Effect of Serial Intralesional Steroid Injections on Risk of Recurrence in Idiopathic Subglottic Stenosis
Funding: This study was funded by a grant from the American Laryngology Association (#1082), an Academic Medical Organization of Southwestern Ontario innovation fund grant (INN21-016), grant support from Department of Otolaryngology—Head and Neck Surgery at University of Toronto and Western University. A.C.N is supported by the Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers. S.Y. is supported by a Canadian Institute of Heath Research Canada Graduate Scholarship-Doctoral.
This study was presented at the 146th Annual Meeting of the American Laryngological Association on May 17, 2025, New Orleans, Louisiana, United States.
Anthony C. Nichols and R. Jun Lin contributed equally to this work.
ABSTRACT
Background
Serial intralesional steroid injections (ILSIs) have been suggested to be an effective adjunct treatment for idiopathic subglottic stenosis (iSGS) by maintaining airway patency and extending inter-surgical intervals. However, evidence for the effectiveness of serial ILSIs remains inconclusive. The current study aimed to assess whether ILSIs reduce the risk of subsequent surgical dilation (i.e., recurrence) in a cohort of patients with iSGS.
Methods
Prospectively collected clinical data for 75 female iSGS patients with 1–4 years of follow-up were analyzed. To assess the effect of ILSI use on the risk of recurrence, we assessed both the time-to-first recurrence using a standard Cox proportional hazards model and all recurrences per patient using a recurrent-events model. Overall, there were 36 patients who had received ILSIs at any point in the follow-up period and 39 patients who had not.
Results
ILSI use was associated with a significantly reduced risk of recurrence in both time-to-first event (hazard ratio (HR) = 0.20, 95% confidence interval (CI) 0.08–0.49) and recurrent events (HR = 0.44, 95% CI 0.26–0.75) multivariate Cox proportional hazard models, along with older age at diagnosis and longer time since diagnosis (all p < 0.05). In the time-to-first event analysis, the median time to recurrence among those who had received ILSIs was 2.5 years compared to 1.4 years among those who had not. The number needed to treat with ILSIs to avoid one recurrence by 2 years follow-up was two.
Conclusion
Serial ILSIs were associated with reduced risk of recurrence, along with older age at diagnosis and longer time since diagnosis.
Level of Evidence
3 (non-randomized controlled cohort/follow-up study).
1 Introduction
Idiopathic subglottic stenosis (iSGS) is a rare disease of unknown etiology, characterized by severe narrowing of the upper airway [1]. The typical age of onset is around 40–60 years, and it affects nearly exclusively white females [2-5]. There are various treatment options for iSGS, including endoscopic dilation and cricotracheal resection [3, 4]. Unfortunately, surgical intervention is not curative for iSGS. Despite repeated dilations or complete resection of the stenosis, scarring often recurs, leading to patient anxiety, financial strain from time off work, and the potential for worsening airway scarring or other complications associated with repeated surgical procedures.
Serial intralesional steroid injections (ILSIs) administered in the clinic setting under local anesthesia have been adopted in many laryngology practices as an adjunct treatment for iSGS [6-14]. Based on evidence that iSGS may be driven by a fibroinflammatory process [2, 15-18] and that corticosteroid injections have been used to manage other fibrotic diseases [19], serial ILSIs have been hypothesized to prolong the time to stenosis recurrence [10, 11]. Nevertheless, evidence for the effectiveness of serial ILSIs remains inconclusive. Small retrospective or case series cohorts with fewer than 25 patients, encompassing various forms of subglottic stenosis, have suggested that ILSIs may nearly double the surgery-free interval compared to the period before ILSI initiation [6, 9, 12]. However, a more recent multicenter prospective study of 290 patients with iSGS found no significant association between serial ILSI use and reduced risk of recurrence [20].
To evaluate the effectiveness of serial ILSIs, we analyzed prospectively collected longitudinal data from a cohort of patients with iSGS (n = 75), which included both patients who had received ILSIs and those who had not. Using multivariate Cox proportional hazards models for both time-to-first event and recurrent event analyses, we assessed the impact of ILSIs on stenosis recurrence risk, adjusting for relevant clinical and demographic covariates. Additionally, we calculated the number needed to treat with ILSIs to prevent one recurrence and summarized the frequency of ILSI administration within our cohort.
2 Methods
2.1 Patient Cohort Description
The study was approved by the research ethics board at both participating institutions (Western HSREB 115746, Unity Health REB 19–324). Written informed consent was obtained from all study participants. Patients 18 years of age or older with a diagnosis of iSGS were ascertained from Unity Health Toronto-St. Michael's Hospital (Toronto, Ontario, Canada) or London Health Sciences Centre (LHSC)—Victoria Hospital (London, Ontario, Canada). Only patients with at least 1 year of prospectively collected clinical data were included. Patients with a history of cricotracheal resection were excluded, given prior evidence indicating a substantially lower risk of recurrence compared to endoscopic dilations [4]. All participants consented to a biopsy of their affected subglottic tissue during a surgical dilation procedure for molecular analyses [2, 15]. The prospective follow-up period was defined as the time from the “index” surgical dilation during which the index biopsy was collected, to the most recent surgical or clinical appointment, until the study cut-off date of July 1, 2024. All data in this study were collected after approval by institutional board review.
All ILSIs were performed in the office under local anesthesia. The majority of the injections were performed trans-nasally via a channeled laryngoscope, and some patients received the steroids via the trans-cricothyroid approach due to a hyperactive gag reflex. Each patient received up to 3 mL of 40 mg/mL of triamcinolone at each injection.
2.2 Study Outcomes and Statistical Analyses
The primary outcome of the study was stenosis recurrence, defined as each surgical dilation following the index dilation that marked the start of the prospective follow-up period. Surgery-free interval was defined as the time between consecutive surgical dilations. To assess the effect of ILSIs administered during the surgery-free interval on the risk of recurrence, we used both a standard (time-to-first event) and the Andersen-Gill extension of the Cox proportional hazard model [21, 22]. First, a standard Cox proportional hazards model was applied to compare the time-to-first recurrence between patients who received one or more ILSI(s) before their first recurrence and those who did not. Second, to include all available longitudinal data points, including when patients experienced multiple recurrences (i.e., three or more surgical dilations), we utilized the Andersen-Gill extension of the Cox proportional hazards model. In this model, ILSI use status was annotated for each surgery-free interval, that is, treated as a time-varying covariate when patients received ILSI(s) during some surgery-free intervals but not others. Thus, specifically the surgery-free intervals where ILSIs were administered were compared with surgery-free intervals where ILSIs were not. Both univariate and multivariate analyses were performed, with the latter adjusting for age at diagnosis, years since diagnosis, body mass index (BMI), and antacid use. Categorical variables required a minimum count of 10 in each category to be included as covariates in the multivariate models. Additionally, years since diagnosis was coded as a time-varying covariate in the recurrent events model, indicating the number of years elapsed since diagnosis at the beginning of each surgery-free interval. For both models, patients were considered censored if their last available record corresponded to an ILSI or clinic appointment. The proportional hazards assumption in the standard Cox proportional hazards model was assessed using Schoenfeld residuals with the cox.zph function [23]. Plots of the scaled Schoenfeld residuals were generated using the ggcoxzph function and visually inspected for a downward or upward trend of the scaled Schoenfeld residuals when the test indicated a statistically significant (p < 0.05) violation of the proportional hazards assumption. Missing data were handled in the models using imputation, using the median value for one missing BMI and the most frequent value for one missing antacid use status.
The number needed to treat (NNT) with serial ILSIs to prevent one recurrence was calculated at multiple time points using Kaplan–Meier estimates of the time-to-first recurrence analysis. The absolute risk reduction (ARR) associated with ILSIs was determined at each time point, and the NNT was derived as the reciprocal of ARR (i.e., NNT = 1/ARR).
All analyses were conducted in R version 4.3.2. The survival package was used to implement the standard and recurrent events Cox proportional hazards models. survfit2 was used to create Kaplan–Meier plots. Statistical significance was defined as p < 0.05.
3 Results
3.1 Cohort Description
In total, there were 75 patients with iSGS with at least 1 year of prospective follow-up data, and no history of cricotracheal resection (Table 1, Figure 1). All patients were female and White. The median age at diagnosis was 47 years (interquartile range (IQR) 34–55 years) and the median BMI was 28.3 kg/m2 (IQR 24.5–34.3). Antacid use was reported by 31 (41.9%) patients. Smoking (current or past history), family history of iSGS (any affected first or second degree relative), and diabetes (type I or II) were infrequent in this cohort (Table 1).
| Patient characteristic (n = 75 total) | |
|---|---|
| Age at diagnosis, years | |
| Median | 47 |
| IQR | 34–55 |
| Range | 18–72 |
| Age at start of follow-up, years | |
| Median | 50 |
| IQR | 38–59 |
| Range | 18–75 |
| BMI, kg/m2a | |
| Median | 28.3 |
| IQR | 24.5–34.3 |
| Range | 18.6–71.1 |
| Smoking ever, No. (%) | 8 (10.7) |
| Antacid use, No. (%)a | 31 (41.9) |
| Family historyb, No. (%) | 4 (5.3) |
| Diabetes (type I or II), No (%) | 5 (6.7) |
| Intralesional steroid injection use, No. (%)c | 36 (48.0) |
| Site, No. (%) | |
| LHSC, London | 36 (48.0) |
| UH, Toronto | 39 (52.0) |
| Surgical dilation rate, dilations per yeard | |
| Median | 0.82 |
| IQR | 0.68–1.21 |
| Range | 0.30–1.87 |
| Follow-up duration, years | |
| Median | 2.2 |
| IQR | 1.6–2.7 |
| Range | 1.1–4.2 |
- Abbreviations: BMI, body mass index; IQR, interquartile range; LHSC, London Health Sciences Centre; UH, Unity Health.
- a Missing data from one patient (n = 74).
- b Defined as any first-degree relative with idiopathic subglottic stenosis.
- c Having received an in-office intralesional steroid injection at any point in the follow-up period.
- d The dilation rate was calculated by dividing the total number of surgical dilations received by the follow-up time in years.
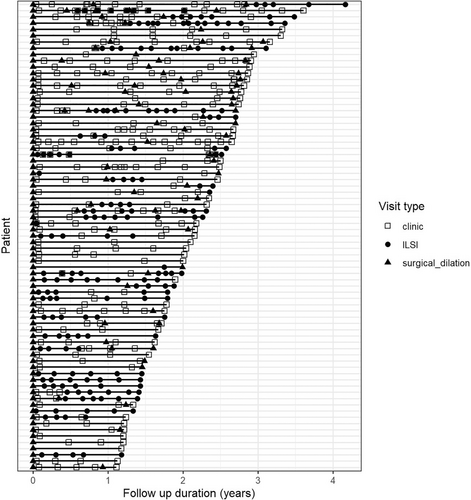
Among these 75 patients, there were 687 recorded surgical or clinical appointments, including 151 surgical dilations (22.5%) and 200 ILSIs (29.8%) (Table S1, Figure 1). Of the 151 surgical dilations, 148 (98.0%) were balloon dilations while the remaining were rigid dilations. Nearly all dilations were performed with microsuspension laryngoscopy and were CO2 laser assisted to divide the stenosis (Table S1). In most cases, intra-lesional steroids were administered at the end of the procedure (Table S1). The overall rate of surgical dilations was 0.82 dilations per year (IQR 0.68–1.21) (Figure S1). There were 30 patients (39.0%) who received only the initial surgical dilation and did not require a second dilation. Twenty-eight patients (37.3%) received two surgical dilations and the remaining received three to five dilations within the 1–4 year follow-up period (Table S1). Approximately half of the cohort (n = 39; 52.0%) had never received an ILSI at any point in the follow-up period while the remaining (n = 36; 48.0%) received between one to 14 ILSIs (Tables 1, S1, and S2).
3.2 Effect of Serial Intralesional Steroid Injections on Risk of Recurrence
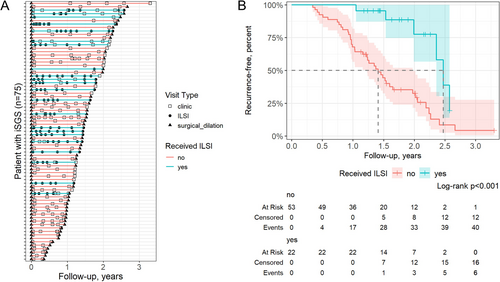
We first compared the time-to-first recurrence, defined as the first subsequent surgical dilation, between patients who received (n = 22) versus those who did not receive (n = 53) one or more ILSI(s) prior to their first recurrence or date of censoring (Figure 2, Table S3). ILSI use was associated with significantly lower risk of recurrence in a univariate model (hazard ratio (HR) = 0.23, 95% confidence interval (CI) 0.10–0.54) and in a multivariate model (HR = 0.20, 95% CI 0.08–0.49) (Table 2). The median time to recurrence among those who received ISLIs was 2.5 years compared to 1.4 years among those who did not (Figure 2). In the multivariate model, older age at diagnosis (HR = 0.96, 95% CI 0.94–0.98) and longer time since diagnosis (HR = 0.88, 95% CI 0.80–0.98) were also associated with significantly reduced risk of recurrence (Table 2). We evaluated the proportional hazards assumption using Schoenfeld residuals and identified a deviation from model assumptions for ILSI use status near the end of the follow-up period, beginning at approximately 2.2 years (Figure S2). To address this, we repeated the analysis censoring all data beyond 2.2 years. This adjustment resulted in minimal differences in the outcomes (Table S4).
| Time-to-first event modela | Recurrent events modelb | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Univariate model | ||||
| Intralesional steroid injectionc | 0.23 (0.10–0.54) | 0.0008 | 0.44 (0.26–0.73) | 0.0014 |
| Multivariate model | ||||
| Intralesional steroid injectionc | 0.20 (0.08–0.49) | 0.0005 | 0.44 (0.26–0.75) | 0.0024 |
| Age at diagnosis | 0.96 (0.94–0.98) | 0.0012 | 0.98 (0.96–0.99) | 0.0026 |
| Years since diagnosisd | 0.88 (0.80–0.98) | 0.0194 | 0.93 (0.88–0.98) | 0.0104 |
| BMI (kg/m2) | 1.00 (0.97–1.03) | 0.9747 | 1.00 (0.98–1.02) | 0.8158 |
| Antacid usee | 1.22 (0.66–2.26) | 0.5329 | 1.15 (0.79–1.66) | 0.4616 |
- Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; HR, hazard ratio.
- a Time-to-first recurrence (i.e., first surgical dilation following the index dilation) in a standard Cox proportional hazards model.
- b Andersen-Gill adapted Cox proportional hazards model applied to all recurrences.
- c Having received one or more in-office intralesional steroid injections during each surgery-free interval.
- d The number of years since diagnosis at the beginning of each surgery-free interval.
- e Active use of antacid medication (e.g., proton pump inhibitors, H2 receptor antagonists) throughout the follow-up period, including during periods of ILSI administration.
We also utilized a recurrent event-adapted Cox proportional hazards model to account for all recurrences in our dataset (Table 2). This analysis included 43 surgery-free intervals (from n = 36 patients) during which one or more ILSI(s) were administered and 102 surgery-free intervals without ILSI use (from n = 55 patients; these include intervals where no ILSI was administered in patients who had received ILSIs during other intervals). Consistent with the findings from the time-to-first event analysis, ILSI use was associated with a significantly lower risk of recurrence in both the univariate model (HR = 0.44, 95% CI 0.26–0.73) and the multivariate model (HR = 0.44, 95% CI 0.26–0.75). Similarly, older age at diagnosis and a longer duration since diagnosis were significantly associated with a reduced risk of recurrence in the multivariate model (Table 2).
3.3 Number Needed to Treat With Intralesional Steroid Injections
We calculated the NNT with ISLIs to prevent one recurrence at 1.0, 1.5, and 2.0 years follow-up, based on the ARR at each time point derived from the Kaplan–Meier estimates in the time-to-first recurrence analysis. The percent of recurrence-free individuals in the ILSI group versus the no-ILSI group at 1.0, 1.5, and 2.0 years was 100.0% vs. 67.9%, 95.5% vs. 44.4%, and 77.6% vs. 32.6%, respectively (Figure 2B). These correspond to an NNT of three, two, and two at 1.0, 1.5, and 2.0 years, respectively (Table S5).
3.4 Frequency of Intralesional Steroid Injections
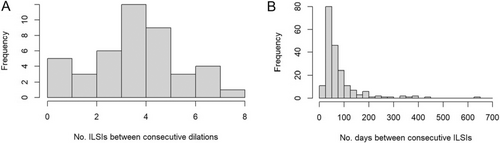
Given that ILSIs are typically administered in a serial manner, we quantified their usage frequency among all patients who had ever received an ILSI (n = 36 patients). Among 43 surgery-free intervals where one or more ILSI(s) were received, either between consecutive surgical dilations or from dilation to censoring, the median number of ILSIs received was four (range 1–8) (Figure 3A). Among the 200 recorded ILSIs, the median time from either the previous surgical dilation or the previous ILSI was 53 days (IQR 42–91) (Figure 3B).
4 Discussion
The current study involved prospectively documented surgical and clinic procedures of 75 patients with iSGS, where a subset of patients was treated with serial ILSIs between endoscopic dilations. ILSI use was associated with a significantly reduced risk of recurrence in both time-to-first event (HR = 0.20) and recurrent event (HR = 0.44) multivariate Cox proportional hazard models. At 2 years follow-up, 78% of patients who did not receive ILSIs experienced recurrence, compared to 33% among patients who did, corresponding to a NNT with ILSIs of two to avoid one recurrence. Notably, within 1 year of the index surgical dilation, no individuals treated with ILSIs experienced recurrence, compared to 30.1% in those not treated with ILSIs. Additionally, the multivariate models showed that older age at diagnosis was associated with a lower risk of recurrence, consistent with previous studies [2, 24, 25], along with a longer time since diagnosis.
In the current study, ILSIs prolonged the median time-to-first recurrence from 1.4 years in those who did not receive ILSIs to 2.5 years in those who did. This effect size appears roughly consistent with previous reports using smaller sample sizes that included other subtypes of subglottic stenosis. For example, the surgery-free interval before versus after ILSI initiation was 6.1 months versus 10.7 months in Neevel et al. [6], 288 days versus 545 days in Pan and Rosow [12], and 10.1 months versus 22.6 months in Bertelsen et al. [9]. Thus, based on the results of the current study and these previous reports, it appears that ILSI use may prolong the time to recurrence by at least approximately 50%. While delaying the time to recurrence, it is also evident from the current study that maintenance of surgery-free intervals using serial ILSIs involves frequent administration, with patients receiving a median of four injections that are each separated by a median of ~2 months between consecutive surgical dilations. Thus, patient preference and accessibility to healthcare may be important factors to consider when making a shared decision on the treatment approach in patients with iSGS.
The results of the current study stand in contrast to a previous large prospective study on patients with iSGS by Hoffman et al. [20], that found a more modest, non-statistically significant risk reduction for recurrence among patients who were treated with serial ILSIs compared to those who were not (HR = 0.68; 95% CI 0.16–2.78). Interestingly, approximately three-quarters of patients remained recurrence-free by 2 years follow-up in the group receiving serial ILSIs in both Hoffman et al. and in the time-to-first recurrence analysis in this study. The apparent difference in the relative effectiveness of serial ILSIs between the two studies may be attributed to the significantly lower recurrence rate observed in the surgical dilation-only group in Hoffman et al. compared to the current study. By 2 years of follow-up, approximately 63% of patients in Hoffman et al.'s surgical dilation-only group remained recurrence-free, compared to 33% in this study. Another important consideration is that given the absence of established guidelines, neither study included standardized indications for initiating serial ILSIs nor standardized protocols for their administration. Differences in the time to initiation of serial ILSIs following surgical dilation, frequency of ILSIs, and selection of patients to undergo serial ISLIs may affect the measured outcomes. Further studies are warranted to determine whether factors such as past history of rapid recurrence, elevated BMI, and challenging surgical exposure may affect selection for treatment with ILSIs and treatment success. Additionally, Hoffman et al. did not require a minimum of 1 year of follow-up for all patients, and their data appear to show substantial censoring before the one-year mark. Less reliably capturing the true number of recurrences that occurred by 1 year follow up could potentially affect the observed treatment group differences [20].
The results of this study are also consistent with previous studies on age and menopause status in iSGS that have suggested the general trend that older age is associated with reduced risk of recurrence [2, 24, 25]. In this study, the patient's age was delineated into two variables: the age at diagnosis and the number of years since diagnosis. Both variables were significant predictors of recurrence in multivariate models suggesting that they may confer additive/independent effects; older age at diagnosis was associated with lower risk of recurrence (~2%–3% reduction in risk per year increase) and additionally the disease severity appeared to generally wane over time (~7%–12% reduced risk of recurrence per year from diagnosis). Given the widely speculated hormonal involvement in the etiology of iSGS, it has been hypothesized that this age-related effect on disease severity may be attributable to cumulative years of estrogen exposure and/or menopause status [24, 25]. While some initial studies have assessed this subject [24, 25], further studies will be needed to disentangle the complex relationship between these variables.
4.1 Advantages and Limitations
To our knowledge, this is one of two prospective studies on the effect of serial ILSIs as an adjunctive treatment for patients with iSGS [20]. This study included 36 patients who received ILSIs and 39 who did not, representing a substantially larger sample size than previous retrospective studies, where fewer than 10 patients with documented surgical dilations prior to ILSI initiation were available for comparison [6, 9, 12]. Additionally, this study exclusively focused on patients with iSGS, in contrast to many previous studies that included more heterogeneous causes of subglottic stenosis [6, 9, 12]. In contrast to a previous prospective study on serial ILSI [20], our data capture the time point that each ILSI was received and the total number of ILSIs received between each recurrence. We applied both time-to-first event and recurrent event models to account for all available surgical events, providing a comprehensive analysis. Furthermore, the availability of detailed clinical data allowed for multivariate models that included other covariates associated with recurrence risk.
This study has several limitations. Although a minimum of 1 year of follow-up was required for inclusion, a longer follow-up period would likely provide a more accurate assessment of the recurrent nature of iSGS. When testing the proportional hazards assumption using Schoenfeld residuals, we observed a trend towards a higher risk of recurrence in the ILSI group after approximately 2.2 years (Figure S2). While this may suggest an upper limit to the efficacy of ILSIs in maintaining surgery-free intervals, it could also represent a spurious trend due to the small number of patients at risk beyond that time point (Figure 1B). Additionally, the relatively higher hazard ratio from the recurrent events model (HR = 0.44, 95% CI 0.26–0.75) compared to the time-to-first event model (HR = 0.20, 95% CI 0.08–0.49) may indicate that the relative effectiveness of serial ILSIs diminishes with successive surgery-free intervals. Longer follow-up with more patients extending beyond the first surgery-free interval will be essential to evaluate the long-term durability of serial ILSIs. While we regard requiring a surgical dilation to be the most relevant clinical outcome of restenosis, our study lacks measurement of functional outcomes, such as peak expiratory flow, to assess direct physiological changes that may be conferred by ILSI use. Future studies will also be necessary to investigate potential side effects of serial ILSIs [6]. Finally, despite adjusting for covariates, the results from this non-randomized study may still reflect bias arising from factors such as variations in surgeon preference, patient access to healthcare, and other demographic features.
5 Conclusion
Serial ILSIs, used as an adjunct therapy to surgical endoscopic dilations, were associated with a significantly reduced risk of recurrence in patients with iSGS. Older age at diagnosis and longer time since diagnosis were also linked to a lower recurrence risk. The number needed to treat (NNT) with serial ILSIs to prevent one recurrence within 2 years post-surgical dilation was two. However, given the observational nature of this study and discordant results in the literature, further studies are still needed to confirm the effectiveness of ILSIs in iSGS.
Acknowledgments
We acknowledge all patients, family members, and caregivers who participated in the current study.
Conflicts of Interest
A.C.N. has research funding from Novartis Canada, Merck Canada, LabCorp, and Droplet Biosciences for studies that are unrelated to the submitted work. He has equity from and is a consultant for NEED Inc. M.J.C. has research funding from Astra Zeneca, Merck and Pfizer. He has received payment for speaker honorarium and/or served on advisory boards for Eli Lilly Merck, Astra Zeneca, and Amgen. M.J.C. has equity from and is a consultant for NEED Inc. A.H.K. has consulted for Merck Inc. and serves on the advisory board for Pentax Inc., both unrelated to the current study. P.Y.F.Z., J.W.B., J.S.M., and A.C.N. hold patents for transcriptional biomarkers in head and neck cancer, unrelated to this work. R.J.L., K.F., H.K., J.A., P.M., A.H.K., S.Y., M.A.J., H.P., V.D. have no conflicts of interest to declare.




