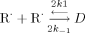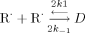Rates and thermodynamic data have been obtained for the reversible self-termination reaction:
Involving aromatic 2-(4′dimethylaminophenyl)indandione-1,3-yl (I), 2-(4′diphenylaminophenyl)indandione-1,3-yl (II), and 2,6 di-tert-butyl-4-(β-phthalylvinyl)-phenoxyl (III) radicals in different solvents. The type of solvent does not tangibly affect the 2
k1 of Radical(I), obviously due to a compensation effect. The log(2
k1) versus solvent parameter
ET(30) curves for the recombination of radicals (II) and (III) have been found to be V shaped, the minimum corresponding to chloroform. The intensive solvation of Radical (II) by chloroform converts the initially diffusion-controlled recombination of the radical into an activated reaction. The log (2
k−1) of the dimer of Radical (I) has been found to be a linear function of the Kirkwood parameter (ε - 1)/(2ε + 1), the dissociation rate increasing with the dielectic constant of the solvent. The investigation revealed an isokinetic relationship for the decay of the dimer of Radical (I), an isokinetic temperature β = 408 K and isoequilibrium relationship for the reversible recombination of Radical (I) with β° = 651 K. For Radical (I) dimer decay In(2
k−1) = const + 0.8 In
K, where
K is the equilibrium constant of this reversible reaction. The transition state of Radical (I) dimer dissociation reaction looks more like a pair of radicals than the initial dimer. The role of specific solvation in radical self-termination reactions is discussed.