The impacts of nutrient addition and livestock exclosure on the soil nematode community in a degraded grassland
Abstract
Nutrient addition and livestock exclosure are two strategies for restoring degraded grassland. Although their impacts on plant community properties are well understood, the effects of fertilization and livestock exclosure on belowground fauna community in degraded grassland remain largely unknown. We examined the main and interactive effects of nutrient addition and livestock exclosure on soil nematode community in a severely degraded grassland of northern China, by recording soil nematode community composition in 0- to 10-cm soil layer in the summer of 2017. Neither nutrient addition nor livestock exclosure had significant effects on the biodiversity of nematode community in this degraded grassland. Livestock exclosure significantly increased the abundances of total nematodes and the maturity index of the nematode community, mainly due to its positive effect on omnivorous + carnivorous nematodes. Nutrient addition weakened the positive effects of livestock exclosure on soil nematode community by reducing soil pH and enhancing soil ammonium concentration. Our results highlight that the positive effects of livestock exclosure on soil nematode community would be reassessed under the scenarios of nutrient enrichment. Given that most grasslands are used for livestock production, the interactive effects of grazing and nutrient enrichment on soil nematode community deserve more attention in further research.
1 INTRODUCTION
Natural grassland is the largest terrestrial ecosystem in China, accounting for 40% of national land area (Kang, Han, Zhang, & Sun, 2007). About 90% of the natural grasslands in China has been degraded due to reclamation and overgrazing (Gang et al., 2014; Nan, 2005). Livestock exclosure and nutrient fertilization are effective and widely used ways to restore the degraded grasslands (Bai et al., 2010; Ren et al., 2018). In the past decades, our mechanistic understanding of the restoration of plant community composition and production following those restoration strategies has been substantially improved by empirical studies from diverse grassland ecosystems (Baer, Blair, Collins, & Knapp, 2004; Bai et al., 2010; Chen et al., 2017). In contrast, we know little about the responses of soil biota, which contribute substantially to the maintenance of ecosystem functioning (Wagg, Bender, Widmer, & Mg, 2014; Wardle et al., 2004).
Soil nematodes are one of the most abundant and diverse groups of soil biota in terrestrial ecosystems, occupying multitrophic levels in the soil detrital food web (Wu, Ayres, Bardgett, Wall, & Garey, 2011; Yeates, 2003). They play an important role in many fundamental ecological processes, such as soil nutrient cycling (Neher, 2001) and plant growth (Alphei, Bonkowski, & Scheu, 1996; Neher, 2001). They are quite susceptible to disturbance and thus being considered as an ideal bioindicator of changes in soil environment (Bongers & Ferris, 1999). Given the impacts of different restoration strategies on ecosystem properties (Baer et al., 2004; Chen et al., 2017), it is reasonable to expect that they would consequently affect the composition and structure of soil nematode community through altering soil characters and primary production.
Primary productivity of global grasslands is co-limited by multiple nutrients (Fay et al., 2015). Consequently, multiple nutrients instead of single nutrient should be used during grassland restoration. There is mounting evidence that nutrient addition reduces the abundance and diversity of nematodes (Liang et al., 2009; Sarathchandra, Ghani, Yeates, Burch, & Cox, 2001). Nutrient addition would impact soil nematode community through several pathways. The enhancement of soil ammonium concentration following N addition is toxic to soil nematodes, especially plant-feeding (PF) nematodes in a temperate steppe (Wei et al., 2012). Soil acidification is another reason for the negative effect of N addition on soil nematode abundance (Li et al., 2010; Liang et al., 2009). Further, nutrient addition would indirectly affect soil nematode community composition through changing plant community composition, because as both plant diversity and identity would alter the production of food sources for nematodes (Deyn, Raaijmakers, Ruijven, Berendse, & Putten, 2004). The changes of nematode community would have top-down regulation effects on other trophic level. For instance, phosphorus fertilization changed the composition of soil nematodes with subsequent effects on soil microbial community structure in a grassland (Chen, Daniell, Neilson, O'Flaherty, & Griffiths, 2014). Given the key role of soil nematodes in driving ecological process (Neher, 2001), understanding the changes of soil nematode community will help fully reveal the impacts of nutrient addition on the ecosystem restoration of degraded grassland.
Our knowledge about the responses of soil nematode community to nutrient addition is from grasslands being fenced to exclude the grazing of large animals, including livestock. The effects of nutrient addition on soil nematode community would depend on whether the ecosystem being grazed or not, because livestock grazing could substantially alter plant community composition, primary productivity, and soil physiochemical characters (Ma, Zhou, & Du, 2013; Sun et al., 2011) and thus change both the quantity and quality of organic matter entering soils (Bardgett & Wardle, 2003). Livestock grazing had negative effects on soil nematode diversity in grasslands (Chen, Zheng, Shan, Taube, & Bai, 2013; Zolda, 2006) through grazer trampling (Bouwman & Wbm, 2000). Moreover, removal of biomass and nutrients contained in it by grazing would alleviate the positive effects of nutrient addition on the soil nutrient accumulation. However, to what extent livestock activities would mediate the effects of nutrient addition on soil nematode community largely remain unknown.
To understand the main and interactive effects of nutrient addition and livestock exclosure on soil nematode community, we measured the changes of abundance, biodiversity, and composition of soil nematode community in response to factorial treatments of nutrient addition and livestock exclosure in a degraded grassland of northern China. We hypothesized that (a) the total abundance and diversity of nematode community would be negatively affected by nutrients addition and positively affected by livestock exclosure and thus (b) nutrient addition and livestock exclosure would interact to affect soil nematode community.
2 MATERIALS AND METHODS
2.1 Study site
The field experiment was carried out at a degraded grassland near the Inner Mongolia Grassland Ecosystem Research Station (IMGERS, 43°38′N, 116°42′E) of the Chinese Academy of Sciences, which is located in the Xilin River Basin of Inner Mongolia Autonomous Region, China. The region has a semiarid continental climate with mean annual temperature of 0.5°C and mean annual precipitation of 336 mm (1970–2009). The soil is a loamy sand texture (Calcic Chernozem according to ISSS Working Group RB, 1998). Artemisia frigida Willd. (a perennial forb), Caragana microphylla Lam. (a shrub), and Kochia prostrata (L.) Schrad. (a sub-shrub) are three dominant species in this degraded grassland under over-grazing by sheep (Xu, Wan, Cheng, & Li, 2008).
2.2 Experimental design
The experiment is a participatory site of the Nutrient Network (Nutnet; http://www.nutnet.umn.edu), a global collaborative research effort to examine the impacts of nutrient addition and herbivore exclosure on grassland function and ecosystem services (Borer et al., 2014). The experiment followed a randomized block design and included 10 treatments with each replicated for three times. A total of 30 sample plots with each area of 5 m × 5 m were set up, and plots were separated by 1-m walkways in July 2016. In this study, we focused on the factorial treatment of nutrient addition and livestock exclosure, that is, control (no nutrient addition and no livestock exclosure), +N (nutrient addition and no livestock exclosure), +E (livestock exclosure but no nutrient addition), and N + E treatment (nutrients addition and livestock exclosure). Multiple nutrients were added to the plots with the treatments of +N and N + E: 10 g N · m−2 · yr−1 as urea [(NH2)2CO], 10 g P · m−2 ·yr −1 as triple-super phosphate [Ca(H2PO4)2], 10 g K · m−2 · yr −1 as potassium sulphate [K2SO4], and 100 g m2 of a micronutrient mix of Fe (15%), S (14%), Mg (1·5%), Mn (2·5%), Cu (1%), Zn (1%), B (0·2%), and Mo (0·05%). Nutrients were dissolved in 2 L of water and sprayed evenly with the spray pot in early May 2017. The plots without nutrient addition received 2 L of water. he 120-cm-tall fences were erected to exclude sheep-grazing in livestock exclosure treatments, and the treatments without livestock exclosure were under sheep-grazing during the growing season from May to September. The grazing intensity was four sheep per hectare (Xu et al., 2008).
2.3 Field sampling and chemical analysis
In each plot, one permanent quadrat (1 m × 1 m) was set up for plant community survey. Plant species richness and abundance in each quadrat were recorded in mid-August 2017. Plant species richness in each quadrat was defined as the total number of vascular species recorded. Percent cover of each species in each quadrat was measured using a 1-m × 1-m metal pane with 100 equal grids and counting the grid junctions whose vertical projections overlapped with plants. Aboveground biomass was sampled by clipping all plants at the soil surface within two 0.1-m × 1-m quadrats near the 1-m × 1-m permanent subplot (Borer et al., 2014). All living plants were oven-dried at 65°C for 48 hr and then weighted.
In mid-August 2017, three soil samples was randomly collected using soil core (diameter 2.5 cm, depth 10 cm) near the 1-m × 1-m permanent monitoring quadrat. After gentle homogenization, soils were sieved through 2-mm mesh to remove roots and separated into two parts. One part was stored in polyethylene bags at 4°C to maintain fresh for the determination of soil nitrate, soil ammonium, and soil moisture, and for extraction of soil nematodes. The other part was air-dried to measure soil total nitrogen, soil total carbon, and soil pH value. For determination of ammonium (NH4+-N) and nitrate (NO3−-N) contents, 8 g of subsample of moist soil was extracted with 40 ml of 2 mol/L KCl, and the filtrates was subjected to determination on flow injection auto analyzer (FIAstar 5000 Analyzer; Foss Tecator, Hillerød, Denmark). Soil moisture was determined using aluminum specimen box as mass loss after drying the soils at 105°C for 24 hr and expressed as percent dry weight. The soil pH was measured in water suspension (soil: water = 1: 2.5) by a pH meter (FE20–FiveEasy; Chen et al., 2013).
2.4 Extraction and identification of soil nematodes
Soil nematodes were extracted from 50 g of fresh soil samples using a modified cotton-wool filter method (Verschoor & Rgmde, 2000). A total number of nematodes were counted after being fixed with 4% formalin solution. The first 100 individuals encountered were identified to genus level using microscope. All nematodes were identified if the total number of individuals is less than 100 in the sample. Nematode abundance is expressed as the number of individuals per 100-g dry soil. All nematodes were divided into four trophic groups according to their feeding habits and esophageal morphology (PF, bacterial-feeding (BF), fungal-feeding (FF), omnivorous, and carnivorous nematodes; Yeates, Bongers, De Goede, Freckman, & Georgieva, 1993). The classification of nematodes is based on the classification system proposed by Jairajpuri and Ahmad (1992) and Bongers (1988). In this study, few genera were identified as carnivorous trophic group, so we merged omnivorous and carnivorous nematodes into one group (omnivorous + carnivorous [OC] nematodes; Chen et al., 2013).
2.5 Data analysis
The species richness (S), Shannon-wiener index (H′), and Simpson index (λ) were used as measures of plant and nematode community diversity.
-
- Species richness
-
- S = the total number of taxa in each plot,
-
- Shannon-Wiener index
-
- H´ = −∑pi ln pi, and
-
- Simpson index
-
- λ = 1-∑pi2,
In addition, the following nematode community abundance were calculated: total nematode abundance, the abundance of different trophic groups: (a) BF, (b) FF, (c) PF, and (d) OC.
The nematode channel ratio was calculated as nematode channel ratio (NCR) = B/(B + F), where B and F are the number of BF nematodes and FF nematodes in the total soil nematode population.
The value of NCR ranged between 1 (completely bacterial-mediated) and 0 (completely fungal-mediated).

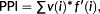
Data were tested for normality using the Kolmogorov–Smirnov test and for homogeneity of variance using Levene's test. Results showed that all data met the requirement of normality and being equal in error variance. Two-way analysis of variance was used to examine the main and interactive effects of nutrient addition and livestock exclosure on soil chemical characters, plant community characters, and the abundance and diversity of soil nematodes communities. The least significant difference test was used when the treatment effects were significant. We performed principal component analyses to identify the relations among plant community characters, soil properties, and nematode community. All statistical analyses were performed by SPSS 17.0 (SPSS Inc., Chicago, IL). Difference at p < .05 level was considered to be statistically significant.
3 RESULTS
3.1 Soil and plant community characters
Nutrient addition significantly enhanced soil moisture (Figure 1a), soil NO3−-N concentrations (Figure 1c), and NH4+-N concentrations (d) but decreased soil pH (Figure 1b). Livestock exclosure significantly reduced soil NH4+-N concentrations (Figure 1d) and had no impact on other soil characters. Nutrient addition and livestock interactively affected soil NH4+-N concentration (Figure 1d), with soil NH4+-N concentration being significantly enhanced by nutrient addition under livestock exclusion conditions but not under grazing conditions.
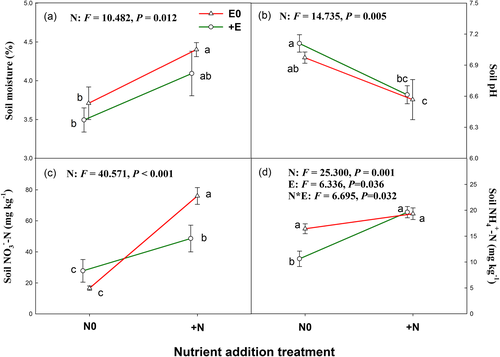
Both nutrient addition and livestock exclosure had no significant effect on species richness, Shannon–Wiener diversity index, and Simpson index of plant community (Figures 2a–2c). Nutrient addition had no significant effect but livestock exclosure significantly increased plant community aboveground biomass (Figure 2d).
3.2 Abundance and composition of soil nematode community
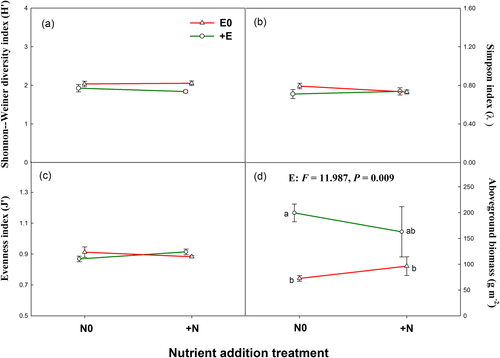
A total of 46 genera from 19 family of soil nematodes were identified (Table A1). The impacts of nutrient addition on the abundance of total nematodes and nematode trophic groups (BF and PF nematodes) were significantly dependent on the status of livestock exclosure (Figure 3), where nutrient addition reduced the abundance of total nematodes under livestock exclosure conditions but had no impact on that under grazing conditions. The abundance of FF nematodes showed no significant response to both nutrient addition and livestock exclosure (Figure 3c). Nutrient addition significantly decreased, but livestock increased the abundance of OC nematodes (Figure 3d). The changes of soil pH and soil ammonium concentrations played an important role in driving the variation of soil nematode abundance (Figure 4).

3.3 Diversity and structure of soil nematode community
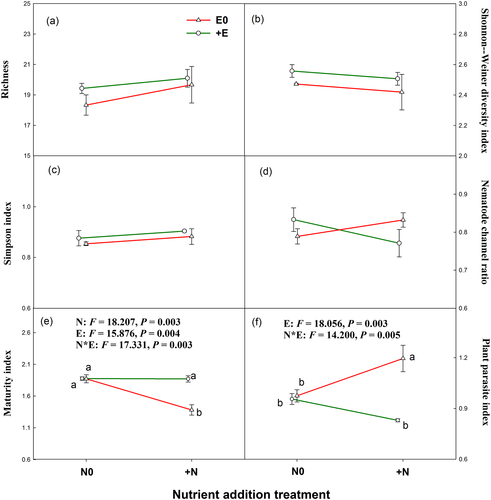
Both nutrient addition and livestock exclosure had no significant effect on diversity of soil nematode community (Figures 5a–5c) and nematode channel ratio (Figure 5d). The variation of soil nematode diversity was mainly driven by Simpson index of plant community (Figure 4). The main and interactive effects of nutrient addition and livestock exclosure on the MI of soil nematode community were statistically significant (Figure 5e). Nutrient addition reduced MI under grazing conditions but had no effect on MI under livestock exclosure conditions. Livestock exclosure significantly reduced PPI of nematode community (0.003; Figure 5f), with such effects being statistically significant only at nutrient addition conditions (0.005; Figure 5f).
4 DISCUSSION
4.1 Effects of nutrient addition on soil nematode communities
Our results showed that soil nematode community in this degraded grassland was resistant to nutrient enrichment, at least in the short term, which is not consistent with our hypothesis on the effects of nutrient addition. Soil acidification and ammonium toxicity have been considered as the main factors accounting for the negative effects of nitrogen addition on the abundance and diversity of soil nematode community (Cheng, Grewal, Stinner, Hurto, & Hamza, 2008; Liang et al., 2009; Ruan et al., 2012; Zhao et al., 2014). Although there was significant reduction of soil pH and enhancement of ammonium concentrations following nutrient addition in this study, such changes had limited impacts on soil nematode community.
We found no significant impacts of nutrient addition on species diversity and aboveground biomass of plant community in this degraded grassland, which would be another factor that helps explain the resistance of soil nematode community to nutrient addition. The shift of plant community caused by fertilization has been found to be the main determinant of the nematode community changes (Hu et al., 2017). Soil nematode diversity is generally positively correlated with plant species diversity (Cesarz et al., 2017; Eisenhauer, Migunova, Ackermann, Ruess, & Scheu, 2011). Furthermore, the community composition of soil nematodes is determined by plant community composition (Veen, Olff, Duyts, & Putten, 2010). Given the impacts of nutrient addition on plant community would be accumulated through time (Zhang et al., 2014), and our results here are the short-term responses of plant community to nutrient addition; long-term impacts of nutrient addition on soil nematodes through altering plant community are reasonably expected.
Nutrient addition significantly reduced nematode MI, which was largely due to the greater abundance of opportunists. Similarly, it was Wei et al. (2012) who documented negative impacts of N addition on MI across a wide-ranged N addition gradient in a nearby ecosystem. The negative impacts of nutrient addition on MI were caused by the replacement of k-strategists with higher cp value by opportunistic strategists with lower cp value (Wasilewska, 1995). For instance, the abundance of OC nematodes, which had higher cp value than other trophic groups, was significantly decreased by nutrient addition. Previous evidence showed that the nematodes of k-strategy (with higher cp value) are more sensitive to disturbance (Liang et al., 2009). The negative effects of nutrient addition on MI suggest that nutrient addition would increase the intensity of soil disturbance (Bongers, 1990) and reduce structural complexity of the soil nematode community and consequently soil food webs (Forge, Bittman, & Kowalenko, 2005; Hu & Qi, 2010).
4.2 Effects of livestock exclosure on soil nematode communities
The hypothesis that livestock exclosure would enhance the diversity of soil nematode community was not supported by our results, as we found no significant impacts of livestock exclosure on all the diversity indices of soil nematode community (Figures 5a–5c). Although many studies reported negative effects of vertebrate grazers on soil nematode diversity in grasslands (Zolda, 2006), such effects would be largely dependent on grazing intensity. For example, it was found that low and moderate grazing intensity (<4.5 sheep per hectare) had no effect on species richness of soil nematode community, whereas higher grazing intensities reduced nematode richness in a temperate steppe (Chen et al., 2013). Our results suggested that the role of livestock exclosure would be limited in affecting soil nematode diversity in degraded. The changes of plant community composition instead of abiotic soil properties have been found to play a more important role in driving the responses of soil nematode community composition to grazing exclusion in a floodplain grassland (Veen et al., 2010). In this study, we found no significant impact of livestock exclosure on both plant community diversity and abiotic soil properties, which may contribute to the resistance of soil nematode community to livestock exclosure.
Livestock exclosure significantly increased the abundances of total nematodes and OC nematodes, which may due to the removal of the suppressive effect of hormones secreted by grazers on nematode communities under livestock exclosure conditions. Previous studies have demonstrated that steroid hormones secreted by mammals had negative effects on the growth and reproduction of nematodes (Höss & Weltje, 2007). The steroid hormones (testosterone, progesterone, and estroned) secreted by mammals could dramatically reduce the total nematode abundance (Hu, Hermann, Pen-Mouratov, Shore, & Steinberger, 2011). Moreover, trampling of grazer would reduce soil pore space and limit air penetration and consequently result in environment uninhabitable for large soil nematodes (Bouwman & Wbm, 2000; Mikola et al., 2009; Sørensen, Mikola, Kytöviita, & Olofsson, 2009), which may reasonably explain the positive effects of livestock exclosure on the abundance of OC nematodes.
The stable NCR indicated that livestock would not change the decomposition pathways of soil organic matter in this ecosystem. The values of NCR were generally greater than 0.5 in this study, suggesting that bacterial energy channel dominated in this degraded grassland. Similar result was observed in seabird colonies that enclosures did not alter NCR, reflecting a weak below-ground effect of enclosure (Wright, Wal, Wanless, & Bardgett, 2010). Consistent with results from other studies (Hu et al., 2015; Mills & Adl, 2011; Schon, Mackay, Yeates, & Minor, 2010; Zolda, 2006), livestock exclosure significantly increased MI and decreased plant parasite index PPI. Such changes were caused by the changes of community composition following livestock exclosure. The proportion of OC (cp 4–5) increased, and that of bacterivores belonging to Cephalobidae (cp 2), fungivores belonging to Leptonchidae (cp 2), and plant parasites belonging to Dolichodoridae (cp 3) decreased in response to livestock exclosure.
4.3 Interactive effects of nutrient addition and livestock exclosure on soil nematode community
We observed strong interactive effects of nutrient addition and livestock exclosure on the abundance of total nematodes, bacterivores, and plant parasites, which supports our second hypothesis. Nutrient addition decreased the total abundance of soil nematodes only under livestock exclosure conditions but not under livestock grazing conditions. Furthermore, the effects of nutrient addition on MI of soil nematode community also depended on the status of livestock exclosure. Most of related studies that examined the effects of nutrient addition on soil nematode communities in grasslands were carried out in mature or undisturbed ecosystems (Li et al., 2013; Wei et al., 2012), with such effects in degraded ecosystems were less known. Our study fills this knowledge gap. Our results highlight the interactive effects of different restoration strategies in affecting soil biota community. Given the fact that most of grasslands are used for livestock production, the combined effects of grazing and nutrient enrichment on soil biota communities deserve more attention in further research.
5 CONCLUSIONS
Although nutrient addition and livestock exclosure did not affect the biodiversity of soil nematode communities, they had significant impacts on the abundance of soil nematodes. Such a result indicates the higher sensitivity of soil nematode abundance than their biodiversity to short-term disturbance. The changes of soil pH and soil inorganic N concentration played an important role in driving the responses of soil nematode abundance following nutrient addition and livestock exclosure. Nutrient addition had negative effects on the abundance of soil nematodes in the fenced treatments but had no effects on that in the grazed treatments, indicating that nutrient addition would be an appropriate restoration strategy for the degraded grasslands caused by livestock grazing.
ACKNOWLEDEGEMENTS
We acknowledge the staff of the Inner Mongolia Grassland Ecosystem Research Station (IMGERS) for their support. This work was supported by the National Key Research and Development Program of China (2016YFC0500601), Natural Science Foundation of China (31770503 and 31822006), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15010403), Youth Innovation Promotion Association CAS (2014174), Doctoral Scientific Research Foundation of Liaoning (20170520179), and Shenyang Science and Technology Bureau (18-013-0-04 and RC180320). Authors declared no conflict of interests.
APPENDIX A
| Family | Genus | Guild | Treatment | |||
|---|---|---|---|---|---|---|
| C | +N | +E | N + E | |||
| Alaimidae | Alaimus | BF4 | 0.0 | 0.7 | 0.3 | 1.3 |
| Cephalobidae | Acrobeles | BF2 | 5.8 | 5.3 | 11.7 | 7.3 |
| Acrobeloides | BF2 | 4.2 | 4.0 | 1.0 | 14.3 | |
| Acrobelophis | BF2 | 0.0 | 0.3 | 0.0 | 0.3 | |
| Cephalobus | BF2 | 0.6 | 3.0 | 9.4 | 0.3 | |
| Cervidellus | BF2 | 8.1 | 9.3 | 4.7 | 12.3 | |
| Chiloplacus | BF2 | 4.9 | 15.0 | 6.4 | 1.7 | |
| Eucephalobus | BF2 | 13.0 | 3.0 | 0.0 | 1.7 | |
| Heterocephalobus | BF2 | 0.0 | 0.0 | 0.0 | 0.7 | |
| Plectidae | Plectus | BF2 | 0.0 | 0.0 | 0.0 | 0.7 |
| Aphelenchidae | Aphelenchus | FF2 | 2.6 | 3.7 | 0.3 | 3.3 |
| Paraphelenchus | FF2 | 0.3 | 1.0 | 0.7 | 0.0 | |
| Leptonchidae | Tylencholaimellus | FF4 | 0.0 | 0.3 | 0.0 | 0.7 |
| Tylencholaimus | FF4 | 6.5 | 3.3 | 6.0 | 7.7 | |
| Tylenchidae | Filenchus | FF2 | 0.3 | 0.0 | 0.0 | 1.0 |
| Aporcelaimidae | Aporcelaimellus | OC5 | 1.0 | 0.0 | 0.0 | 0.7 |
| Paraxonchium | OC5 | 0.0 | 0.0 | 0.3 | 0.0 | |
| Campydoridae | Campydora | OC4 | 0.0 | 0.0 | 1.7 | 0.0 |
| Geslacht | Discolaimium | OC5 | 3.2 | 1.0 | 0.0 | 0.0 |
| Discolaimus | OC5 | 0.0 | 0.3 | 0.0 | 0.3 | |
| Nordiidae | Pungentus | OC4 | 2.3 | 0.3 | 1.7 | 0.0 |
| Thornia | OC4 | 0.0 | 0.0 | 0.7 | 0.0 | |
| Qudsianematidae | Dorydorella | OC4 | 1.3 | 0.0 | 1.0 | 0.0 |
| Eudorylaimus | OC4 | 0.0 | 0.0 | 0.3 | 0.0 | |
| Kochinema | OC4 | 0.3 | 0.0 | 1.0 | 0.0 | |
| Microdorylaimus | OC4 | 0.3 | 0.7 | 6.7 | 0.0 | |
| Thonus | OC4 | 11.0 | 5.3 | 9.4 | 13.7 | |
| Thornenematidae | Mesodorylaimus | OC4 | 0.0 | 0.0 | 0.3 | 0.0 |
| Anguinidae | Anguina | PF2 | 0.0 | 0.3 | 0.0 | 0.0 |
| Ditylenchus | PF2 | 0.0 | 1.0 | 0.3 | 0.0 | |
| Belondiridae | Axonchium | PF5 | 0.0 | 0.0 | 0.0 | 0.3 |
| Dorylaimellus | PF5 | 0.0 | 0.3 | 0.0 | 0.0 | |
| Dolichodoridae | scutylenchus | PF3 | 23.6 | 28.2 | 17.7 | 12.7 |
| Hoplolaimidae | Helicotylenchus | PF3 | 0.0 | 0.3 | 0.7 | 1.0 |
| Pararotylenchus | PF3 | 1.3 | 1.0 | 2.0 | 2.7 | |
| Rotylenchus | PF3 | 2.0 | 0.3 | 1.0 | 0.7 | |
| Longidoridae | Longidorus | PF5 | 0.0 | 0.3 | 0.3 | 0.0 |
| Nordiidae | Longidorella | PF4 | 0.6 | 0.3 | 0.0 | 0.0 |
| Pratylenchidae | Pratylenchus | PF3 | 0.6 | 0.0 | 0.0 | 0.7 |
| Tylenchidae | Aglenchus | PF2 | 0.0 | 0.0 | 0.0 | 1.7 |
| Basiria | PF2 | 0.3 | 1.7 | 1.3 | 0.7 | |
| Boleodors | PF2 | 1.3 | 1.0 | 0.3 | 0.7 | |
| Lelenchus | PF2 | 4.2 | 7.0 | 12.0 | 10.3 | |
| Malenchus | PF2 | 0.3 | 0.7 | 0.0 | 0.0 | |
| Neopsilenchus | PF2 | 0.0 | 1.0 | 0.3 | 0.3 | |
| Tylenchus | PF2 | 0.0 | 0.0 | 0.3 | 0.3 | |
- Note. Functional guilds of soil nematodes characterized by feeding habits and life history characters. Numbers following the functional groups represent the cp values.
- Abbreviations: C: control; +N: nutrient addition; +E: livestock exclosure; N + E: combined nutrient addition and livestock exclosure. BF: bacterial-feeding; FF: fungal-feeding; OC, omnivores + carnivorous; PF: plant-feeding.




