Pain After Ear Surgery: A Prospective Evaluation of Endoscopic and Microscopic Approaches
Editor's Note: This Manuscript was accepted for publication on July 31, 2020.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objectives/Hypothesis
Assumed advantages of a minimally invasive endoscopic transmeatal approach in ear surgery are less postoperative pain, faster healing, and preservation of functional anatomy. We evaluated pain after ear surgery and compared endoscopic transmeatal, microscopic endaural, and retroauricular approaches.
Study Design
Prospective cohort study.
Methods
A prospective evaluation of pain during 3 weeks after ear surgery was performed. Three groups were defined: endoscopic transmeatal, microscopic endaural, and retroauricular. Data from 20 fully completed questionnaires (Brief Pain Inventory–Short Form) per group were analyzed with Bayesian and frequentist statistics.
Results
For all approaches, low pain scores were found, not exceeding 4 on a scale of 0 to 10. Analysis of the worst, least, and average pain scores documented per 24 hours showed no statistically significant difference nor equality between groups. With Bayesian statistics, a Bayes factor of 1.07, 0.25, and 0.51 was found, respectively. With frequentist statistics a p value of .092, .783, and 0.291 was found, respectively. Small, but statistically significant, differences were found for sleep, natural sleeping position, normal work, and pain medication taken. The location of pain correlates with the incision site.
Conclusions
The results of this study show that the surgical approach has no clinically relevant influence on postoperative pain after ear surgery. The statistically significant differences on natural sleeping position, sleep, normal work, and amount of pain medication taken are small and should be interpreted with caution. Therefore, these should not be decisive factors in the choice of surgical approach in ear surgery.
Level of Evidence
3 Laryngoscope, 131:1127–1131, 2021
INTRODUCTION
Several surgical approaches can be used to access the middle ear. These range from the minimally invasive transmeatal, to the more invasive endaural, and most invasive retroauricular approach. Each of these approaches is associated with specific soft and hard tissue trauma. These days, a surgical trend toward minimally invasive surgery is advocated. This has been associated with faster healing, better postoperative quality of life, and comparable surgical results.1, 2
Within the field of otology, this trend has gained traction with the introduction of endoscopic ear surgery. To achieve sufficient view of the operating field, the microscope needs a more traumatic approach. The endoscope hypothetically will achieve the same—or better—surgical view with less trauma needed.3 It would seem reasonable to assume that the aforementioned benefits would apply to endoscopic ear surgery. Other benefits of endoscopic ear surgery that have been postulated are a reduction of residual disease in cholesteatoma surgery,4-11 reduction of surgery time,12-14 preserving functional anatomy,15-18 and better cost-effectiveness.10, 19, 20 Comparable results in myringoplasty have been demonstrated, suggesting that the outcome remains equal.21
Only a few studies have been conducted investigating these benefits. One comparative study was found regarding time needed to heal between microscopic and endoscopic ear surgery.22 Only two studies compared both types of surgery with respect to postoperative pain.23, 24 To strengthen the claims that endoscopic ear surgery has true benefits over microscopic ear surgery, more comparative studies with high quality are needed.
The aim of our study was to prospectively compare postoperative pain in patients following either endoscopic or microscopic ear surgery. More specifically, we aimed to compare the three mentioned approaches for increasing tissue damage.
MATERIALS AND METHODS
Participant Selection
Three groups were defined. The first group was comprised of patients with a transmeatal endoscopic approach, the second group consisted of patients with an endaural approach, and the third group consisted of patients with a retroauricular approach. To be eligible for inclusion, patients had to be older than 18 years and have sufficient understanding of the Dutch language to complete the questionnaire. Patients were excluded if they suffered from chronic pain complaints, migraine or other forms of headache, and if they mentioned the chronic use of painkillers.
No randomization was performed, as inclusion and informed consent was acquired after surgical approach and planning were completed. All consecutive patients listed for an operation and eligible for inclusion were asked to participate until a total of 20 patients in every group were included.
Pain Assessment
The Brief Pain Inventory–Short Form (BPI-SF) was used to measure postoperative pain.25-27 BPI-SF is a validated pain questionnaire and uses a scale from 0 (no pain/influence) to 10 (worst pain/influence). Three nonvalidated questions were added separately to specify pain due to the pressure bandage applied in the retroauricular group, influence of pain on normal sleeping position, and pain medication used (type, frequency, dose). Relief of pain by medication is measured as a percentage from 0% to 100%. Additionally, a drawing of a human head was added to enable patients to specify areas of paresthesia and/or numbness. A baseline measurement was attained 1 day preoperatively. We repeated the questionnaires daily during the first week and on day 10, 14, and 21 postoperatively. Localization and extent of pain were recorded on a drawing of a human head.
Data Analysis
Repeated-measures analysis of variance (ANOVA) was performed at all 10 time points to evaluate the difference of pain perception and influence of pain on daily life activities among the three groups. These analyses were performed with Bayesian statistics (primary analysis) as well as with frequentist statistics (secondary analysis) using JASP version 0.9.0.1 (https://jasp-stats.org). For the Bayesian analysis, default priors were used.28
With Bayesian statistics, smaller datasets can be analyzed without losing power while retaining precision.29 Such analyses result in Bayes factors (BFs), which express relative support for one model over another model given the data. We used Lee and Wagenmakers' classification scheme for interpretation of the BF.30 A BF higher than 1 favors the model that allows a difference between groups, whereas a BF lower than 1 favors the model in which there is no difference between groups. A BF between 1 and 0.1 should be interpreted as anecdotal to moderate evidence, whereas a BF smaller than 0.1 can be interpreted as strong evidence for the no-difference model. A BF between 1 and 10 can be seen as anecdotal to moderate evidence, whereas a BF larger than 10 should be interpreted as strong evidence for the difference model.
To facilitate comparison with literature, we secondarily determined significance with frequentist statistics between groups using JASP. We performed repeated-measures ANOVA to analyze differences of pain perception and influence of pain on daily life activities. Differences in pain medication taken were determined by one-way ANOVA. A P value<.05 was considered statistically significant.
Comparison of the location of pain and paresthesia to the surgical approach was done on the day of worst pain by visual evaluation of the drawing in the questionnaire. The study was conducted according to the current version of the Declaration of Helsinki (Edinburgh, Scotland, October 2000). Institutional review board approval was attained (W16_283 # 16.333).
RESULTS
Participants
Inclusion started March 2017 and was completed in June 2018. Due to dropouts and incomplete questionnaires, a total of 72 eligible participants were asked to participate before all groups were complete. The demographics of the final study groups are shown in Table I. The endoscopic group consisted of 18 tympanoplasties (86%) and two transmeatal atticotomies (9%) and had a dropout of one tympanoplasty (5%). The endaural microscopic group was comprised of 11 stapedotomies (40%), eight tympanoplasties (30%), and one transmeatal atticotomy (4%). The seven dropouts (26%) were evenly divided: four tympanoplasties, two stapedotomies, and one transmeatal atticotomy. The retroauricular group consisted of 15 canal wall up mastoidectomies (63%), four cochlear implants (17%), and one canalplasty (4%). Four canal wall up mastoidectomies dropped out (16%).
| Study Population, N = 60 | Group 1, Transmeatal Endoscopic, n = 20 | Group 2, Endaural Microscopic, n = 20 | Group 3, Retroauricular Microscopic, n = 20 | |
|---|---|---|---|---|
| Gender | ||||
| Male | 31 | 11 | 9 | 11 |
| Female | 29 | 9 | 11 | 9 |
| Age, yr, mean | 50 | 39 | 54 | 58 |
| Surgery, n = 60 | 18 tympanoplasties, 2 atticotomies | 8 tympanoplasties, 1 atticotomy, 11 stapedotomies | 15 canal wall up mastoidectomies, 4 cochlear implants, 1 canalplasty | |
Pain Assessment
The mean scores with its standard deviations of the individual questions of the BPI-SF are shown in Table II with their BF and P values. Overall visual analog scale (VAS) scores at all time points did not exceed 4. Additionally, no BF was found to be lower than 0.1 or higher than 10 for all subquestions, which implies that no evidence was found for one of both models. Frequentist statistics, on the other hand, showed that patients from the retroauricular group experienced significantly more impact on their sleep (P = .032), and patients from the endaural group were less impaired in performing normal work (P = .011). The additional questions did result in a BF of more than 10 when asked if pain influenced the normal sleeping position (Table II). A mean VAS score of 3.83 ± 3.08 was found in the retroauricular group regarding pain by the pressure bandage.
| Group 1, Transmeatal Endoscopic, Mean Score ± SD | Group 2, Endaural Microscopic, Mean Score ± SD | Group 3, Retroauricular Microscopic, Mean Score ± SD | P Value of Between Groups Effect of RM ANOVA | Bayes Factor of Between Groups Effect of RM ANOVA | |
|---|---|---|---|---|---|
| BPI-SF | |||||
| Worst pain | 2.26 ± 2.02 | 1.42 ± 1.72 | 2.72 ± 1.85 | .092 | 1.07 |
| Least pain | 0.91 ± 1.30 | 0.91 ± 1.62 | 1.16 ± 0.85 | .783 | 0.25 |
| Mean pain | 1.51 ± 1.58 | 1.11 ± 1.68 | 1.87 ± 1.28 | .291 | 0.51 |
| General activities | 1.96 ± 2.29 | 0.98 ± 1.25 | 2.00 ± 1.49 | .126 | 0.75 |
| Mood | 1.33 ± 1.29 | 0.66 ± 1.27 | 1.53 ± 1.36 | .096 | 0.81 |
| Walking ability | 0.82 ± 1.09 | 0.55 ± 0.92 | 0.85 ± 1.07 | .629 | 0.18 |
| Normal work | 2.66 ± 2.47 | 1.07 ± 1.54 | 3.17 ± 2.41 | .011* | 4.84 |
| Relations with other people | 1.23 ± 1.52 | 0.62 ± 1.22 | 1.14 ± 1.09 | .308 | 0.32 |
| Sleep | 1.82 ± 1.62 | 1.29 ± 2.07 | 2.82 ± 1.72 | .032* | 2.11 |
| Enjoyment of life | 0.94 ± 0.96 | 0.85 ± 1.75 | 1.49 ± 1.48 | .311 | 0.28 |
| Additional questions | |||||
| Influence on natural sleeping position | 1.95 ± 1.54 | 1.31 ± 1.75 | 3.34 ± 1.80 | .001* | 25.43* |
| Pain by pressure bandage | — | — | 3.83 ± 3.08 | — | — |
- Overall scores do not exceed 4. No significance was found between the groups for worst, least, and mean pain. Moreover, Bayes factors are between 0.1 and 10. The pressure bandage resulted in a mean score of 3.83 on the first day in the retroauricular group. Sleep was significantly influenced in the retroauricular group (P = .032), whereas the endaural group was less impaired in performing normal work (P = .011). Patients in the retroauricular group experienced more influence on their natural sleeping position (Bayes factor = 25.43, P = .001).
- * Statistically significant value.
- ANOVA = analysis of variance; BPI-SF = Brief Pain Inventory–Short Form; RM = repeated measures; SD = standard deviation.
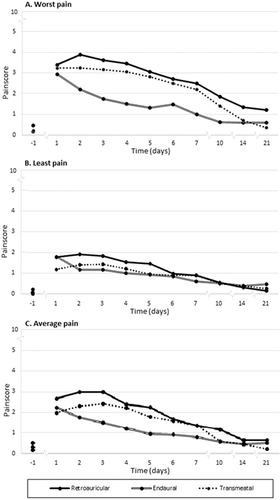
We illustrate how pain is scored over time in Fig. 1. The worst and least pain are distributed just above and below the average pain. A significant (P < .001) increase is present in experienced pain after surgery for the worst and average pain compared to the baseline measurement. Pain levels quickly receded, and after 10 days pain scores were below 2. Analysis of the worst, least, and average pain scores measured per 24 hours showed a BF of 1.07, 0.25, and 0.51, respectively, between groups in the first week and on day 10, 14, and 21 after surgery.
Approximately twice the amount of pain medication was used in the retroauricular and endoscopic transmeatal group, compared to the endaural group (Table III). This difference was statistically significant (P < .001). More powerful opioid painkillers were not used in the endoscopic group when compared to the microscopic groups. The number of patients not needing painkillers was evenly distributed over all investigated groups (P = .650). The areas of experienced pain and paresthesia correspond with the incisions of the surgical approaches.
| Group 1, Transmeatal Endoscopic, Average per Patient ± SD | Group 2, Endaural Microscopic, Average per Patient ± SD | Group 3, Retroauricular Microscopic, Average per Patient ± SD | |
|---|---|---|---|
| Paracetamol 1 g | 10.1 ± 10.7 | 5.6 ± 9.0 | 11.3 ± 9.6 |
| NSAID (ibuprofen 400 mg, diclofenac 50 mg, naproxen 250 mg) | 3.2 ± 8.6 | 0.7 ± 1.56 | 4.6 ± 7.5 |
| Opioid (tramadol 50 mg, oxycodone 5 mg) | 0 | 0.7 ± 3.1 | 0.7 ± 2.5 |
| Mean relief of pain, % | 54.5 ± 29.7 | 64.4 ± 26.6 | 67.1 ± 18.3 |
- Approximately twice the amount of pain medication per patient was used in the retroauricular and endoscopic transmeatal group, compared to the endaural group. Opioid painkillers were taken in the endaural and retroauricular group (average 0.7 per patient), but not in the endoscopic transmeatal group. Relief of pain was 54.5%, 64.4%, and 67.1%, respectively, for the endoscopic transmeatal, endaural, and retroauricular group (P = .704).
- NSAID = nonsteroidal anti-inflammatory drug; SD = standard deviation.
DISCUSSION
Overall pain perception in all groups was very low, especially when compared with other pathologies measured with the BPI-SF such as osteoarthritis of the hip and knee or a malignancy with bone metastases.26, 31 Others have also demonstrated that ear surgery in general can be considered to be near to painless.32 Even the pressure bandage in the retroauricular group did not negatively influence pain scores (mean VAS score = 3.83 ± 3.08). We used only fully completed questionnaires, because inclusion of pain scores from incomplete questionnaires from dropouts had no influence on results.
Differences between groups are evaluated with Bayesian and frequentist statistics. Bayesian statistics makes it possible to evaluate differences and equality in small groups without losing power while retaining precision.29 Frequentist statistics solely test differences and cannot determine equality. Bayesian analysis neither showed equality nor difference between the three groups. This says that there is anecdotal evidence or a relationship by chance that the amount of pain is different or equal between groups. Frequentist analysis did not show significant differences in pain. Because differences in pain scores are small, much larger groups would be needed to achieve statistical significance. It is our belief that these large numbers needed to treat do not reflect clinically relevant differences. The statistically significant differences found between groups, such as the small disadvantages for sleep and natural sleeping position in the retroauricular group, should be interpreted with caution. Because these differences are small, the clinical relevance of these differences is questionable. We believe this also applies to the difference of pain scores found by Kakehata et al., which were mean 1.1 ± 0.9 for transmeatal endoscopic versus 2.8 ± 2.6 for retroauricular microscopic approach.23
In this study, more dropouts were present in the microscopic group. Additionally, there is a difference in performed surgeries; the amount of tissue damage can be considered to differ among our three groups. Because Kakehate et al. demonstrated that type of surgery did not influence pain within their study population,23 we believe that groups are comparable. They found that between the endoscopic and microscopic group, the amount of resected bone was not an influence on postoperative pain. Our patient selection minimalized inclusion bias but did not completely eliminate it.
Our study population was not hospitalized for more than 24 hours, which is different from the study conducted in Japan.23 This could theoretically influence pain perception. Therefore, our study group cannot be considered easily comparable to the Japanese group. Others found no difference in pain measured after 2 weeks postoperatively.24 Mean pain scores of 3.6 and 4 for endoscopic transmeatal and retroauricular microscopic surgery, respectively, are relatively high compared to the Japanese group and our results. No information about hospitalization is mentioned.24
The role of postoperative pain medication appears to be limited, because few painkillers were taken, and similar relief percentages were reported (P = .704). We have no explanation why patients from the endaural group used half the amount of pain medication compared to the other two groups. Opioid painkillers, although in low amounts (average = 0.7 per patient), were taken in the endaural and retroauricular group. Surprisingly, none were used in the endoscopic group. As expected, areas of pain and paresthesia correspond with the incisions of the surgical approaches. This supports Kakehata's finding that type of surgery has no influence on the level of pain.23
CONCLUSION
The results of this study show that the surgical approach has no clinically relevant influence on postoperative pain after ear surgery. The statistically significant differences on natural sleeping position, sleep, normal work, and amount of pain medication taken are small and should be interpreted with caution. Therefore, these should not be decisive factors in the choice of surgical approach in ear surgery.
ACKNOWLEDGMENT
A generous contribution of the Heinsius-Houbolt Foundation.




