Association between Estrogen Exposure and Idiopathic Subglottic Stenosis
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Funding from NHLBI grant no. R01HL146401 (Alexander Gelbard), 2018 Burroughs Wellcome Fund Physician-Scientist Institutional Award to Vanderbilt University (ID: 1018894), and North American Airway Collaborative is supported by Patient-Centered Outcomes Research Institute (1409-22214).
American Laryngological Association. Combined Otolaryngology Society Meetings. Boston, MA, USA. May 2023.
Abstract
Objective
Idiopathic subglottic stenosis (iSGS) is a rare, recurrent, fibroinflammatory disease affecting the larynx and proximal trachea. Given it occurs primarily in adult females, estrogen is speculated to play a central pathophysiological role. This study aimed to evaluate relationships between estrogen exposure, disease progression, and recurrence.
Methods
North American Airway Collaborative (NoAAC) data of adults with iSGS obstructive airway lesions, who underwent index endoscopic airway dilation, were used to identify associations between estrogen exposure, disease characteristics, and time to recurrence (TTR), and interventions were analyzed using Kruskal–Wallis test and Pearson coefficient. Cox proportional hazards regression models compared hazard ratios by estrogen exposure. Kaplan–Meier curves were plotted for TTR based on menopausal status.
Results
In all, 533 females had complete estrogen data (33% premenopausal, 17% perimenopausal, 50% postmenopausal). Median estrogen exposure was 28 years. Overall, there was no dose–response relationship between estrogen exposure and disease recurrence. Premenopausal patients had significantly shorter time from symptom manifestation to diagnosis (1.17 vs. 1.42 years perimenopausal vs. 2.08 years postmenopausal, p < 0.001), shorter time from diagnosis to index endoscopic airway dilation (1.90 vs. 2.50 vs. 3.76 years, p = 0.005), and higher number of procedures (1.73 vs. 1.20 vs. 1.08 procedures, p < 0.001).
Conclusions
We demonstrate premenopausal patients may have a more aggressive disease variant than their peri- and postmenopausal counterparts. However, it is unclear as to whether this is related to reduced estrogen in the peri- and postmenopausal states or the age-related physiology of wound healing and inflammation, regardless of estrogen.
Level of Evidence
3 Laryngoscope, 134:825–830, 2024
INTRODUCTION
Idiopathic subglottic stenosis (iSGS) is a rare, fibroinflammatory upper airway disease that affects predominantly Caucasian women with a mean age of 50.35 years.1 The pathophysiology of iSGS has yet to be elucidated; however, the sex propensity suggests that estrogen may play an important role. Estrogen's role in the pathophysiology is biologically plausible as it (1) affects scar formation and wound healing2, 3 and (2) regulates tissue inflammation4 and (3) estrogen receptors (estrogen beta, ERβ) are dysregulated in mucosal iSGS specimens.5
Currently, no animal models exist to test the biological role of estrogen in iSGS. Thus, the potential role of estrogen in iSGS biology is inferred from the sex and hormonal differences elucidated in other related inflammatory respiratory conditions (e.g., asthma).6 Animal models have demonstrated increased allergic airway inflammation in females.7 Conversely, oophorectomized models have few eosinophils (less inflammation) that dramatically increase in numbers with exposure to exogenous estrogen.8, 9 At present, the two theories regarding the role of estrogen in iSGS are that (1) estrogen is a required initiating factor for iSGS onset, and/or (2) longer estrogen exposure increases the risk of developing and the severity of iSGS, and thus the peak incidence in middle-aged women. The present study leverages the large prospective North American Airway Collaborative (NoAAC) patient cohort to investigate the relationship between years of estrogen exposure and severity of disease.
METHODS
The Vanderbilt University Medical Center Institutional Review Board (ID: 1018894) approved study uses data from the iSGS1000 patient cohort, a prospective multi-institutional cohort established in 2014 by the NoAAC, housed within the NoAAC Data Coordinating Center. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Patient Population
NoAAC inclusion criteria are detailed elsewhere.10 In brief, participants included were age > 18 years with an obstructive airway lesion involving the subglottis without a history of prior traumatic, thermal, or caustic laryngotracheal injury or neck radiation. Excluded were patients with history of intubation or tracheotomy within two years of symptom onset, positive antinuclear cytoplasmic antibody titer, or presence of vasculitis or collagen vascular disease. We limited the study population to participants whose index procedure was an endoscopic dilation to minimize treatment related variability. Subsequent inclusion was limited to those with recorded estrogen exposure data in baseline and follow-up surveys.
Study Protocol
The study followed a pre-specified protocol.10 At enrollment, patients completed surveys collecting baseline sociodemographic characteristics. Disease and treatment data were abstracted from electronic health records. Enrolled patients underwent standard-of-care surgery at their respective medical centers. Each patient's “index” or most recent operation (if last one predated study inception) was defined as time zero (T0). After enrollment, study patients completed an electronic health status survey every 3 months. We collected the date of first subsequent operative intervention (T1, if applicable). The time between T0 and T1 was termed time to recurrence (TTR). The TTR allows us to perform time-to-event Kaplan–Meier analyses to investigate effects of sociodemographic, disease-related, and treatment-related modifying factors on recurrence.
Outcome Variable
Recurrence, the primary outcome and proxy for severity of disease, was defined as receipt of repeat operative intervention. Consistent with prior publications,10, 11 operative intervention was limited to endoscopic dilation (excluding tracheal resection and endoscopic resection). Endoscopic dilation herein subsumes all techniques using dilation including via balloon or rigid instruments and variations in the operation, including radial incisions with cold knife or laser, steroid injection, and/or mitomycin-C application. Peak expiratory flow (PEF) is the secondary outcome. PEF data were included to mitigate the fact that younger patients have higher respiratory function and activity levels, prompting increased sensitivity to airway patency changes. PEF is agnostic to patient-reported factors. PEF values did not differ by menopausal status group indicating that changes in subjective airway patency leading to recurrent dilation were representative of anatomic airway narrowing. The participants measured PEF on a handheld device and recorded results using mobile device software.
Exposure
Estrogen exposure was defined as years from menarche to enrollment or menopause (whichever came first) and termed estrogen-years. A second categorical estrogen exposure variable was menopausal status at T0 (i.e., pre-, peri-, or postmenopausal). Premenopausal was defined as having regular menstrual cycles at time of baseline survey; perimenopausal patients reported irregular cycles; and postmenopausal patients reported cessation of menstrual cycles for at least 12 months. Additional estrogen-related variables collected included history of hormone replacement therapy (y/n) and number of full-term pregnancies.
Covariates
Multiple variables were used to adjust for the association between estrogen exposure and iSGS recurrence including sociodemographic variables (age at index procedure, education level, and Charlson Comorbidity Index) and disease-related variables. Body mass index also was included as a covariate due to the link between adiposity and estrogen production.13
Statistical Analysis
Median and interquartile ranges and percentages summarized continuous and dichotomous variables, as appropriate. We performed comparative analyses to evaluate the relationship between estrogen exposure and recurrence. The first analysis, using Wilcoxon rank sum and Pearson coefficient, compared median estrogen-years between patients with and without recurrence. The second analysis evaluated the association between recurrence and patient menopausal status (premenopausal, perimenopausal, and postmenopausal) at T0 using Kruskal–Wallis one-way analysis of variance and Pearson coefficient.
Kaplan–Meier and Adjusted Analyses
Hazard ratios (HR) and 95% confidence intervals (CIs) were calculated using weighted Cox proportional hazards regression models comparing estrogen-year quartiles using the lowest quartile as the referent. Kaplan–Meier analyses evaluated the TTR as a function of menopausal status at index procedure, estrogen-years quartile, and age at presentation (diagnosis with disease). The secondary outcome, PEF, stratified by menopausal status was plotted over time using Loess curves of mixed-effects model-fitted values to quantify disease severity among those not needing recurrent operation, because PEF is representative of degree of anatomic stenosis.12
RESULTS
A total of 603 women met inclusion criteria. Of these, 533 had adequate data for calculation of estrogen-years. These patients were Caucasian and had a median age of 49 years at T0 (IQR 42–57; Table I). The participants had symptoms a median of 1.5 years before diagnosis with iSGS (IQR 0.7–3.6) and had a median of three airway operations prior to study enrollment (IQR 2–7). Median estrogen-years exposure was 28 (IQR 20–34) and 50% of women were postmenopausal at T0. In all, 169 of 603 (28%) of patients experienced recurrence during the 3-year study period.
| N | Endoscopic Dilation (N = 603) | |||
|---|---|---|---|---|
| Estrogen exposure | 533 | 20 | 28 | 34 |
| Estrogen exposure (grouped by quartiles) | 533 | |||
| [0,20.5] | 26% | 136 | ||
| [20.5,28.1] | 25% | 135 | ||
| [28.1,33.6] | 25% | 131 | ||
| [33.6,48.5] | 25% | 131 | ||
| Menopausal status at index procedure | 563 | |||
| Premenopausal | 36% | 204 | ||
| Perimenopausal | 14% | 79 | ||
| Postmenopausal | 50% | 280 | ||
| Full-term pregnancy count | 550 | |||
| 0 | 25% | 139 | ||
| 1 | 15% | 81 | ||
| 2 | 37% | 203 | ||
| 3 | 16% | 88 | ||
| More than 3 | 7% | 39 | ||
| Hormone replacement therapy | 78 | |||
| Yes | 17% | 13 | ||
| No | 83% | 65 | ||
| Age at menarche | 539 | |||
| Less than 12 | 17% | 91 | ||
| 12 | 31% | 167 | ||
| 13 | 29% | 154 | ||
| 14 | 11% | 57 | ||
| 15 or older | 13% | 70 | ||
| Age at menopause | 261 | 43 | 49 | 52 |
| Age at presentation | 448 | 37 | 45 | 52 |
| Age at presentation (grouped by quartiles) | 448 | |||
| [17,37] | 26% | 115 | ||
| [37,45] | 27% | 120 | ||
| [45,52] | 24% | 108 | ||
| [52,84] | 23% | 105 | ||
| Age (at index procedure) | 564 | 42 | 49 | 57 |
| Age (at index procedure by quartile) | 564 | |||
| [22,42] | 26% | 146 | ||
| [42,49] | 25% | 142 | ||
| [49,57] | 26% | 144 | ||
| [57,85] | 23% | 132 | ||
| Hormonal birth control | 279 | |||
| Yes | 32% | 89 | ||
| No | 68% | 190 | ||
| Number of prior surgeries (total treatments) | 533 | 2 | 3 | 7 |
| Time from first symptom date to diagnosis date (years) | 446 | 0.66 | 1.5 | 3.57 |
| Time from diagnosis through enrollment date (years) | 439 | 0.7 | 2.6 | 5.8 |
| Frequency of number of prior procedures | 414 | 0.77 | 1.37 | 2.79 |
| BMI | 550 | 23 | 27 | 33 |
| BMI—30 and above (obese) | 550 | |||
| Yes | 36% | 200 | ||
| No | 64% | 350 | ||
- Note: a, b, c represent the lower quartile a, the median b, and the upper quartile c for continuous variables. N is the number of nonmissing values. Numbers after proportions (%) are frequencies.
Estrogen-Years and Recurrence
There was no difference in estrogen-years between women who recurred or did not (recur: median 28 [20–33] vs. no recur: 28 [21–34], p = 0.28; Table II). Similarly, no difference was observed in menopausal status, full-term pregnancy count, use of hormone replacement therapy or hormonal birth control, or age at menarche between those that recurred or did not. Age at first iSGS symptom presentation did not differ (recur: 44 years [36–53] vs. no recur: 45 [38–52]; p = 0.85) nor did age at T0 (both: 49 years [42–57]; p = 0.98). Patients who experienced recurrence during the study had more prior iSGS operations (recur: 4 operations [2–8] vs. no recur: 3 [1–6]; p = 0.04).
| N | Recurrence: Yes (N = 169) | Recurrence: No (N = 434) | Combined (N = 603) | Test Statistic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen exposure | 533 | 20 | 28 | 33 | 21 | 28 | 34 | 20 | 28 | 34 | F1,531 = 1.1 | p = 0.281 |
| Estrogen exposure (grouped by quartiles) | 533 | p = 0.562 | ||||||||||
| [0,20.5] | 29% | 46 | 24% | 90 | 26% | 136 | ||||||
| [20.5,28.1] | 24% | 38 | 26% | 97 | 25% | 135 | ||||||
| [28.1,33.6] | 26% | 42 | 24% | 89 | 25% | 131 | ||||||
| [33.6,48.5] | 22% | 35 | 26% | 96 | 25% | 131 | ||||||
| Menopausal status at index procedure | 563 | p = 0.812 | ||||||||||
| Premenopausal | 38% | 64 | 35% | 140 | 36% | 204 | ||||||
| Perimenopausal | 13% | 22 | 14% | 57 | 14% | 79 | ||||||
| Postmenopausal | 49% | 82 | 50% | 198 | 50% | 280 | ||||||
| Full-term pregnancy count | 550 | p = 0.712 | ||||||||||
| 0 | 26% | 42 | 25% | 97 | 25% | 139 | ||||||
| 1 | 15% | 25 | 14% | 56 | 15% | 81 | ||||||
| 2 | 36% | 59 | 37% | 144 | 37% | 203 | ||||||
| 3 | 18% | 29 | 15% | 59 | 16% | 88 | ||||||
| More than 3 | 5% | 8 | 8% | 31 | 7% | 39 | ||||||
| Hormone replacement therapy | 78 | p = 0.652 | ||||||||||
| Yes | 14% | 3 | 18% | 10 | 17% | 13 | ||||||
| No | 86% | 19 | 82% | 46 | 83% | 65 | ||||||
| Age at menarche | 539 | p = 0.722 | ||||||||||
| Less than 12 | 20% | 32 | 16% | 59 | 17% | 91 | ||||||
| 12 | 31% | 51 | 31% | 116 | 31% | 167 | ||||||
| 13 | 26% | 43 | 30% | 111 | 29% | 154 | ||||||
| 14 | 9% | 15 | 11% | 42 | 11% | 57 | ||||||
| 15 or older | 14% | 23 | 13% | 47 | 13% | 70 | ||||||
| Age at menopause | 261 | 43 | 48 | 52 | 44 | 50 | 53 | 43 | 49 | 52 | F1,259 = 3.2 | p = 0.0741 |
| Age at presentation | 448 | 36 | 44 | 53 | 38 | 45 | 52 | 37 | 45 | 52 | F1,446 = 0.04 | p = 0.851 |
| Age at presentation (grouped by quartiles) | 448 | p = 0.732 | ||||||||||
| [17,37] | 27% | 35 | 25% | 80 | 26% | 115 | ||||||
| [37,45] | 26% | 34 | 27% | 86 | 27% | 120 | ||||||
| [45,52] | 21% | 27 | 25% | 81 | 24% | 108 | ||||||
| [52,84] | 26% | 33 | 23% | 72 | 23% | 105 | ||||||
| Age (at index procedure) | 564 | 42 | 49 | 57 | 42 | 49 | 57 | 42 | 49 | 57 | F1,562 = 0 | p = 0.981 |
| Age (at index procedure by quartile) | 564 | p = 0.992 | ||||||||||
| [22,42] | 26% | 43 | 26% | 103 | 26% | 146 | ||||||
| [42,49] | 26% | 44 | 25% | 98 | 25% | 142 | ||||||
| [49,57] | 25% | 42 | 26% | 102 | 26% | 144 | ||||||
| [57,85] | 23% | 39 | 23% | 93 | 23% | 132 | ||||||
| Hormonal birth control | 279 | p = 0.972 | ||||||||||
| Yes | 32% | 27 | 32% | 62 | 32% | 89 | ||||||
| No | 68% | 58 | 68% | 132 | 68% | 190 | ||||||
| Number of prior surgeries (total treatments) | 533 | 2 | 4 | 8 | 1 | 3 | 6 | 2 | 3 | 7 | F1,531 = 4.4 | p = 0.0371 |
| Time from first symptom date to diagnosis date (years) | 446 | 0.58 | 1.67 | 3.88 | 0.67 | 1.5 | 3.46 | 0.66 | 1.5 | 3.57 | F1,444 = 0.07 | p = 0.791 |
| Time from diagnosis through enrollment date (years) | 439 | 0.86 | 2.67 | 5.84 | 0.65 | 2.53 | 5.84 | 0.7 | 2.63 | 5.84 | F1,437 = 0.01 | p = 0.941 |
| Frequency of number of prior procedures | 414 | 0.84 | 1.31 | 2.65 | 0.75 | 1.39 | 2.83 | 0.77 | 1.37 | 2.79 | F1,412 = 0.34 | p = 0.561 |
| BMI | 550 | 23 | 26 | 34 | 23 | 27 | 33 | 23 | 27 | 33 | F1,548 = 0.47 | p = 0.491 |
| BMI - 30 and above (obese) | 550 | p = 0.52 | ||||||||||
| Yes | 39% | 62 | 35% | 138 | 36% | 200 | ||||||
| No | 61% | 99 | 65% | 251 | 64% | 350 | ||||||
- Note: a, b, c, represent the lower quartile a, the median b, and the upper quartile c for continuous variables. N is the number of non-missing values. Numbers after proportions (%) are frequencies. Tests used: 1Wilcoxon test; 2Pearson test.
Kaplan–Meier Analyses
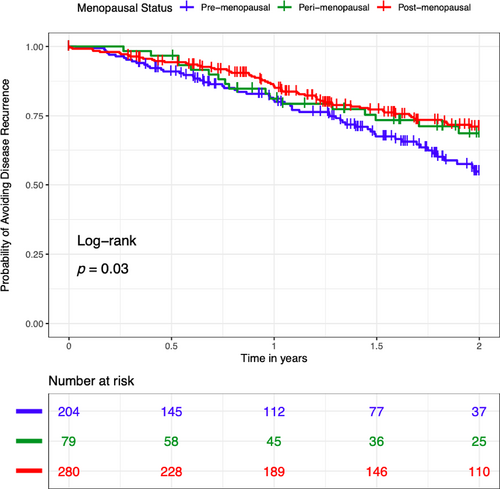
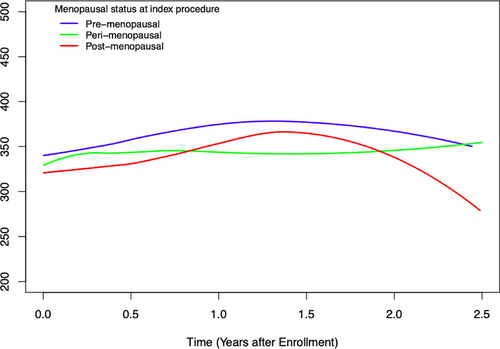
Kaplan–Meier analyses showed that 45% of premenopausal women recurred within 2 years of T0 compared with 30% of perimenopausal or postmenopausal women with iSGS (log-rank p = 0.03) (Figure 1). Kaplan–Meier analyses of probability of recurrence based on estrogen-years quartiles and age at presentation demonstrated no significant difference between quartiles; the same was true of Kaplan–Meier analysis based on age at presentation. Using a Loess Smooth Curve of mixed effect model, the peak flow meter values were similar across menopausal status from T0, indicating that each comparison group had similar baseline breathing and airway patency (Figure 2).
Adjusted Analysis
There was an inverse relationship between estrogen-years and likelihood of recurrence. Specifically, there was a 45% reduced hazard of recurrence for those women in the highest estrogen-year quartile compared with those in the lowest quartile (HR 0.55, CI 0.32–0.93; p = 0.03).
DISCUSSION
The almost exclusively female population diagnosed with iSGS indicates that estrogen regulation may play a yet undefined role in its pathophysiology. These results demonstrated that women with active estrogen exposure had recurred faster and more frequently. Estrogen-years did not impact the likelihood or timing of disease recurrence. The results refute the hypothesis that there is a dose–response relationship between years of estrogen exposure and risk of recurrence. Instead, it seems the absence of estrogen in the postmenopausal period reduces the likelihood of recurrence.
Age Versus Estrogen Exposure
Age confounds the relationship between estrogen exposure and recurrence; premenopausal patients are younger than postmenopausal patients. It is challenging to disentangle whether estrogen exposure, age, or both factors are associated with risk of recurrence. Thus, it is possible that iSGS presentation at a younger age increases the likelihood of recurrence independent of duration of estrogen exposure. However, Kaplan–Meier analysis did not show that recurrence differed between age quartile among women with iSGS.
Age alone has been associated with significant effects on inflammation and wound healing. Increased age results in increased cellular senescence, which impacts collagen fiber remodeling and proportion of different types of collagen in extracellular matrices. Age is also shown to be associated with reduction in focal adhesion sites in tissue, diminishing fibroblast migration and function for wound healing. Additionally, matrix metalloproteinases increase with age, resulting in increased collagen degradation.14 In fact, cutaneous female wound samples have demonstrated that with increased age, there is (1) reduced cutaneous wound healing, (2) reduced levels of transforming growth factor-β1 immunostaining, and (3) steady TGF-β1 mRNA.2 With this observation, it is also plausible that age alone is associated with decreased wound healing, and subsequent decreased recurrence of iSGS scar in older patients following endoscopic dilation.
Estrogen and Wound Healing
Estrogen's role in wound healing is also well documented. Cellular senescence and decreased cell division have been further associated with the absence of estrogen, specifically 17β-estradiol.3 This translates into decreased wound healing through diminished inflammation and granulation.3 In the previously mentioned study on aged cutaneous female wound samples, dermal fibroblast secretion of TGF-β1increased along with wound healing rates with the administration of hormone replacement therapy.2 These inflammatory signals are theorized to work via an interplay of estrogen receptor alpha (ERα) and beta (ERβ). In iSGS, IL-17A has specifically been found to drive scar fibroblast proliferation and synergize with TGF-β1 to promote production of collagen and fibronectin.15 Taken together, this evidence suggests that presence of estrogen is associated with more inflammation related to wound healing. This could explain the lower rates of recurrence in postmenopausal women after dilation for iSGS.
Prior literature supports the theory that older age may reduce inflammation and scar formation. It has been observed that recurrence of stenosis slows in women as they become older, especially postmenopausal.16 However, it remains unclear as to whether estrogen and/or age is the main factor contributing to inflammation and scar formation in iSGS. It remains highly plausible that estrogen acts as a cofactor in the development of this disease; however, understanding this requires mechanistic investigation.
Limitations
This was a retrospective analysis with an approximately 3-year follow-up for patients; therefore, we could not assess likelihood of recurrence beyond that threshold. Additionally, the measurement of estrogen exposure assumed that years of exposure and quantity of estrogen present in individuals is equivalent among all women. Women may have variable individual physiologic estrogen production per unit time, not accounted for by our definitions of estrogen-years. This was a secondary analysis of prospective data from a large international cohort study, thus making it infeasible to collect further data related to estrogen exposure. Additional data on estrogen status could provide further insight into types of hormonal variations and if/how they influence TTR, including history of estrogen or progesterone receptor modulator therapy, other hormonal diagnoses (e.g., polycystic ovarian syndrome and ovarian cancer). Menopausal status was queried only upon study enrollment rather than time of iSGS diagnosis. Finally, the parent study did not track whether patients had changes in menopausal status or other acquired hormone exposure metrics between diagnosis, study enrollment, and study end date. However, it is unlikely that many patients transitioned between menopausal status groups within the 3-year study period.
CONCLUSION
iSGS is a rare disease manifesting primarily in middle-aged, Caucasian females, leading to the proposition of estrogen as an influencing factor. The idea of a dose–response relationship is refuted in our analysis. In fact, premenopausal, and thus, younger patients, had a higher likelihood of recurrence and over a shorter disease-free interval compared with their peri- and postmenopausal counterparts. This demonstrates that younger patients have more aggressive disease and may require closer surveillance and different intervention strategies.




