centrifuged at 1500 rpm for 5 min, and poured onto 10-cm Teflon plates. The films were dried at 30°C for 24 h and subsequently conditioned in a desiccator with a relative humidity of 52.80 ± 0.20% at 23°C until they reached a constant weight. | Improved water vapor barrier and mechanical properties | Mostafavi et al. (2016) | |
|
Whey protein isolate (WPI) Glycerol |
Film-forming solutions containing 5% whey protein isolate (WPI) and 2% glycerol were prepared. Various concentrations of locust bean gum (LBG) (0%, 0.025%, 0.05%, and 0.1%) were tested after stirring for 4 h. | Improved flexibility and reduced solubility of the films, resulting in lower gas permeability and decreased transparency | Silva et al. (2016) |
3 Extension of Shelf Life
Edible coatings are important in preserving the shelf life and quality of various food products. They achieve this by reducing oxidation and regulating the respiration rate of the food. These coatings offer mechanical, physical, and biological properties, while also providing protection against light and ultraviolet rays. A prolonged shelf life is accomplished by creating a semipermeable protective layer on the surface of fruits and vegetables. This layer alters the surrounding gaseous environment (O2 and CO2), reducing the respiration rate and ethylene biosynthesis, thereby delaying the biochemical changes associated with ripening. Furthermore, coating fruits or vegetables can fill cracks on the pericarp, leading to the closure of stomata and lenticels, which can delay the development of physiological disorders such as weight loss (Yadav et al. 2022). Edible coatings possess significant potential for incorporating active ingredients like antibrowning agents, flavors, colorants, spices, nutrients, and antimicrobial compounds. These additives can effectively prolong the shelf life and reduce the microbial growth on products (Rojas-Graü, Soliva-Fortuny, and Martín-Belloso 2009). Additionally, studies have shown that edible coatings help decrease respiration rates and ethylene production in fruits, resulting in delayed senescence and longer storage periods (Dhall 2013) (Table 2).
| Legume | Plasticizer/additives | Coated food | Key findings | Reference |
|---|---|---|---|---|
| Soy protein concentrate (SPC) | Cassava starch | Groundnuts | Prolonged the shelf life of toasted groundnuts by 14 days when stored at a temperature of 27 ± 1°C | Chinma, Ariahu, and Abu (2014) |
| Soy protein Isolate (SPI) | Chitosan | Apricots | Significantly decreased weight loss and improved firmness and texture of the fruit at 2°C; also prevented the breakdown of pectin | L. Zhang et al. (2018) |
| Cinnamon and clove essential oil | Pork | Reduced the rate of water loss and total bacterial count during cold storage, resulting in an enhanced shelf life | Ju et al. (2019) | |
| Chitosan and soybean oil | — | Eggs | Prolonged the shelf life of eggs by 5 weeks at 25°C. Coatings significantly improved the shelf life of egg compared to uncoated egg at both room and refrigerated conditions. | Wardy et al. (2011) |
| Pea starch and guar gum (PSGG) | Glycerol | Oranges | Overall flavor and freshness after storage compared to uncoated fruit | Saberi et al. (2018) |
| Glycerol lipid mixture containing shellac and oleic acid | Oranges | Reduced respiration rate, ethylene production, weight loss, firmness loss, peel pitting, and fruit decay rate | Saberi et al. (2018) | |
| Guar gum (GG) | Potassium sorbate | Apples, cucumbers, and tomatoes | Enhances the antifungal activity | Mehyar et al. (2011) |
| Potassium sorbate | Selected vegetables | Effective against molds isolated from selected vegetables and protects potassium sorbate from disappearing during storage | Saha et al. (2016) | |
| Carboxymethyl guar gum, potassium sorbate, cinnamon oil, glycerol, water emulsifying agent | Cucumber | Reduced weight loss, decay loss, acidity, and total phenolics, while maintaining antioxidant activity. It also reduced microbial infection and improved the shelf life | Saha et al. (2016) | |
| Glycerol | Roma tomatoes | Improved firmness, minimized weight loss, slowed changes in soluble solids content, delayed the reduction in total acidity, and reduced the respiration rate. This approach positively affected the physicochemical, microbial, and sensory attributes and postponed the ripening process at ambient temperature | Ruelas-Chacon et al. (2017) | |
| Chitosan | Shiitake mushrooms | Had beneficial effects on maintaining the quality of the mushrooms compared with those that were uncoated during storage at 4°C | Huang et al. (2019) | |
| Tamarind seed starch (TAM) | Kinnow fruits (orange) |
Reductions in physiological weight loss, decay incidence, and respiration rate. Decrease in the activity of pectin methyl esterase and lipoxygenase. Coated fruits exhibited higher fruit firmness, total phenols, titratable acidity, ascorbic acid, and antioxidant activity along with receiving superior sensory scores. Prolongs the shelf life for up to 25 days while retaining their desirable quality attributes during ambient storage |
Bhan et al. (2022) | |
| Tomato | Lowest physiological weight loss was observed in tomatoes coated with 6% guar gum followed by 8% guar gum. Sensory evaluation indicated that the 6% guar gum coating effectively maintained the overall quality of tomato fruits during storage for up to 32 days by delaying the ripening process | Ghosh et al. (2014) | ||
|
Chitosan Coconut oil, clove bud oil, and cinnamon bark oil |
Khasi mandarins | Exhibited reduced decay and maintained postharvest quality attributes | Goswami et al. (2024) | |
| Citric acid and mint leaf extract | Ber fruit | Coated fruit exhibiting superior sensory and physical and chemical parameters | P. Kumar, Kumar, et al. (2023) | |
| Almond gum and oregano essential oils | Okra | 75% guar gum and 25% almond gum with 0.15% oregano essential oil, exhibiting superior properties, including pH, antioxidant activity, particle size, zeta potential, and antimicrobial efficacy against common pathogens | Shinde et al. (2024) | |
| Mung bean starch (MBS) |
Guar gum Sunflower seed oil Soybean oil |
Rice cake | Delayed the staling of rice cakes | Lee, Lee, and Han (2020) |
|
MBS Guar gum |
Sunflower seed oil Grad seed extract (GSE) |
Rice cake | Showed antimicrobial effects by controlling the growth of Bacillus cereus and Penicillium citrinum during storage | Lee, Song, et al. (2020) |
| Mung bean starch soybean polysaccharide | Nanoemulsified clove and cinnamon oil | Steamed buns |
Increased antimicrobial activity Exhibited better sensory qualities and lower hardness |
Li et al. (2020) |
| Gum arabic | — | Mango | Slowed weight loss, postponed the rise in total soluble solids (TSS) and β-carotene development, while preserving ascorbic acid levels in mangoes during storage | Daisy et al. (2020) |
| — | Tomato | Exhibited changes in weight, firmness, soluble solids concentration, titratable acidity, ascorbic acid content, decay percentage, and color development | Ali et al. (2010) | |
|
Gum arabic Soybean gum |
Jojoba wax, glycerol | Anna apple | Delayed changes in weight loss, firmness, total soluble solids, titratable acidity, decay, and color | El-Anany, Hassan, and Ali (2009) |
| Locust bean gum (LBG) |
Beeswax Shellac Polysorbate 80 Glycerol |
Fortune mandarins | Enhanced postharvest quality by controlling the weight loss and improving the gloss | Rojas-Argudo, del Río, and Pérez-Gago (2009) |
| Polyethylene glycol (PEG) 200, PEG 400, glycerol | Sausages | Extended the shelf life | Dilek et al. (2010) | |
|
Silver nanoparticles Egg white proteins |
Cherries and apricots | Extended the shelf life by inhibiting activity against Bipolaris sorokiniana and Fusarium graminearum | Akyüz et al. (2023) | |
| Sodium alginate | Turbot fish | Inhibited the formation of undesirable compounds with off flavor and maintained the freshness in the refrigerator | W Liu, Mei, and Xie (2021) | |
| Peanut protein |
Cinnamon essential oil Chitosan |
Fish | Exhibited effective inhibition against Escherichia coli, Staphylococcus aureus, and Pseudomonas fluorescens | Ju et al. (2019) |
| Defatted peanut flour (DPF) |
Glycerol Butylated hydroxytoluene (BHT) |
Roasted peanuts | Extended the shelf life by delaying the lipid oxidation process and enhancing sensory property preservation | Paula Martín et al. (2019) |
4 Future Scope
Even though edible coatings offer significant advantages, their widespread commercial adoption is still limited. Improvements are needed in enhancing the mechanical strength, water resistance, and barrier properties of biopolymer films (Nayik, Majid, and Kumar 2015). Continued research into creating new formulations by integrating bioactive compounds into pea starch guar gum (PSGG)–based coatings shows great potential for advancing edible packaging. This includes studying how these coatings affect microbial growth and the physiological processes of various fruits and vegetables (Saberi et al. 2018). Also, there is a need to focus on expanding production techniques and improving cost efficiency to enable wider industrial acceptance. There is a focus on sustainability in packaging, influenced by environmental concerns, which highlights the significance of developing edible films from pea protein and PS (Farshi et al. 2023). It is essential to find new potential biopolymers without using common ingredients to improve the mechanical properties, barrier properties, and antimicrobial properties of these films and coatings (Bhattacharya 2021).
They are favored for their easy availability, cost-effectiveness, and biodegradability, meeting the growing consumer demand for sustainable options in packaging fresh produce. Ongoing research aims to improve edible packaging and films by incorporating bioactive substances that can enhance the taste, nutritional value, and overall sensory experience of perishable foods. However, there is a need for more studies to improve the mechanical strength, barrier properties, rheological characteristics, and optical qualities of these coatings (Saha et al. 2017). Currently, the majority of research on edible coatings has been carried out at the laboratory level. Therefore, there is a need for further studies on an industrial scale to enable the widespread commercial application of edible coatings for preserving a broader range of foods (Saha et al. 2017).
Additionally, efforts to create edible coatings with enhanced antibacterial characteristics are expected to persist. The utilization of nanotechnology in the production of edible films and coatings is considered a promising area for future research. As these materials advance and become more widely adopted in the food industry, food packaging and preservation are likely to become more eco-friendly and sustainable (Matloob et al. 2023). Furthermore, there is a need for further research to understand the complex interactions between active ingredients and coating materials in the development of new edible film and coating applications. The incorporation of active ingredients such as antimicrobials, antioxidants, and nutrients can significantly impact the mechanical, sensory, and functional properties of these coatings. However, studies in this area are limited, highlighting the necessity for more comprehensive investigations to enable the preparation of novel coating applications with improved properties and better sensory attributes (Rojas-Graü, Soliva-Fortuny, and Martín-Belloso 2009).
Ruelas-Chacon et al. (2017) emphasized the importance of improving water vapor barrier properties in GG-based edible coatings by incorporating specific lipid component to increase the postharvest storage quality at both ambient and cold temperatures. Plant-based gums are becoming increasingly popular as substitutes for synthetic packaging materials. Further investigation is required to understand the impact of gum arabic coating on microbial proliferation and the fruit ripening physiology (Ali et al. 2010). LBG can be further incorporated with other biopolymers and functional ingredients such as nanoparticles, antibiotics, bacteriocins, essential oils, polyphenol-rich plant extracts, biocontrol agents, and probiotics to develop new functional films (Yuan et al. 2020).
Moreover, it has been proven that the integration of microorganisms with antimicrobial properties is effective in mitigating pathogen growth. The success of incorporating living microorganisms into edible films to prevent or delay food product contamination can vary based on factors such as the combination of materials, methods of microorganism incorporation, production processes, strains of microorganisms, storage conditions, and application in food products. However, further studies are required to understand the impact of these variables on the effectiveness of edible films containing living microorganisms (Guimarães et al. 2018). Further research should be directed towards the biodegradability of edible films while maintaining their mechanical strength and barrier properties over specific periods (F. Hassan, Mu, and Yang 2024).
5 Conclusion
In conclusion, there is a good potential to use legume-based edible coatings as an effective and sustainable solution to extend shelf life and maintain the quality of various products. Legumes are a rich source of protein, polysaccharides, fibers, and bioactive compounds. Therefore, they can be used to develop films with desirable mechanical and functional properties. Additionally, the physicochemical properties of legume-based edible coatings can be modified by adjusting various factors including the concentration of plasticizers, cross-linking agents, and other additives. Furthermore, the addition of bioactive compounds, such as essential oils and antioxidants, into these coatings can improve their functionality, particularly in terms of antimicrobial and antioxidant activities.
Further understanding about the interactions between different components of legume-based coatings, optimization of processing conditions to improve the properties of coatings, and exploration of potential new legume sources are required. More studies are essential to commercialize the different new leguminous sources as sustainable and effective for the food industry.
Author Contributions
A. K. A. N. W. M. R. K. Thamarsha: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft. data curation, validation. Kiran Mor: data curation, validation. Ashutosh Upadhyay: project administration, resources, validation, visualization. Venkata Sarath Pamu: validation, visualization.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




