Pemetrexed-induced pseudocellulitis
Peripheral edema, also known as pseudocellulitis, is a common side effect for patient treating with gemcitabine. But pseudocellulitis caused by pemetrexed has rarely been reported. Herein, we report a case of pemetrexed-induced pseudocellulitis.
A 75-year-old man with underlying disease of non-small cell lung cancer presented to our department with painful rash distributed on the bilateral legs for 4 days. The patient was treated with the sixth times combination therapy of cisplatin and pemetrexed for non-small cell lung cancer, without adverse cutaneous reaction. Four days prior to the development of the rash, the patient was treated with the seventh times chemotherapy of pemetrexed only. Painful rash appeared on the bilateral shins initially without fever. Subsequently the lesions spread to the bilateral ankles and dorsal feet within 1 day. Physical examination revealed ill-defined, erythematous plaques symmetrically distributed along shins to dorsal feet, associated with grade 3 pitting edema (Figure 1A). The patient complained very painful upon palpation but not aggravated by stretching his toes. Laboratory data demonstrated mild leukopenia (3130/mm3; normal > 4000), C-reactive protein (CRP) elevation (11.43 mg/dL; normal < 1), D-Dimer mild elevated (5.46 μg/mL; normal < 5) and eosinophil counts within normal range (0.1%). No clinical improvement was observed under the treatment of oral augmentin 1000 mg every 12 hours for 4 days. Skin biopsy was not performed because the patient worried about the possibility of poor wound healing. After discontinuation of antibiotic, treatment with 20 mg prednisolone daily and clobetasol propionate ointment (twice daily) were administered for 3 days, the decreased affected areas and resolution of symptoms were noticed. Venous doppler ultrasonography showed no evidence of deep vein thrombosis. Under the clinical presentation and response to the treatment, the diagnosis of pemetrexed-induced pseudocellulitis was made, without evidence of infection contribution to the presentation. Treatment with 20 mg prednisolone daily was continued for 2 weeks and pemetrexed was suspended. The lesions became softer and without tenderness and edema, associated with diffuse desquamation (Figure 1B).
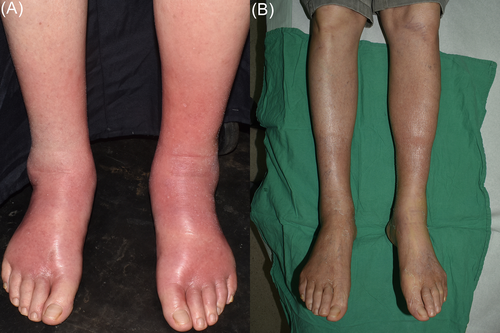
Pemetrexed, an antifolate and cytotoxic agent, is widely be used as the combination therapy with cisplatin for non-small cell lung cancer, other solid organ and hematologic malignancies. Its cutaneous side effects include pruritis, conjunctivitis, periorbital edema and edema of the legs.1 Few publications discussed pemetrexed-induced pseudocellulitis after the literature review.2-5 The diagnosis of pemetrexed-induced pseudocellulitis is based on clinical features that unresponsive to antibiotic and with obvious improvement under systemic or topical steroid. The main difference between pemetrexed-induced pseudocellulitis and cellulitis is that the affected lesions are always bilateral. The histopathology revealed drug hypersensitivity reaction and interface dermatitis,1 while squamous metaplasia of the eccrine ducts2 and eosinophilic infiltration were also reported.3 The underlying pathomechanism of pemetrexed-induced pseudocellulitis was still unclear. Many hypotheses were postulated, including drug hypersensitivity, vascular damage, impaired lymphatic drainage and the patients' nutrition status. Prophylaxis can be managed with steroid and folic acid supplement, but the occurrence of this adverse cutaneous reaction was still noticed on previous report.4 Due to the limited number of cases, no predisposing factors could be found, and both sexes were equally reported. The onset of this adverse reaction is between the second and the seventh courses. Further studies are needed for clarifying the exact cause.
In conclusion, we report a case of pemetrexed-induced pseudocellulitis. The unfamiliarity of dermatologists with this adverse cutaneous reaction may cause diagnostic difficulty. This case highlights the importance to raise the awareness of physicians to this diagnosis process.
CONFLICT OF INTEREST
All authors declare no conflict of interest.




