Transcriptomic Features of Recurrence Rates in Idiopathic Subglottic Stenosis
Funding: This work was supported by a grant from the American Laryngology Association ALVRE Grant (#1082), an Academic Medical Organization of Southwestern Ontario innovation fund grant (INN21-016). A.C.N is supported by the Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers. S.Y. is supported by a Canadian Institute of Heath Research Canada Graduate Scholarship-Doctoral.
Anthony C. Nichols and R. Jun Lin contributed equally to this study.
This study was presented at the 146th Annual Meeting of the American Laryngological Association; May 17, 2025; New Orleans, Louisiana.
ABSTRACT
Objective
Idiopathic subglottic stenosis (iSGS) is a rare disease characterized by narrowing of the upper airway and affects near-exclusively females. Patients often experience recurrent disease and require repeated surgical dilations. The pathophysiology underlying the broad spectrum of disease severity within iSGS remains unknown. In the current study, we sought to identify transcriptomic differences between iSGS patients with markedly different recurrence rates.
Methods
Prospectively collected clinical and bulk RNA sequencing data from subglottic tissues of 56 female iSGS patients with 1–4 years of follow-up were analyzed. DESeq2 was used to perform differential expression analysis, comparing samples from the highest (1.19–1.87 dilations/year) versus the lowest (0.30–0.65 dilations/year) quartile of surgical dilation rate (i.e., high vs. low recurrence groups).
Results
In total, 220 genes were significantly differentially expressed between the high and low recurrence groups (adjusted p < 0.1 and log2 fold change > |1|). Pathway enrichment analyses showed that the high recurrence group had significantly increased expression of genes involved in adaptive immune responses (e.g., immunoglobulin subunit genes) and extracellular matrix organization (e.g., COMP, NID2) (adjusted p < 0.1). In contrast, the low recurrence group had significantly increased expression of genes involved in cilia structure and function (e.g., CFAP43, DNAI2) (adjusted p < 0.1), suggesting a relatively increased abundance of respiratory cilia.
Conclusion
Transcriptomic profiling suggests that lower recurrence rates in iSGS are associated with retention of respiratory cilia, while adaptive immune responses and increased extracellular matrix deposition are present in those with higher recurrence rates. These results hold promise for the development of prognostic markers and identification of therapeutic targets for iSGS.
1 Introduction
Idiopathic subglottic stenosis (iSGS) is a rare disease of unknown etiology, characterized by narrowing of the upper airway that can lead to potentially life-threatening shortness of breath [1]. iSGS affects almost exclusively white females, with the typical age of onset being around 40–60 years [2, 3]. Patients are most commonly treated using endoscopic surgical dilation to re-establish airway patency [2-4]. However, this procedure is non-curative and most patients experience recurrence, requiring repeated surgical dilations [3-8]. Intriguingly, the duration of time between dilations (i.e., recurrence rate) can vary widely between patients, indicating substantial disease heterogeneity, likely driven by clinical and tissue-specific cellular and molecular factors [3-8].
iSGS is pathologically characterized by the localized formation of a fibrous luminal scar, driven by infiltration of collagen-secreting myofibroblasts and elevated levels of proinflammatory cytokines [8, 9]. The overlying epithelium exhibits reduced thickness and diminished expression of cell–cell adhesion proteins and cilia [3, 10, 11]. Although drivers behind such a fibroinflammatory cascade remain unclear and are likely multifactorial, evidence suggests potential roles for hyper-responsiveness to androgen [8], microbial infection [12, 13], and genetic predisposition [3].
While previous studies have characterized differences between the subglottic tissues of patients with iSGS and unaffected controls [3, 8-11, 13-15], molecular features that may underlie the heterogeneity in disease severity among patients with iSGS remain largely unexplored. In this study, we sought to identify transcriptomic differences between patients with markedly different recurrence rates. We examined bulk RNA sequencing data from diseased subglottic tissue from iSGS patients [3, 8] in the top versus bottom quartile of surgical dilation rates. Subsequent pathway analyses were conducted to identify mechanisms that may potentially contribute to higher recurrence rates.
2 Methods
2.1 Patient Cohort Description
The study was approved by the research ethics board at both institutions (Western HSREB 115746, Unity Health REB 19-324). Written informed consent was obtained from all study participants. This study involved patients of 18 years of age or older with a diagnosis of iSGS, ascertained from Unity Health Toronto—St. Michael's Hospital (Toronto, Ontario, Canada) and London Health Sciences Centre (LHSC)—Victoria Hospital (London, Ontario, Canada) (Table S1). We included only patients with (1) available bulk RNA sequencing data of the affected subglottic tissue, (2) at least 1 year of prospectively documented surgical procedure and clinical appointment data, and (3) no history of cricotracheal resection. All biopsies of the affected subglottic tissue were obtained at the first surgical dilation required by the patient (i.e., “index” dilation) following research ethics board approval and informed consent. One biopsy of the subglottic tissue per patient was included in this study. The prospective follow-up period was defined as the date of subglottic biopsy to the most recent surgical or clinic appointment date, prior to the study cutoff date of July 1, 2024.
2.2 Surgical Dilation Rate and Clinical Variables
By nature of the study design, where the subglottic tissue biopsy was obtained during a surgical procedure, all patients received at least one dilation during the prospective follow-up period (Table 1, Table S1). Surgery at both centers entailed CO2 laser incision of subglottic scar, rigid or balloon dilatation, and Triamcinolone injection. A subset of patients received in-office intralesional steroid injections between surgical dilations (Tables 1 and S1).
| Continuous variables | High recurrence (n = 14)a | Low recurrence (n = 14)a | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age at diagnosis (years) | 33 | 30–49 | 54 | 47–57 | 0.0051 |
| Years from diagnosis to biopsy |
1.67 |
1.23–4.43 | 2.28 | 0.84–3.64 | 0.5295 |
| BMI (kg/m2) | 27.6 | 24.2–35.3 | 28.4 | 25.0–30.7 | 0.3674 |
| Categorical variables | N | % | N | % | p |
|---|---|---|---|---|---|
| Smoker (ever)b | 2 | 14.3 | 1 | 7.1 | 1 |
| Antacid use | 8 | 57.1 | 6 | 42.9 | 0.7064 |
| Family historyc | 1 | 7.1 | 1 | 7.1 | 1 |
| Diabetes (type I or II) | 2 | 14.3 | 1 | 7.1 | 1 |
| Intralesional steroid injectiond | 4 | 28.6 | 8 | 57.1 | 0.2519 |
| Site: Unity Health Torontoe | 5 | 35.7 | 9 | 64.2 | 0.2568 |
- Note: Comparisons between the high and low recurrence groups were performed using a Welch's t test for continuous variables and a Fisher's exact test for categorical variables.
- Abbreviations: BMI, body mass index; IQR, interquartile range.
- a The high and low recurrence groups were defined as patients in the fourth and first quintiles of surgical dilation rate, respectively, among the total cohort with RNA sequencing data (n = 56).
- b Includes patients who are current smokers or have a history of smoking.
- c Defined as having any first or second degree relative with an iSGS diagnosis.
- d Defined as having received one or more in-office intralesional steroid injections between surgical dilations.
- e Patients were ascertained from Unity Health in Toronto or London Health Sciences Centre in London, Ontario.
The surgical dilation rate for each patient was determined by dividing the total number of surgical dilations received during the follow-up period by the length of the follow-up period in years, expressed as dilations per year. Differences in baseline clinical and demographic variables between recurrence groups (see section below) were compared using Welch's t test for continuous variables and a Fisher's exact test for categorical variables (Table 1).
2.3 Transcriptomic Analysis of Bulk RNA Sequencing Data
To identify transcriptomic differences between patients with markedly different dilation rates, we performed differential expression analysis comparing samples from patients in the fourth (high recurrence) versus first (low recurrence) quartile of surgical dilation rate (Table 1, n = 28), using DESeq2 v1.42.1 [16] Bioconductor/R package.
The Supporting Information Methods detail RNA sequencing, preprocessing, and differential expression analysis protocols. In brief, all RNA sequencing data in this study were previously generated in Lin et al. [3] and data preprocessing was performed identically. Standard quality control and gene pre-filtering was performed. Batch correction for five sequencing batches was performed using ComBat-seq in sva v3.50.0 [17]. Bioconductor/R package (Table S2, Figure S1). In addition to high versus low recurrence status (the main variable of interest), the DESeq design formula for differential expression analysis adjusted for age at diagnosis, years from diagnosis to biopsy, and use of in-office intralesional steroid injections during the follow-up period. Age at diagnosis was significantly associated with recurrence rate in a previous analysis [3] (see Supporting Infomation Methods for detailed rationale of model covariate inclusion). The DESeq function was used with default parameters. To obtain reliable results for the differentially expressed genes, we performed shrinkage of log2 fold changes (log2FC) using ashr [18] for robust effect size estimates and p values were adjusted for multiple testing hypothesis using Benjamini–Hochberg false discovery rate [19] (“adjusted p value”) and independent hypothesis weighting [20], as implemented in DESeq2 Bioconductor/R package. Genes with an adjusted p < 0.1 and an absolute log2FC > 1 were defined as significantly differentially expressed. ClusterProfiler v4.10.1 [21]. Bioconductor/R package was used to perform gene-set overrepresentation and gene-set enrichment analyses of differentially expressed genes using gene sets from Gene Ontology [22].
All statistical and differential expression analyses were performed in R version 4.3.2. All comparisons were two-tailed. Statistical significance was defined as p < 0.05 for nominal significance and adjusted p < 0.1 when corrected for multiple testing hypotheses.
3 Results
3.1 Cohort Description
Among 56 female white patients with iSGS who had bulk RNA sequencing data available for the affected subglottic tissue and at least 1 year of prospective follow-up, the median dilation rate was 0.84 dilations per year (range: 0.30–1.87). Most patients (39 out of 56; 70.0%) underwent 1–2 dilations during the follow-up period (up to a maximum of 4.2 years), while the remaining patients required 3–5 dilations. See Table S1 for a summary of the clinical and demographic characteristics.
To identify transcriptomic differences that underly markedly different surgical dilation rates, we compared the subglottic tissue from iSGS patients in the fourth versus first quartile of surgical dilation rate (Table 1). The range of dilation rates in the fourth quartile (i.e., high recurrence group) was 1.19–1.87 dilations per year compared to 0.30–0.65 dilations per year in the first quartile (i.e., low recurrence group) (Figure S2). There were no significant differences in clinical/demographic features between these two groups except for a significantly younger age at diagnosis in the high recurrence group (median age 33 vs. 54 years, p = 0.005) (Table 1), consistent with a previous analysis of an overlapping cohort [3].
3.2 Transcriptomic Analysis of Recurrence Rates in Idiopathic Subglottic Stenosis
In total, 220 genes were significantly differentially expressed between the high and low recurrence groups (adjusted p < 0.1 and log2FC > |1|) (Figure 1, Table S3). There were 128 genes that had higher expression in the high recurrence group (log2FC > 1; over-expressed) while 92 genes had higher expression in the low recurrence group (log2FC < −1; under-expressed).
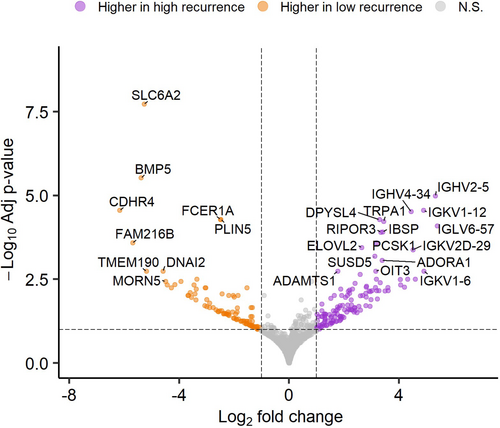
We subsequently performed gene-set overrepresentation analyses to characterize the biological functions of these differentially expressed genes (Figure 2). Genes with increased expression in the high recurrence group were significantly overrepresented in 57 gene sets from Gene Ontology (adjusted p < 0.1), forming two main connectivity groups in an enrichment map (Figure 2A). First, the strongest overrepresentation was for gene sets capturing adaptive immune responses, which included genes encoding immunoglobulin subunits (e.g., IGHV4-34, IGHV2-5, IGHG4) (Table S4). Second, there was a series of gene sets characterizing extracellular matrix components (Figure 2A). These included genes encoding fibroblast-expressed collagen binding proteins that have previous evidence of driving tissue fibrosis, such as COMP [23] and NID2 [24], and metalloproteinases (ADAMTS1, ADAMTS4, ADAMTS18, MMP14), which have established roles in extracellular matrix turnover and remodelling [25] (Table S4).
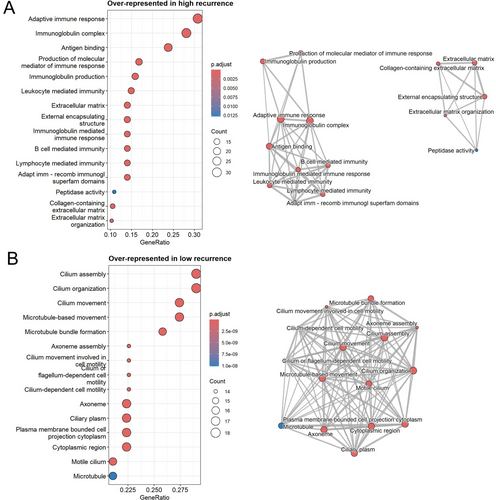
In contrast, genes with higher expression in the low recurrence group were overrepresented in 76 gene sets (adjusted p < 0.1), almost all of which characterize cilia structure and function (Figure 2B). These included genes belonging to the cilia and flagella associated protein (CFAP) family (e.g., CFAP210, CFAP221) and genes encoding dynein subunits (e.g., DNAI2, DNAH11, DNAI1) (Table S5).
We also applied a complementary approach, gene-set enrichment analysis, using a list of all genes tested in differential expression analysis ranked by shrunken (ashr) log2FCs. Twenty-eight sets were significantly enriched (adjusted p < 0.1) (Table S6), showing similar results as the overrepresentation analysis; genes with increased expression in the high recurrence groups (positive enrichment score) were enriched in gene sets related to high immunoglobulin expression, and genes with increased expression in the low recurrence group (negative enrichment score) were enriched in cilia-related gene sets (Table S6).
4 Discussion
Patients with iSGS can vary widely in disease severity and the corresponding intensity of treatment required. In this RNA sequencing study of patients with iSGS, we uncover substantial differences in the transcriptomic profile between patients with divergent recurrence rates. Patients with high recurrence rates exhibited transcriptional markers of an adaptive immune response and elevated extracellular matrix deposition, while those with low recurrence rates had increased cilia expression.
Previous studies on the etiology of iSGS have uncovered molecular and cellular differences between patients with iSGS and unaffected controls [3, 8-11, 13-15]. Many of these differences appear to be mirrored in the spectrum of disease severity within patients with iSGS. Consistent with previous evidence of myofibroblast activity leading to excessive collagen deposition and fibrotic scar formation in iSGS [8, 9, 14], those with higher recurrence rates showed increased expression of genes related to collagen-rich extracellular matrix components, suggesting a relatively elevated fibrotic scar-forming process occurring in those with more severe disease. Interestingly, we also observed a large number of immunoglobulin subunits over-expressed in patients with higher recurrence rates, suggesting the possible involvement of a B cell-mediated immune response. There is previous evidence that iSGS may be initiated by pathogen entry through a disrupted epithelial barrier and that a subsequent adaptive immune response may be involved in tissue remodelling [12, 13]. Likewise, in idiopathic pulmonary fibrosis, a related respiratory disease of unknown etiology, microbial antigen stimulation of B cells has been shown to promote fibroblast migration and activation [26]. Conversely, we found that cilia expression was highly enriched in those with low recurrence rates. Resembling the direction of previous studies that found reduced cilia expression in iSGS compared to unaffected controls [8, 11], these results suggest that relatively greater loss of cilia, compared to the healthy phenotype, may correspond to more severe disease.
Our study leveraged, to our knowledge, the largest available bulk RNA sequencing dataset of patients with iSGS [3]. Prospectively documented surgical dilations with detailed clinical data enabled us to accurately capture a wide spectrum of recurrence rates. The substantial transcriptomic differences uncovered suggest that the development of a prognostic biomarker based on gene expression may be feasible. Future studies, with the availability of an independent validation cohort, may explore whether a gene signature comprising a small number of genes can effectively capture the gene sets enriched in the opposing recurrence rate groups, and be implemented on a cost-effective and clinically feasible platform. The elevated adaptive immune response and fibrotic activity observed in patients with higher recurrence rates suggest that targeting these processes could be a promising treatment option for iSGS in the future [8]. However, confirming the involvement of an immune-mediated fibrotic process will require further functional studies, along with human clinical trials to establish the efficacy of these potential novel therapeutic approaches.
This study also has several limitations. Although we capture tissue-specific effects in disease using biopsies of the subglottis, bulk RNA sequencing is unable to capture the cell-type specificity of these signals. Single-cell RNA sequencing may be useful to further explore the cellular heterogeneity in iSGS. Furthermore, it is unclear whether the associations identified in this study, such as the relatively lower cilia abundance in the high recurrence group, play a direct role in contributing to recurrence or whether it occurs as a consequence of other underlying pathology driving restenosis. This study also includes a subset of patients who received in-office intralesional steroid injections during the follow-up period. Such adjunct procedures were performed based on previous evidence suggesting that they can potentially prolong the time to subsequent restenosis [27-30]. While intralesional steroid injection use was adjusted for as a covariate in the differential expression analysis in this study, future studies with larger sample sizes may be sufficiently powered to stratify analyses by this potential confounder. Finally, in this study, we focused on capturing transcriptomic features that could most likely be attributable to differences in recurrence rates by comparing only those in the outer quartiles of recurrence rates. This approach, however, likely limits the discovery of other patterns of heterogeneity residing in this data that further exploratory analyses utilizing the full cohort may uncover.
5 Conclusion
In summary, we identified transcriptomic differences in the subglottic tissue of iSGS patients with varying recurrence rates. Patients with high recurrence rates exhibited elevated expression of genes associated with B cell immunity and extracellular matrix components, whereas those with low recurrence rates demonstrated greater retention of respiratory cilia.
Acknowledgments
We acknowledge all patients, family members, and caregivers who participated in the current study. We would like to thank Drs. Danielle MacNeil and Adrian Mendez who collected specimens (< 5) for this study. Ms. Sabrina Rashid and Ms. Silkan Bains helped with specimen handling and transportations at Unity Health Toronto, St. Michael's Hospital. Ms. Lama Elkadri helped with sample transportation and data collection.
Conflicts of Interest
A.C.N. has research funding from Novartis Canada, Merck Canada, LabCorp, and Droplet Biosciences for studies that are unrelated to the submitted work. He has equity from and is a consultant for NEED Inc. M.J.C. has research funding from Astra Zeneca, Merck, and Pfizer. He has received payment for speaker honorarium and/or served on advisory boards for Eli Lilly Merck, Astra Zeneca, and Amgen. M.J.C. has equity from and is a consultant for NEED Inc. A.H.K. has consulted for Merck Inc. and serves on the advisory board for Pentax Inc., both unrelated to the current study. P.Y.F.Z., J.W.B., J.S.M., and A.C.N. hold patents for transcriptional biomarkers in head and neck cancer, unrelated to this work. The other authors declare no conflicts of interest.




