Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients
Abstract
Objectives/Hypothesis
Olfactory dysfunction has been observed as one of the clinical manifestations in COVID-19 patients. We aimed to conduct a systematic review and meta-analysis to estimate the overall pooled prevalence of olfactory dysfunction in COVID-19 patients.
Study Design
Systematic review and meta-analyses.
Methods
PubMed, Scopus, Web of Science, Embase, and Google Scholar databases were searched to identify studies published between 1 December 2019 and 23 July 2020. We used random-effects model to estimate the pooled prevalence with 95% confidence intervals (CIs). Heterogeneity was assessed using the I2 statistic and Cochran's Q test. Robustness of the pooled estimates was checked by different subgroup and sensitivity analyses This study is registered with PROSPERO (CRD42020183768).
Results
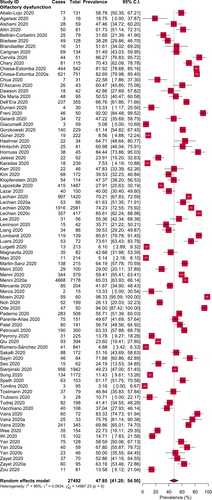
We identified 1162 studies, of which 83 studies (n = 27492, 61.4% female) were included in the meta-analysis. Overall, the pooled prevalence of olfactory dysfunction in COVID-19 patients was 47.85% [95% CI: 41.20–54.50]. We observed olfactory dysfunction in 54.40% European, 51.11% North American, 31.39% Asian, and 10.71% Australian COVID-19 patients. Anosmia, hyposmia, and dysosmia were observed in 35.39%, 36.15%, and 2.53% of the patients, respectively. There were discrepancies in the results of studies with objective (higher prevalence) versus subjective (lower prevalence) evaluations. The discrepancy might be due to false-negative reporting observed in self-reported health measures.
Conclusions
The prevalence of olfactory dysfunction in COVID-19 patients was found to be 47.85% based on high-quality evidence. Due to the subjective measures of most studies pooled in the analysis, further studies with objective measures are advocated to confirm the finding.
Level of Evidence
2 Laryngoscope, 131:865–878, 2021
INTRODUCTION
The world has recently been afflicted by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19). China witnessed the first case of pneumonia of unknown origin reported on 8th December 2019 from Wuhan City, Hubei province,1 and within a very short period, it started to spread globally. World Health Organization (WHO) declared COVID-19 a public health emergency of international concern on 30th January 2020 and a global pandemic disease on 11th March 2020. As of 23rd October 2020, it has become a global pandemic with over 1.1 million deaths and 41.5 million confirmed cases worldwide.2 As its nature and characteristics are unknown, understanding its presenting symptoms may help in earlier diagnosis. Current accumulated data indicate fever, cough, dyspnea, myalgia, arthralgia, and diarrhea to be the most predominant symptoms of SARS-CoV-2 infection.1, 3
Initially, a handful of studies reported the observation of olfactory dysfunction in COVID-19 patients.4-6 Following that the Ear, Nose, and Throat Society of UK and British Rhinological Society came up with an anecdotal report on the association between SARS-CoV-2 infection and olfactory dysfunction, in addition to urging new-onset anosmia to be investigated for SARS-CoV-2 infection while taking precautionary isolation.7 Similarly, the American Academy of Otolaryngology on 22 March 2020 advocated anosmia, hyposmia, and dysgeusia to be added as symptoms upon screening for COVID-19 with measure such as precautionary isolation advised.8 With the mounting evidence of olfactory dysfunction as a plausible symptom of COVID-19, the Centers for Disease Control and Prevention has added olfactory dysfunction as part of COVID-19's list of presenting symptoms.9
With more cases being reported,10 it is becoming apparent that the prevalence of olfactory dysfunction in COVID-19 patients varies widely across the range. An earlier meta-analysis by Tong et al.11 revealed the prevalence of olfactory dysfunction in COVID-19 patients was 52.73% based on 10 studies with 1627 patients available at that time. Remarkably, the authors stated that this figure is an underestimation due to the different type of assessment tools, which may be compounded by the smaller number of studies. Hence, another meta-analysis evaluating newer available studies and a larger pool of patients is required to present a more representative figure of the global prevalence of olfactory dysfunction among COVID-19 patients.
MATERIALS AND METHODS
We conducted a systematic review and meta-analysis of the literature in accordance with the PRISMA guideline to identify studies that presented the prevalence of olfactory dysfunction in patients with COVID-19, worldwide.12 This study is registered with PROSPERO, number CRD42020183768.
Data Sources and Searches
PubMed, Scopus, Web of Science, Embase, and Google Scholar databases were searched to identify studies published between 1 December 2019 and 23 July 2020 without language restrictions. The following key terms were searched: coronavirus, COVID-19, COVID19, nCoV, SARS-CoV-2, SARS-CoV2, olfaction, olfactory, smell, anosmia, hyposmia, dysosmia, cacosmia, and parosmia. Complete details of the search strategy are in the Supporting Table 1. In addition to the published studies, preprints were also considered if data of interest were reported. Review articles, case reports, opinions, and perspectives were excluded. Data reported by news reports and press releases or data collected from websites or databases were not considered. To ensure a robust search procedure, references of the included studies were also reviewed. Duplicate studies were excluded by using EndNote X8 software.
Study Selection
To identify eligible studies, articles of interest were screened based on the title and abstract, followed by full text by two authors (J.S. and M.A.I.) independently. Disagreements about inclusion were discussed and resolved by consensus.
Data Extraction and Quality Assessment
Data extraction was done independently by two authors (J.S. and M.A.I.). From each eligible study, we extracted the following information into a predefined Excel spreadsheet: first author's last name; study design; country of the participants; data collection period; total number of COVID-19 patients; number of female COVID-19 patients; age; COVID-19 confirmation procedure; confirmatory procedure of olfactory dysfunction; olfactory symptoms after the onset of illness; and number of recovered patients from olfactory dysfunction.
Random-effects model was used to obtain the pooled prevalence and 95% confidence intervals (CIs) of olfactory dysfunction in patients with COVID-19. The quality of included studies was assessed independently by two authors (J.S. and M.A.I.) using the Joanna Briggs Institute (JBI) critical appraisal tools.13 The studies were classified as low-quality (high-risk of bias) if the overall score was ≤50%.14 To assess publication bias, a funnel plot presenting prevalence estimate against the standard error was constructed and the asymmetry of the funnel plot was confirmed with Egger's test.
Data Synthesis and Analysis
Heterogeneity between studies was assessed using the I2 statistic (I2 > 75% indicating substantial heterogeneity) in addition to using the Cochran's Q test to identify the significance of heterogeneity. As subgroups, the prevalence of olfactory dysfunction in COVID-19 patients from different geographical regions and in different types, including anosmia, hyposmia, and dysosmia were analyzed. To identify the source of heterogeneity and to check the robustness of the results, sensitivity analyses were performed through the following strategies: i) excluding small studies (n < 100); ii) excluding the low-quality studies (high-risk of bias); iii) excluding studies not reporting COVID-19 confirmation assay; iv) considering only cross-sectional studies, and v) excluding outlier studies. In addition, to identify the outlier studies and the sources of heterogeneity, a Galbraith plot was constructed. All the analyses and plots were generated by using metaprop codes in meta (version 4.11–0) and metafor (version 2.4–0) packages of R (version 3.6.3) in RStudio (version 1.2.5033).15
RESULTS
Study Selection
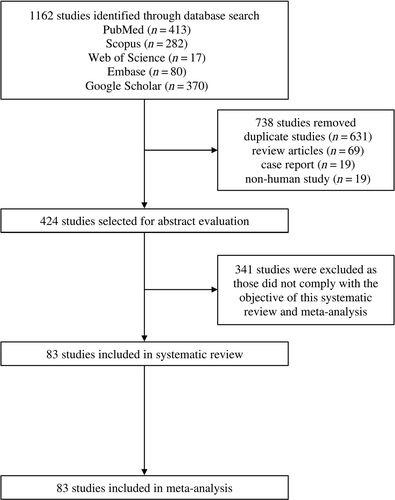
Our search initially identified 1162 studies. After removing 738 studies [duplicate studies (n = 631), review articles (n = 69), case reports (n = 19), and non-human studies (n = 19)]; titles and abstracts of 424 studies were screened for eligibility, of which 341 studies were excluded as those did not comply with the objective of this study. Therefore, 83 studies were included in the systematic review and meta-analysis (Fig. 1).
Study Characteristics
Detailed characteristics and references of the included studies are presented in Table I. Overall, this meta-analysis reports data from 27492 COVID-19 patients (61.4% female). Ages of the COVID-19 patients included in this meta-analysis ranged from 28.0 ± 16.4 to 70.2 ± 13.9 years. Studies were from 27 countries, including Spain, Germany, Italy, France, Ireland, Belgium, Romania, Switzerland, UK, Netherlands, Poland, Israel, China, Saudi Arabia, Turkey, Iraq, Iran, Pakistan, Singapore, Korea, Uruguay, Argentina, Bolivia, Venezuela, Australia, Canada, and USA. Among the included studies, 97.5% confirmed COVID-19 patients by using the RT-PCR method, whereas the method was not reported in two of the studies.
| No. | Study IDReference | Study Design | Country | Data Collection Period | Total Number of COVID-19 Patients (Female) | Age (years) (Mean ± SD/Median (IQR)/Range | COVID-19 Confirmation Procedure | Type of Assessment for Olfactory Dysfunction (Subjective/Objective) | Method of Assessment for Olfactory Dysfunction |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abalo-Lojo 202016 | Cross-sectional | Spain | NR | 131 (75) | 50.4 ± NR | RT-PCR | Subjective | Self-reported |
| 2 | Agarwal 202017 | Cross-sectional | USA | 1 March–4 April 2020 | 16 (4) | 67.0 (38.0–95.0) | RT-PCR | NR | NR |
| 3 | Alshami 202018 | Cross-sectional | Saudi Arabia | 16 March–18 April 2020 | 128 (69) | 39.6 ± 15.5 | RT-PCR | Subjective | Telephone questionnaire survey |
| 4 | Altin 202019 | Case–control | Turkey | 25 March–20 April 2020 | 81 (40) | 54.1 ± 16.9 | RT-PCR | Objective | Sniffin' Sticks test |
| 5 | Beltrán-Corbellini 202020 | Case–control | Spain | 23–25 March 2020 | 79 (31) | 61.6 ± 17.4 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 6 | Biadsee 202021 | Cross-sectional | Israel | 25 March–15 April 2020 | 128 (70) | 36.2 ± NR | RT-PCR | Subjective | Telephone questionnaire survey |
| 7 | Brandsetter 202022 | Cross-sectional | Germany | NR | 31 (26) | 18.0–65.0 | RT-PCR | Subjective | Self-reported |
| 8 | Carignan 202023 | Case–control | Canada | 10–23 March 2020 | 134 (81) | 57.2 (42.6–64.4) | RT-PCR | Subjective | Telephone interview |
| 9 | Cervilla 202024 | Cross-sectional | Spain | March–May 2020 | 51 (44) | 43.8 ± 10.7 | RT-PCR | Subjective | Telephone questionnaire survey |
| 10 | Chary 202025 | Cross-sectional | France | 25 March–18 April 2020 | 115 (81) | 47.0 (20.0–83.0) | RT-PCR | Subjective | Telephone interview |
| 11 | Chiesa-Estomba 202026 | Cross-sectional | Spain, Uruguay, Argentina, and Venezuela | NR | 542 (324) | 34.0 ± 11.0 | RT-PCR | Subjective | Online questionnaire survey |
| 12 | Chiesa-Estomba 2020a27 | Cross-sectional | Spain, Belgium, France, Canada, and UK | NR | 751 (477) | 41.0 ± 13.0 | RT-PCR | Subjective | Online questionnaire survey |
| 13 | Chua 202028 | Cross-sectional | Singapore | 23 March–4 April 2020 | 31 (NR) | NR | RT-PCR | Subjective | Self-reported |
| 14 | D'Ascanio 202029 | Cross-sectional | Italy | 1 February–24 April | 43 (14) | 58.1 ± 15.7 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 15 | Dawson 202030 | Cross-sectional | USA | March–April 2020 | 42 (NR) | NR | RT-PCR | Subjective | Self-reported questionnaire survey |
| 16 | De Maria 202031 | Cross-sectional | Italy | NR | 95 (NR) | NR | RT-PCR | Subjective | Self-reported questionnaire survey |
| 17 | Dell'Era 202032 | Cross-sectional | Italy | 10–30 March 2020 | 355 (163) | 50.0 (40.0–59.5) | RT-PCR | Subjective | In person interview or telephone questionnaire survey |
| 18 | Durrani 202033 | Cross-sectional | Pakistan | 20 March–10 April 2020 | 30 (6) | 44.0 (7.0–81.0) | RT-PCR | Subjective | Self-reported |
| 19 | Freni 202034 | Cross-sectional | Italy | NR | 50 (20) | 37.7 ± 17.9 | RT-PCR | Subjective | Online questionnaire survey |
| 20 | Gelardi 202035 | Cross-sectional | Italy | NR | 72 (33) | 49.7 (19.0–70.0) | RT-PCR | Subjective | Self-reported |
| 21 | Giacomelli 20204 | Cross-sectional | Italy | 19 March 2020 | 59 (19) | 60.0 (50.0–74.0) | NR | Subjective | Self-reported questionnaire survey |
| 22 | Gorzkowski 202036 | Cross-sectional | France | 1 March–31 March 2020 | 229 (147) | 39.7 ± 13.7 | RT-PCR | Subjective | Telephone questionnaire survey |
| 23 | Güner 202037 | Cross-sectional | Turkey | 10 March–10 April 2020 | 222 (90) | 50.6 ± 16.5 | RT-PCR | Subjective | Self-reported |
| 24 | Haehner 202038 | Cross-sectional | Germany | NR | 34 (16) | 43.2 ± 11.6 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 25 | Hintschih 202039 | Cross-sectional | Germany | NR | 41 (28) | 37 (NR) | RT-PCR | Subjective | Online questionnaire survey |
| 26 | Hornuss 202040 | Cross-sectional | Germany | April 2020 | 45 (20) | 56.0 ± 16.9 | RT-PCR | Objective | Sniffin' Sticks test |
| 27 | Jalessi 202041 | Cross-sectional | Iran | February–March 2020 | 92 (30) | 52.9 ± 13.2 | RT-PCR | Subjective | Self-reported |
| 28 | Karadaş 202042 | Cross-sectional | Turkey | April–May 2020 | 239 (106) | 46.4 ± 15.4 | RT-PCR | Subjective | Self-reported |
| 29 | Kerr 202043 | Cross-sectional | Ireland | 24 March 2020 | 46 (27) | 36.5 (27.0–48.0) | RT-PCR | Subjective | Self-reported |
| 30 | Kim 202044 | Cross-sectional | Korea | 12–16 March 2020 | 172 (106) | 26.0 (22.0–47.0) | RT-PCR | Subjective | Self-reported questionnaire survey |
| 31 | Klopfenstein 202045 | Cross-sectional | France | 1–17 March 2020 | 114 (36) | 47.0 ± 16.0) | RT-PCR | NR | NR |
| 32 | Lapostolle 202046 | Cross-sectional | France | 24 March–6 April 2020 | 1487 (752) | 44.0 (32.0–57.0) | RT-PCR | Subjective | Telephone interview |
| 33 | Lazar 202047 | Cross-sectional | Romania | 28 March 2020 | 100 (49) | 41.0 (NR) | RT-PCR | Subjective | Medical record review |
| 34 | Lechien 202048 | Cross-sectional | France, Italy, Spain, Belgium, and Switzerland | 22 March–10 April 2020 | 1420 (962) | 39.0 ± 12.0 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 35 | Lechien 2020a49 | Cross-sectional | Belgium | NR | 86 (56) | 41.7 ± 11.8 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 36 | Lechien 2020b50 | Cross-sectional | European countries | 22 March–3 June 2020 | 2581 (1624) | 44.5 ± 16.4 | RT-PCR | Subjective | Self-reported |
| 37 | Lechien 2020c51 | Cross-sectional | Belgium, Italy, France, and Spain | NR | 417 (263) | 36.9 ± 11.4 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 38 | Lee 202052 | Cross-sectional | Canada | 16 March–15 April 2020 | 56 (33) | 38.0 (31.8–47.2) | RT-PCR | Subjective | Telephone questionnaire survey |
| 39 | Levinson 202053 | Cross-sectional | Israel | 10–23 March 2020 | 42 (19) | 34.0 (15.0–82.0) | RT-PCR | Subjective | Telephone questionnaire survey |
| 40 | Liang 202054 | Cross-sectional | China | 16 March–12 April 2020 | 86 (42) | 25.5 (6.0–57.0) | RT-PCR | Subjective | Self-reported questionnaire survey |
| 41 | Lombardi 202055 | Cross-sectional | Italy | 24 February–31 March 2020 | 139 (82) | NR | RT-PCR | Subjective | Self-reported |
| 42 | Luers 202056 | Cross-sectional | Germany | 22–28 March 2020 | 72 (31) | 38.0 ± 13.0 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 43 | Luigetti 202057 | Cross-sectional | Italy | 14 March–20 April 2020 | 213 (76) | 70.2 ± 13.9 | RT-PCR | Subjective | Self-reported |
| 44 | Magnavita 202058 | Cross-sectional | Italy | 27 March–30 April 2020 | 82 (56) | NR | RT-PCR | Subjective | Self-reported questionnaire |
| 45 | Mao 202059 | Cross-sectional | China | 16 January–19 February 2020 | 214 (127) | 52.7 ± 15.5 | RT-PCR | NR | NR |
| 46 | Martin-Sanz 202060 | Case control | Spain | 15 March–7 April 2020 | 215 (171) | 42.9 ± 0.6 | RT-PCR | Objective | VAS |
| 47 | Meini 202061 | Cross-sectional | Italy | April 2020 | 100 (40) | 65.0 ± 15.0 | RT-PCR | Subjective | Telephone interview |
| 48 | Menni 20205 | Cross-sectional | UK | 24–29 March 2020 | 579 (400) | 40.79 ± 11.84 | RT-PCR | Subjective | Smartphone-based App survey |
| 49 | Menni 2020a62 | Cross-sectional | UK | 24 March–21 April 2020 | 6452 (4638) | 41.2 ± 12.1 | RT-PCR | Subjective | Smartphone-based App survey |
| USA | 726 (567) | 44.6 ± 14.3 | |||||||
| 50 | Mercante 202063 | Cross-sectional | Italy | 5–23 March 2020 | 204 (94) | 52.6 ± 14.4 | RT-PCR | Subjective | Telephone questionnaire survey |
| 51 | Merza 202064 | Cross-sectional | Iraq | 18 March–7 April 2020 | 15 (6) | 28.0 ± 16.4 | RT-PCR | NR | NR |
| 52 | Moein 202065 | Case–control | Iran | 21–23 March 2020 | 60 (20) | 46.5 ± 12.1 | RT-PCR | Objective | UPSIT |
| 53 | Noh 202066 | Cross-sectional | Korea | NR | 199 (130) | 38.0 ± 13.1 | RT-PCR | Subjective | In person interview |
| 54 | Otte 202067 | Cross-sectional | Germany | NR | 50 (NR) | 43.2 (23.0–69.0) | RT-PCR | Objective | Sniffin' sticks test |
| 55 | Paderno 202068 | Cross-sectional | Italy | 27 March–1 April 2020 | 508 (223) | 55.0 ± 15.0 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 56 | Parente-Arias 202069 | Cross-sectional | Spain | 3–24 March 2020 | 151 (98) | 55.2 (18.0–88.0) | RT-PCR | Subjective | Self-reported questionnaire survey |
| 57 | Patel 202070 | Cross-sectional | UK | 1 March–1 April 2020 | 141 (58) | 45.6 (20.0–93.0) | RT-PCR | Subjective | Telephone interview |
| 58 | Petrocelli 202071 | Cross-sectional | Italy | 16 April–2 May 2020 | 300 (225) | 43.6 ± 12.2 | RT-PCR | Objective | Olfactory threshold and identification test |
| 59 | Peyrony 202072 | Cross-sectional | France | 9 March–4 April 2020 | 225 (150) | 62.0 (48.0–71.0) | RT-PCR | Subjective | Self-reported |
| 60 | Qiu 202073 | Cross-sectional | China, France and Germany | 15 March–5 April 2020 | 394 (NR) | NR | RT-PCR | Subjective | Self-reported questionnaire survey |
| 61 | Romero-Sánchez 202074 | Cross-sectional | Spain | 1 March–1 April 2020 | 841 (368) | 66.4 ± 14.9 | RT-PCR | Subjective | Medical record review |
| 62 | Sakalli 202075 | Cross-sectional | Turkey | NR | 172 (88) | 37.8 ± 12.5 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 63 | Sayin 202076 | Case–control | Turkey | NR | 64 (39) | 37.7 ± 11.3 | RT-PCR | Subjective | Self-reported questionnaire survey |
| 64 | Seo 202077 | Cross-sectional | Korea | 28 April 2020 | 62 (NR) | NR | RT-PCR | Objective | CC-SIT |
| 65 | Sierpiński 202078 | Cross-sectional | Poland | 17–18 April 2020 | 1942 (1169) | 50.0 (NR) | RT-PCR | Subjective | Telephone interview |
| 66 | Song 202079 | Cross-sectional | China | 27 January–10 March 2020 | 1172 (595) | 61.0 (48.0–68.0) | RT-PCR | Subjective | Telephone interview |
| 67 | Speth 202080 | Cross-sectional | Switzerland | 3 March–17 April 2020 | 103 (53) | 46.8 ± 15.9 | RT-PCR | Subjective | Telephone interview |
| 68 | Tomlins 202081 | Cross-sectional | UK | 10–30 March 2020 | 95 (35) | 75.0 (59.0–82.0) | RT-PCR | NR | NR |
| 69 | Tostmann 202082 | Cross-sectional | Netherlands | 10–30 March 2020 | 79 (NR) | NR | NR | Subjective | Self-reported questionnaire survey |
| 70 | Trubiano 202083 | Cross-sectional | Australia | 1–22 April 2020 | 28 (14) | 55.0 (46.0–63.5) | RT-PCR | Subjective | Medical record review |
| 71 | Tudrej 202084 | Cross-sectional | Switzerland | 24 March–14 April 2020 | 198 (NR) | NR | RT-PCR | Subjective | Self-reported questionnaire survey |
| 72 | Vacchiano 202085 | Cross-sectional | Italy | NR | 108 (46) | 59.0 (18.0–83.0) | RT-PCR | Subjective | Telephone questionnaire survey |
| 73 | Vaira 202086 | Cross-sectional | Italy | 31 March–6 April 2020 | 72 (45) | 49.2 ± 13.7 | RT-PCR | Objective | CCCRC |
| 74 | Vaira 2020a87 | Cross-sectional | Italy | 9–10 April 2020 | 33 (22) | 47.2 ± 10 | RT-PCR | Objective | CCCRC |
| 75 | Vaira 2020b88 | Cross-sectional | Italy | NR | 345 (199) | 48.5 ± 12.8 (23–88) | RT-PCR | Objective | CCCCRC |
| 76 | Wee 202089 | Cross-sectional | Singapore | 26 March–10 April 2020 | 154 (NR) | NR | RT-PCR | Subjective | Self-reported questionnaire survey |
| 77 | Wi 202090 | Cross-sectional | Korea | 15 April 2020 | 111 (57) | 41.3 ± 19.0 | RT-PCR | Subjective | Medical record review |
| 78 | Yan 202091 | Cross-sectional | USA | 3 March–8 April 2020 | 128 (67) | 53.5 (40.0–65.0) | RT-PCR | Subjective | Self-reported |
| 79 | Yan 2020a92 | Cross-sectional | Germany, USA, Bolivia and Venezuela | NR | 59 (29) | 18.0–79.0 | RT-PCR | Subjective | Online questionnaire survey |
| 80 | Yan 2020b93 | Cross-sectional | USA | 9 March–29 April 2020 | 46 (NR) | NR | RT-PCR | Subjective | Medical record review |
| 81 | Zayet 202094 | Cross-sectional | France | 26 February–14 March 2020 | 70 (41) | 56.7 ± 19.3 | RT-PCR | Subjective | Self-reported questionnaire |
| 82 | Zayet 2020a95 | Case–control | France | 30 March–3 April 2020 | 95 (79) | 39.8 ± 12.2 | RT-PCR | Subjective | Medical record review |
| 83 | Zou 202096 | Cross-sectional | China | 1 February–3 March 2020 | 81 (43) | 58.0 (50.0–68.5) | RT-PCR | Subjective | Medical record review |
- AAO-HNS = American academy of otolaryngology–head and neck surgery; CC-SIT = cross-cultural smell identification test; CCCRC = Connecticut chemosensory clinical research center orthonasal olfaction test; IQR = interquartile range; NR = not reported; RT-PCR = reverse transcription polymerase chain reaction; SD = standard deviation; UPSIT = University of Pennsylvania smell identification test; VAS = visual analog scale..
Outcomes
Overall, the pooled prevalence of olfactory dysfunction in COVID-19 patients was 47.85% [95% CI: 41.20–54.50] (Fig. 2). From the subgroup analyses, we observed olfactory dysfunction in 54.40% European, 51.11% North American, 31.39% Asian, and 10.71% Australian COVID-19 patients (Table II, Supporting Figure 1). In addition, anosmia, hyposmia, and dysosmia were observed in 35.39%, 36.15%, and 2.53% of the COVID-19 patients, respectively (Table II, Supporting Figure 2). Interestingly, the prevalence of olfactory dysfunction was observed higher in COVID-19 patients on objective rather than subjective evaluations (72.10% vs. 44.53%) (Table II, Supporting Figure 3). Based on the clinical severity, olfactory dysfunction was higher in non-severe patients compared to severe patients with COVID-19 (47.48% vs. 9.02%) (Table II, Supporting Figure 4).
| Subgroups of COVID-19 Patients | Olfactory Dysfunction Prevalence [95% CIs] (%) | Number of Studies Analyzed | Total Number of COVID-19 Patients | Heterogeneity | Publication Bias, Egger's Test (P Value) | |
|---|---|---|---|---|---|---|
| I2(%) | P Value | |||||
| Olfactory dysfunction in different regions | ||||||
| Europe | 54.40 [46.19–62.61] | 49 | 20,738 | 99 | <.0001 | .19 |
| North America | 51.11 [41.10–61.13] | 7 | 1,148 | 87 | <.0001 | NA |
| Asia | 31.39 [18.26–44.51] | 22 | 3,477 | 99 | <.0001 | .66 |
| Australia | 10.71 [0.00–22.17] | 1 | 28 | NA | NA | NA |
| Different types of olfactory dysfunction | ||||||
| Anosmia | 35.39 [27.73–43.04] | 43 | 10,979 | 99 | <.0001 | .11 |
| Hyposmia | 36.15 [27.65–44.64] | 24 | 5,200 | 98 | <.0001 | .003 |
| Dysosmia | 2.53 [0.0–6.0] | 1 | 79 | NA | NA | NA |
| Evaluation types of olfactory dysfunction | ||||||
| Subjective | 44.53 [37.59–51.47] | 73 | 26,229 | 99 | <.0001 | .60 |
| Objective | 72.10 [59.41–84.79] | 10 | 1,263 | 97 | <.0001 | .33 |
| Olfactory dysfunction based on clinical severity | ||||||
| Severe | 9.02 [2.67–15.38] | 4 | 687 | 85 | .001 | NA |
| Non-severe | 47.48 [21.34–73.62] | 8 | 5,135 | 100 | <.0001 | NA |
- CIs = confidence intervals; NA = not applicable.
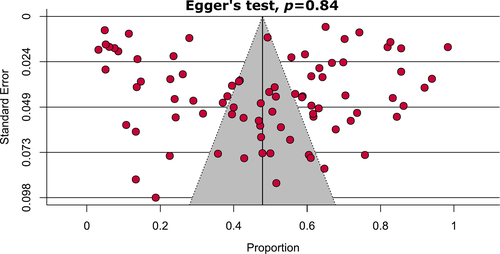
Detailed quality assessment of the included studies is shown in the Supporting information (Supporting Table 2, Supporting Table 3). Briefly, 95.1% of the included studies were of high-quality (low-risk of bias). Overall, very high levels of heterogeneity (ranging from 87% to 99%) were observed during the estimation of olfactory dysfunctions in the main analysis as well as in different subgroup analyses. Visual inspection of the funnel plot and Egger's test results showed that there was no significant publication bias (P = .84) (Fig. 3).
Sensitivity analyses on assessing olfactory dysfunction in COVID-19 patients excluding small studies, low-quality studies, studies where COVID-19 confirmation test was not reported, considering only cross-sectional studies, and excluding outlier studies showed very marginal differences in overall pooled prevalence (Table III, Supporting Figure 5). Overall, our sensitivity analyses indicated that the results of olfactory dysfunction prevalence in COVID-19 patients are robust and reliable. As the source of heterogeneity, from the Galbraith plot, three studies were identified as the source of heterogeneity (Supporting Figure 6).
| Strategies of Sensitivity Analyses | Olfactory Dysfunction Prevalence [95% Cis] (%) | Difference of Pooled Prevalence Compared to the Main Result | Number of Studies Analyzed | Total Number of COVID-19 Patients | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2(%) | P Value | |||||
| Excluding small studies | 46.03 [37.08–54.97] | 3.8% lower | 43 | 25,162 | 100 | <.0001 |
| Excluding low-quality studies | 49.03 [42.21–55.85] | 2.5% higher | 79 | 27,146 | 99 | <.0001 |
| Excluding studies where COVID-19 confirmation test was not reported | 48.40 [41.67–55.12] | 1.1% higher | 81 | 27,354 | 99 | <.0001 |
| Considering only cross-sectional studies | 46.66 [39.87–53.44] | 2.5% lower | 77 | 26,979 | 99 | <.0001 |
| Excluding outlier studies | 47.28 [40.61–53.95] | 1.2% lower | 80 | 27,297 | 99 | <.0001 |
- CIs = confidence intervals.
DISCUSSION
The route of entry of SARS-CoV-2 to the olfactory neuron is via the olfactory epithelium found at the nasal roof.97 This region is exposed the most to inspired air during inspiration after it passes the nasal valve and moves upwards. The sensory neurons found at the olfactory epithelium are accountable for detecting as well as transmitting information of odors to the brain. It is noteworthy that the unique property of olfactory epithelium is its basal cell, which can regenerate throughout life.98, 99
The novel SARS-CoV-2 infection was discovered and delineated by Zhou et al.100 on 3rd February 2020. They described that SARS-CoV-2 enters the cell through angiotensin-converting enzyme 2 (ACE2). It is postulated that SARS-CoV infiltrates cells via the interplay between its spike (S) protein and the ACE2 protein on the target cells.101, 102 Interestingly, the number of ACE2 cells is similar both in nasal and oral tissues, as well as lung and colon tissues,103 although it is postulated that nasal and oral tissues may be the first site of entry by SARS-CoV-2. The two genes accountable for anosmia following SARS-CoV-2 infection are ACE2 and TMPRSS2.104 SARS-CoV-2 has been shown to enter the brain via olfactory bulb on transgenic mice causing transneuronal spread and was discovered abundantly in the olfactory bulb following infection.105 In addition, autopsy samples taken from patients with SARS showed SARS-CoV-2 in the brain samples. The mode of entry into the brain is postulated to be via olfactory bulb.106, 107 Previous experience had led to a revelation that coronaviruses have shown to share a similar structure as well as an infective pathway.108 Hence, structural changes in the olfactory bulb ought to be assessed.109 It is noteworthy that, reduction in the volume of olfactory bulb has been reported to result from a prior infection-related olfactory dysfunction.110 There are several possible mechanisms for olfactory dysfunction following SARS-CoV-2 infection. Among the countless existing theories, the most notable ones include olfactory cleft syndrome and postviral anosmia syndrome.111 The former theory advocates on mucosal obstruction at the olfactory cleft results in conduction impairment of smell,112 while the latter proposes on a neural loss mechanism whereby direct injury to the olfactory sensory neurons preceding viral infection.113
It is noteworthy that postviral olfactory loss (PVOL) is not a novel phenomenon. Numerous virus has been advocated to enable olfactory dysfunction, including influenza virus, adenovirus, parainfluenza virus, respiratory syncytial virus, coxsackievirus, adenovirus, poliovirus, enterovirus, and herpesvirus.114-117 Suguira et al.115 in an earlier study supported parainfluenza virus (PIV) type 3 to be the primary virus responsible for PVOL. Subsequent research revealed a similar finding, whereby PIV-3 was the leading culprit behind PVOL.116 Tian et al.117 studied the Sendai virus (SeV), the murine counterpart of the PIV on olfactory function and regenerative ability of the olfactory epithelium. In addition, they found that SeV impairs olfaction and persists in the olfactory epithelium and olfactory body, thus hindering the regenerative ability as well as the normal physiologic function of olfactory sensory neurons.
Suzuki et al.114 found rhinovirus to be the predominant cause of PVOL followed by PIV-2, Epstein–Barr virus, and coronavirus, which was identified in one patient. PIV-3 was not, however, studied in their sample. Coronavirus was not considered in many studies as the involvement of coronavirus in PVOL was not extensively reported, and it is challenging to isolate coronavirus.115 In addition, the challenge faced by many researchers in identifying the virus responsible for PVOL is following the delay of patients with the olfactory loss to visiting the clinic, believing the notion that PVOL will resolve spontaneously. A noteworthy study by Potter et al.118 shed more light on the interaction between virus and host in PVOL related condition. Potter et al. suggested that a seasonal pattern emerged among influenza and non-influenza related PVOL indicating not only variations of potency and virulence of virus but also on host susceptibility as a factor in determining the progression and manifestation of the infection. Olfactory disorders related to non-influenza virus peaked in warmer months compared to colder months.
In our meta-analysis, all 83 studies revealed a strong association between olfactory dysfunction and SARS-COV-2 infection. Overall nasal symptoms among COVID-19 positive patients have been scarcely reported.3, 119 Chen et al.3 in their series, reported only 4% of their patients had rhinorrhea; while Guan et al.119 reported 5% of their patients demonstrated nasal obstruction. Scanty reported data on olfactory dysfunction had been attributed by either overlooked nasal symptoms by physicians,51 or the possibility of different virus sequences leading to the various presentations.120 The latter theory was supported based on a study by Benvenuto et al.120 who compared genomes of 15 virus sequences from patients in various regions in China with other coronaviruses. The possibility that olfactory, as well as gustatory dysfunction, prevails among the European community has emerged.51 in addition, lack of awareness among Asian patients in addition to unnoticed olfactory loss could have contributed to the low number of reported cases among Asian patients. Recent epiphany on olfactory dysfunction among Asian patients accruing the surge in cases has enabled olfactory dysfunction to be included in suspect case criteria for SARS-CoV-2 infection, allowing test to be carried out in these patients, while isolation is implemented concomitantly.28
Female predominance was revealed among our patients (61.4%). Similarly, previous studies have shown olfactory loss postviral prevails among female patients.121, 122 This notion is attributed to gender-related variation in the inflammatory process.123 Increase in numbers of female patients can be attributed by greater tendency of females to volunteer for studies. In addition, female patients are found to be more sensitive in detecting chemosensory alteration.
Most studies involved online questionnaire either through an online application, online survey, smartphone-based App filled up by patients or clinicians, whereas objective assessment of olfactory assessment was utilized in four studies whereby Sniffin test, University of Pennsylvania smell identification test (UPSIT), and Connecticut chemosensory clinical research center orthonasal olfaction test (CCCRC) were performed. It is noteworthy that, in our meta-analysis, we found prevalence of olfactory dysfunction among objectively evaluated studies to be higher (72.10%) as compared to the subjectively evaluated studies (44.53%). This could be attributed by the fact that most COVID-19 patients are unaware of their olfactory dysfunction leading to possibility of underestimation. Moein et al.65 reported 98% of their patients were found to have olfactory dysfunction post UPSIT, of which only 35% were initially aware of their symptoms. Generally, loss of smell is only perceived upon significant loss of smell such as anosmia. Thus, it is worth noting that the prevalence of olfactory dysfunction may be higher if tested objectively. Quantitative testing of olfactory disturbance may provide rapid and cheap modality to screen COVID-19 in a large population. Interestingly, Moein et al.124 reported that time of testing is the most important factor in explaining the prevalence variations among studies apart from variations in question and types of olfactory testing. They found that 61% of the earlier 96% of patients who demonstrated olfactory disturbance, when retested during the late acute phase showed an improvement.
Although the jarring increase in the number of cases daily, which led to a surge in research as well as publications, we obtained only 83 studies on olfactory dysfunction in SARS-CoV-2 infection. This may be attributed by the fact that the substantial available peer-reviewed studies report on hospitalized patients, which means that the self-limiting,125 as well as the mild group of patients, are omitted from the various studies. The notion that olfactory manifestation predominately affects the milder form of SARS-CoV-2 infection is inevitable. Yan et al.92 found that most patients with olfactory disturbance with positive SARS-CoV-2 infection were treated as out-patient or ambulatory and not requiring hospitalization. Yet, it is imperative to keep in mind that the nature of this virus is yet to be explored, and owing to the varying genome in virus sequencing, all SARS-CoV-2 infection positive patients with olfactory disturbance should not be taken lightly. Villalba et al.126 reported on two patients who presented with anosmia as the initial symptom of SARS-CoV-2 infection had to be hospitalized, and unfortunately, one patient succumbed. Varying reports are available on the outcome following the PVOL. Yan et al.92 and Klopfenstein et al.45 demonstrated 74% and 98% resolution of olfactory symptoms and linked this short-lived manifestation to the unique ability of olfactory epithelium to regenerate and repair following viral clearance.
In our meta-analysis, none of the authors mentioned on specific treatment directed to smell impairment. The role of intranasal steroids is debatable in this situation accruing the possibility of triggering upper respiratory tract infection. Oral steroids used traditionally to treat idiopathic anosmia ought to be averted by all means to avoid further risk of immunosuppression in SARS-CoV-2 infection patients.112 The outcome of olfactory loss revealed persistence of symptoms mentioned in some of the studies. Duration of olfactory dysfunction remains a conundrum as the nature of this novel pandemic is still a mystery. Heretofore, PVOL habitually has been shown to have a good prognosis. Despite still premature, several anecdotal reports have revealed on total or partial recuperation of olfactory loss over a few months.127 This is owing to the fact that a longer time for regeneration following damage to olfactory neurons is required. Albeit considered innocuous, olfactory disturbance has been related to a number of detrimental effects notably on quality of life, impacts social interaction, and depression. Astonishingly, several high-profile studies have related olfactory disturbance to a 5-year mortality rate.128-131 The unique neuroplasticity potential found in olfactory system opens to novel possibility of olfactory recovery via numerous modalities such as olfactory training.132
Implications for Clinical Practice
The characteristics of an ideal screening tool are high probability of detecting disease (highly sensitive) and high probability of excluding disease when it is negative (highly specific). Besides being reliable, it must be cost-effective, simple to perform, and widely available.133, 134 Moreover, an effective screening requires engagement of both target populations and health care providers. As olfactory dysfunction can be simply detected by using questionnaire,135 it fulfills all these criteria and can be a useful screening tool besides temperature surveillance. Applying a specific questionnaire to detect olfactory dysfunction, especially in those with suspicious flu-like symptoms, travel history from affected countries, and contact with COVID-19 patients may enhance the pick-up rate of infected patients. Furthermore, questionnaire-based screening tool may easily be assimilated in the global health care system and more so in developing countries where cost is a factor.
Implications for Research
As there is no standardized questionnaire available to screen for olfactory dysfunction, a consensus is required to determine the most suitable questionnaire for a reliable detection. Perhaps a more refined questionnaire based on the available questionnaires can be developed by selecting the relevant questions and compare by comparing them with an objective smell test to choose the most consistent questions. Researches need to be conducted employing the more objective smell test, which will provide us information on specific odor affected by this infection. By identifying the specific associated odor link to the infection, a simple smell test can be developed particularly to screen for COVID-19. Olfactory dysfunction may serve as prognosticators to triage and stratify patients according to different categories of severity, which can help to detect those who need immediate and urgent hospitalization. Research into this may help in preventing death among COVID-19 patients.
Strengths
Our study has several strengths. This meta-analysis was conducted with significant number of studies and hence including a considerable number of participants, resulting in more robust estimates. Majority of the included studies confirmed COVID-19 subjects by using the RT-PCR technique, which strengthens our findings. None of the analyses represented significant publication bias demonstrating that we were unlikely to have missed studies that could have altered the findings. All the conducted sensitivity analyses generated similar results to the main findings indicating the robustness of the meta-analysis results. Based on the quality assessments, 95.1% of the studies were of high methodological quality (low-risk of bias), which ensured a reliable result.
Limitations
Nevertheless, there are several notable limitations. Based on the search strategy and considered time period, this meta-analysis could include participants from 27 countries from four continents; therefore, the prevalence may not represent at a global scale and generalization of the findings should be done with care. One of the major limitations in this meta-analysis is the presence of substantial degrees of heterogeneity. Even though we examined the sources of heterogeneity by subgroup, sensitivity analyses and Galbraith plot, source of heterogeneity could not be fully explained by the factors included in the analyses. Although we comprehensively investigated the prevalence of olfactory dysfunction from the first eight-month data of the COVID-19 outbreak, we have somewhat characterized olfactory dysfunctions in severe versus non-severe COVID-19 patients due to the limited number of studies.
Another major limitation is majority of the studies used self-reported data. When self-reported health measures are used, both underestimation due to false negative reporting and overestimation due to false positive reporting may possibly transpire, and the results should be interpreted with caution. A meta-analysis involving studies with large number of patients may minimize the potential bias but an amplification of the compromised methodology cannot entirely be excluded.
CONCLUSION
This meta-analysis found the prevalence of olfactory dysfunction was 47.85% of the COVID-19 patients based on the high quality of evidence, which suggests it as a significant initial symptom of SARS-CoV-2 infection. Due to the subjective measures of most studies pooled in the analysis, further studies with objective evaluations are recommended to confirm the finding.




