A Programming Algorithm for Optimizing Hypoglossal Nerve Stimulator Respiratory Entrainment
This work is supported by National Institutes of Health (R01HL161635).
D.K. has received research support from Laborie Medical Technologies Corp, Invicta Medical, Inspire Medical, and Nyxoah SA. He is a consultant to Invicta Medical and Nyxoah SA, and is a scientific advisory board member with Nyxoah SA. H.B.: None. K.G. has received research support from Inspire Medical. P.H. has received research support from Nyxoah SA and Inspire Medical and is a consultant for Inspire Medical. A.S. has received research support from Inspire Medical and is a consultant for XII Medical. M.S. is a consultant to Inspire Medical and Nyxoah SA.
Abstract
Hypoglossal nerve stimulation (HNS) is a surgical treatment for obstructive sleep apnea that activates in a phasic manner. The most widely available HNS device has respiratory entrainment programming settings that are not widely utilized. We present an algorithm for office-based respiratory sensing adjustments to optimize HNS respiratory entrainment. Laryngoscope, 135:1532–1536, 2025
INTRODUCTION
Hypoglossal nerve stimulation (HNS) is an increasingly popular surgical treatment alternative to positive airway pressure for obstructive sleep apnea (OSA), but approximately one-third of patients struggle with incomplete responses to therapy.1
The pharynx exhibits phasic changes in collapsibility throughout the respiratory cycle.2 It is most compliant during expiration, and prior work suggests that HNS timing affects therapy efficacy.3 The most widely available HNS device's programming manual states that it is designed to activate during late expiration (pg. 109) and recommends adjusting respiratory detection settings if proper therapy timing is not observed.4 These settings are rarely changed in standard clinical practice and, anecdotally, our group observed that the default device settings often poorly entrain respiration in both the wake and sleep states, causing the device to largely operate in a 4 s on, 1.5 s off fashion that stimulates over approximately 70% of the respiratory cycle (Fig. 1). We therefore iteratively developed an algorithm for office-based respiratory sensor adjustments to optimize individual patient respiratory entrainment (Fig. 2). A comprehensive review of the proposed algorithm is beyond the scope of this brief case review, although it is important to note that explanations for the sensor programming graphical indicators, as well as the sensitivity, threshold, and “off” timer settings can be found in the physician programming manual on pages 15–17 and 77–81. Review of these definitions will give interested clinicians an excellent basis for understanding the flow of the proposed algorithm's logic.
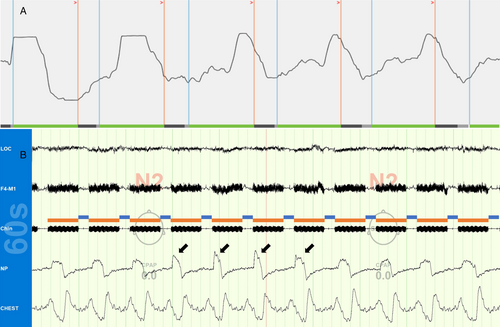
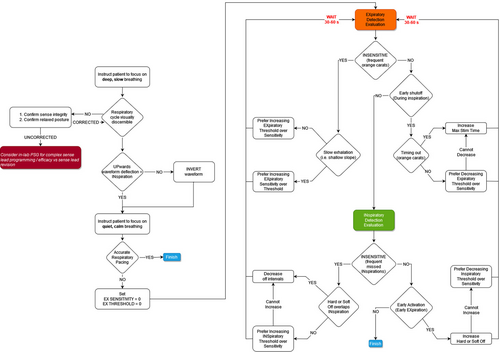
Examples of our initial experiences are presented herein to empower other practitioners with a framework for exploring respiratory entrainment settings and further algorithm refinement. Each patient met relevant clinical screening criteria and underwent uneventful HNS implantation (Inspire IV; Inspire Medical Systems, Inc).
Case 1
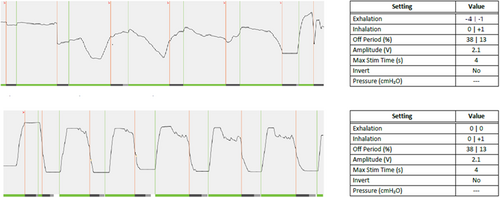
A 55-year-old male had a history of moderate OSA with a body mass index (BMI) of 27 kg/m2 and an apnea-hypopnea index (AHI) of 26.2 events/h. After HNS implantation, he self-titrated therapy in the default electrode configuration over a period of several months but developed a gagging sensation at 2.1 V despite incomplete resolution of OSA symptoms. Awake endoscopy did not reveal significant differences with changes in various stimulation parameters. Suboptimal stimulation timing was observed, and expiratory settings were changed to sensitivity = 0 and threshold = 0 (Fig. 3). At follow-up, the patient reported his gagging sensation had resolved and HNS was comfortable. Titration polysomnography (PSG) revealed a residual AHI of 12.9 events/h at 2.2 V.
Case 2
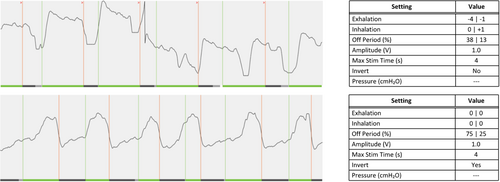
A 47-year-old male had a history of severe OSA with a BMI of 29 kg/m2 and an AHI of 51.0 events/h. After HNS implantation, he underwent several rounds of HNS adjustment for residual OSA and persistent subjective symptoms. Further PSG testing revealed fragmented sleep with multiple arousals and a residual AHI of 30.2 events/h at 2.0 V. At his subsequent office visit, he was observed to have an inverted respiratory sensor waveform with HNS activating at end-inspiration (Fig. 4). After waveform inversion and further entrainment changes, he reported an immediate improvement in subjective respiratory synchronization, overall sleep quality, and therapy tolerance. He further self-titrated HNS to 2.8 V, and his residual AHI on repeat PSG was 10.9 events/h.
Case 3
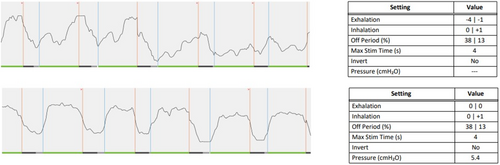
A 66-year-old male had a history of severe OSA with a BMI of 29 kg/m2 and an AHI of 34.4 events/h. He experienced rapid subjective benefit after HNS implantation, but he had persistent moderate OSA (AHI 19 events/h on a home sleep apnea test) in the default electrode configuration at 1.5 V after multiple therapy adjustments. Suboptimal stimulation timing was observed at a subsequent office visit, which was corrected by changing the expiratory sensitivity and threshold settings to 0 (Fig. 5). Home sleep testing with the new respiratory sensor settings demonstrated AHI of 11.3 events/h.
DISCUSSION
This case series demonstrates improvements in patient tolerance and efficacy of HNS with office-based adjustments to respiratory sensing parameters that affect stimulation timing. Our initial experiences suggest that respiratory sensor changes can impact patient comfort and response to HNS.
The algorithm presented herein was designed for use in the awake state as repeated HNS titration polysomnography studies are onerous for patients. The functions of the different programming parameters can be found within the manufacturer's programming manual (pgs 15–17).4 We found it important to evaluate patients in the recumbent position so that involuntary postural adjustments did not contaminate respiratory sensing. In general, the algorithm prioritizes appropriate expiratory phase detection, which resolved most entrainment issues in our experience.
The optimal timing for intermittent HNS therapy remains unknown. Mucosal surface tension forces and airway narrowing upon onset of inspiration contribute to increased collapsibility,2 and it is generally assumed that HNS activation at end-expiration therefore minimizes the amount of energy required to open the pharynx. Interestingly, our team has observed that the current HNS device does not algorithmically anticipate end-expiration, such as by using a prespecified delay from the peak inspiratory deflection. It instead requires an upward deflection of the pressure waveform meeting trigger criteria signaling the beginning of inspiration to activate. This behavior can be observed by asking a patient without cardiac artifact in the respiratory signal to hold their breath (we have observed cardiac artifact to frequently cause spurious activation).
This early evaluation of respiratory sensing parameters has significant limitations. The cases presented herein were selected from a larger, heterogenous convenience sample of HNS nonresponders, because they primarily underwent respiratory sensing changes that affected their HNS experience. We think it possible that there are groups of absolute responders and nonresponders to HNS therapy who will not achieve meaningful changes in their therapy experience because of other, more robust determinants of clinical response.5 There is likely an as-yet-undefined group of borderline responders for whom the timing of stimulation onset affects clinical response, but larger sample sizes will be needed to quantify this cohort. We hope that our initial experience with respiratory sensing lead changes motivates the wider sleep community to begin identifying these patients. For the sake of clinical efficiency, most nonresponders seen in our clinics underwent electrode configuration changes at the same time as respiratory sensing adjustments, which currently impedes a broader analysis of the independent effects of HNS respiratory entrainment. Post-implant management in HNS nonresponders can be challenging, and many providers pursue a variety of different therapy adjustments and adjunct therapies tailored to the individual patient.5
CONCLUSION
Optimizing HNS entrainment in the office setting may help improve patient comfort and response to therapy. The ultimate value of respiratory sensing parameter adjustments remains unknown, and we do not currently suggest that most patients will require programming changes for success with therapy. Further work is required to ascertain the true benefit of respiratory sensing changes. We encourage other investigators to apply and further refine our proposed algorithm.




