Successful Treatment of Erythroderma in a Psoriasis Patient, Developed After Anti-IL-17, Anti-IL-23 and Anti-IL-4/IL-13 Treatment, With Deucravacitinib
ABSTRACT
We present the case of a 79-year-old female patient diagnosed with psoriasis who developed paradoxical drug reactions after administration of anti-IL17 and anti-IL-23 inhibitors. Having shifted towards a rather eczematous phenotype, the patient received an anti-IL-4/IL-3 inhibitor, subsequently developing erythroderma. This patient had a history of lichen planus 20 years prior, which was not present clinically during the following years. The skin lesions in this patient resolved significantly after initiation of the TYK2 inhibitor deucravacitinib, possibly because this drug inhibits inflammatory factors typical of psoriasis (IL-17, IL-23) as well as key cytokines of lichen planus (CXCL10).
1 Introduction
Recent advancements in targeted therapies have significantly improved the management of inflammatory skin diseases, such as psoriasis and atopic dermatitis. However, paradoxical drug reactions emerged as a challenge, leading to a shift in the clinical phenotype of a diseas. The so-called “flip-flop” phenomenon, wherein patients with atopic dermatitis develop psoriasis under biologic therapy, is well documented [1]. These complex paradoxical reactions can involve alterations in the inflammatory pathways, such as type I, II and III immune responses [2]. Understanding these underlying mechanisms is crucial to optimizing treatment strategies for patients in selecting the most effective therapy each individual. Here, we present a case of a 79-year-old female with psoriasis who experienced a paradoxical shift in her disease pattern, ultimately requiring a broader immunomodulatory approach.
2 Case Report
A 79-year-old female patient presented to our outpatient unit with erythroderma and scaling of the entire integument with severe affection of the scalp and 24 h peak point pruritus of 10/10 on the Numeric Rating Scale (NRS). The patient had been diagnosed with psoriasis 2 years previously. At that time point she only had psoriasis-typical plaques at the extensor-sides her extremities and the lesional histology was in accordance with this diagnosis (Figure 1a–c). Treatment with an IL-17 inhibitor (bimekizumab) was initiated, but resulted in no clinical improvement. Therefore, the systemic therapy was switched to an anti-IL-23 inhibitor (risankizumab) together with an intensified topical steroidal therapy and UVB light therapy. Under this therapy she developed a further clinical deterioration, specifically with an eczematous suberythroderma, so that the diagnosis of a paradoxical drug reaction with now new manifestation of atopic dermatitis was made. Systemic therapy was subsequently switched to an anti-IL-4/IL-13 inhibitor (dupilumab), under which the overall picture continued to aggravate, developing erythroderma and severe pruritus. At this timepoint, she was presented to our department.
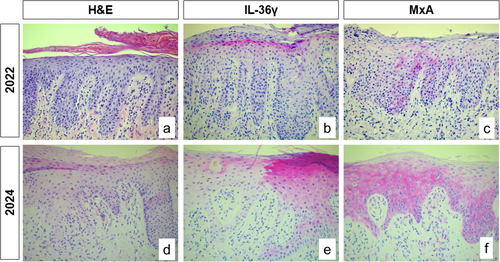
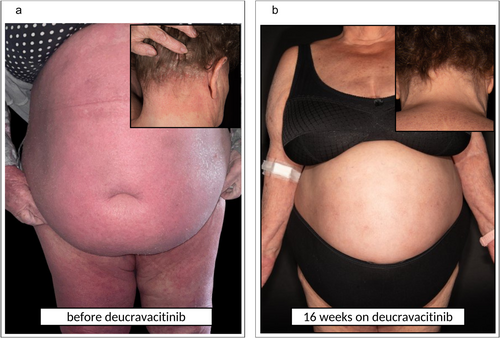
We performed skin biopsies, that revealed a pronounced inflammatory skin infiltrate with patterns of different skin diseases: in addition to psoriasis-like skin manifestation including IL-36γ expression, and focal spongiosis, an interface dermatitis with sawtooth-like acanthosis and a strong interferon (IFN) signature was present, as typically seen in lichen planus (LP) (Figure 1d–f). As part of the detailed anamnesis, the patient reported that she had been diagnosed with lichen planus 20 years ago. However, the skin lesions had improved spontaneously at that time and completely regressed without further systemic intervention. Due to these findings, we decided to switched systemic therapy to deucravacitinib. This drug is a TYK2 inhibitor with broader effector mechanism, including not only anti-psoriatic pathways but also IFN-associated pro-inflammatory cytokines, which are typically seen in LP [4]. This therapy resulted in a very good overall therapeutic response with a complete remission of erythema and reduction of the peak point pruritus to 2/10 NRS within a few weeks (Figure 2).
3 Discussion
Paradoxical drug reactions are rare but can be potentially severe, and they may occur with any biologic therapy. These reactions often lead to shifts in the clinical phenotype of the disease, presenting challenges in patient management. They can involve alterations in inflammatory pathways, such as type I, II and III immune responses, requiring careful consideration in treatment selection.
Our patient developed eczema after treating her psoriasis, a type III disease, with anti-IL-17 and anti-IL-23 inhibitors followed by treatment with the anti-IL-4/IL-13 inhibitor, which has been developed to treat atopic dermatitis, a type II disease. It is tempting to speculate why she developed erythrodermia, but the fact that she had a history of LP could be the key to understanding this: LP is a type I disease (characterized by a strong activation of the type I IFN system) that usually does not respond to therapy with anti-IL-17, anti-IL-23 or anti IL-4/IL-13 inhibitors. LP-like drug reactions have already been described under therapy with anti-IL-17, anti-IL-23 and anti-IL-4/IL-13, agents [5-7]. For this reason, we opted for a broader anti-inflammatory therapy, in this case with deucravacitinib, when the patient presented to our clinic. Another effective and more cost-efficient option would be treatment with methotrexate, which also provides broad anti-inflammatory properties. However, this was not feasible due to the patient's multiple pre-existing conditions, including impaired kidney function.
Deucravacitinib is a highly selective inhibitor of the JAK family member TYK2. The FDA approved deucravacitinib for adults with moderate-to-severe plaque psoriasis in September 2022 followed by the European Commission (EMA) in March 2023. TYK2 plays an important role in JAK-STAT-mediated signal transduction of various ligands, including IL-23, IL-12 and, in particular, type I IFN [1]. Unlike other JAK inhibitors, deucravacitinib has a unique binding mechanism, specifically targeting the regulatory pseudokinase domain of TYK2 and stabilizing an inhibitory interaction with the catalytic domain. This blockade prevents receptor-mediated activation of TYK2 and inhibits downstream cellular functions, distinguishing it from nonselective JAK inhibitors that target the conserved active site of the kinase domain, resulting in a distinct profile of effects and side effect [1]. On the one hand, these mechanisms lead to the downregulation of typical psoriasis-cytokines such as IL-17 and IL-23, but on the other hand also have a targeted effect on type I-associated pro-inflammatory cytokines such as CXCL9 and CXCL10, which play a decisive role in lichen planus [8-10].
Another important differential diagnosis to consider in cases of erythroderma unresponsive to therapy is malignant erythroderma, such as mycosis fungoides or Sézary syndrome. If the diagnosis is uncertain, multiple biopsies and evaluation of T-cell receptor clonality should be carried out.
Our case highlights the challenge of selecting the optimal drug for the individual patient with a specific skin disease. Antibodies targeting individual cytokines have the advantage of a high specificity, but still can induce unwanted side effects such as the paradoxical drug reactions described here. Small molecules, which usually lead to the blockade of entire inflammatory pathways, are more broadly effective, but tend to be associated with more systemic side effects. Ideally, a comprehensive molecular characterization of individual patients before initiating therapy should be made to identify underlying overlap diseases and the specific inflammatory pattern.
Author Contributions
T.G., J.W., N.N. and D.W.T. have treated the patient. J.W. and L.d.V.H. have reviewed the histopathology. T.G. and J.W. prepared the manuscript. L.d.V.H., D.W.T. and N.N. have revised the manuscript.
Acknowledgements
We thank the patient for granting permission to publish this information. The authors received no specific funding for this work.
Ethics Statement
The patient in this manuscript has given written informed consent for use of his deidentified, anonymized, aggregated data and their case details (including photographs) for publication. Ethical Approval: not applicable.
Conflicts of Interest
Joerg Wenzel has received funding from BMS for a clinical research project and fees for consulting services. Natalija Novak and Dagmar Wilsmann-Theis have also received funding from BMS. The remaining authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.