The efficacy of anti-IL-1 targeted therapy in PAPA and PASH syndrome
[Correction added on 16 November 2022, after first online publication: Acknowledgement has been changed to Ethics Statement as appropriate.]
Abstract
Introduction
Pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) and pyoderma gangrenosum, acne and hidradenitis suppurativa (PASH) are rare autoinflammatory diseases. Treatment of PAPA and PASH is difficult. Conventional immunosuppressants and tumor necrosis factor (TNF) antagonists fail to control skin lesions in many patients.
Materials and Methods
We conducted a retrospective observational study of patients treated with interleukin-1 (IL-1) antagonists for PAPA or PASH syndrome. We included all PASH and PAPA treated with the IL-1 antagonists canakinumab and anakinra in Toulouse, Brest and Montpellier, France and report on the patients' response to treatment.
Results
Four patients were treated with anakinra (one patient) and/or canakinumab (three patients) for 6 months to 10 years. Treatment was associated with sustained improvement of skin lesions: hidradenitis suppurativa in four patients including full resolution of skin lesions in three patients, acne with full resolution in one patient, pyoderma gangrenosum in three patients who experienced full resolution of skin lesions. Anti-IL-1 treatment was also associated with full resolution of symptoms associated with spondyloarthritis in one patient. One patient who had been receiving oral corticosteroids for years was able to stop treatment while on IL-1 antagonist.
Discussion
IL-1 antagonists, especially canakinumab, are useful alternatives to treat patients with PAPA or PASH syndrome, especially patients who are non-responders to conventional treatment and TNF antagonists.
1 INTRODUCTION
Pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) and pyoderma gangrenosum, acne and hidradenitis suppurativa (PASH) are two rare autoinflammatory diseases caused by dysregulation of the innate immune system with an overproduction of interleukin-1β (IL-1β).1-4
PAPA syndrome is an autosomal dominant hereditary disease associated with mutations in the PSTPIP1 gene.1, 5-10 The disease usually develops during childhood. PASH syndrome was identified more recently than PAPA syndrome.11 It can be distinguished from PAPA by the absence of pyogenic sterile arthritis. Hidradenitis suppurativa (HS) is a polygenic, inflammatory skin disease that causes painful nodules, abscesses, sinus tracts, and hypertrophic scars in the apocrine gland-bearing areas. HS has been associated with dysregulation of PSTPIP1 expression, mutations in the MEFV (Mediterranean fever) and nicastrin (NCSTN) genes, and in the NOD2 (nucleotide-binding oligomerisation domain-containing protein 2).1, 9, 11-14
Pathophysiologically, there is a dysregulation of IL-1 production and signalling in patients with PAPA and PASH.3 Therefore, anti-IL-1 therapy holds promise for the treatment of autoinflammatory disorders. Currently, two anti-IL-1 agents are available: Anakinra (an IL-1 receptor antagonist, administered daily) and Canakinumab (a neutralising monoclonal anti-interleukin-1β antibody, administered in one injection every 1–2 months).
Anakinra is the agent most studied in the literature and has been reported to be a treatment of PAPA and PASH syndromes.
There are mixed reports on its use in PAPA and PASH. In one patient, early termination of anakinra treatment occurred because of intolerable local reactions at the injection site.7 Three patients had very good responses in the treatment of arthritis and pyoderma gangrenosum.15-17 The dose of anakinra used varied between 1 mg/kg and 200 mg per day. Two patients with PASH failed to achieve complete remission of pyoderma, acne and hidradenitis despite a dose of 200 mg daily.11, 18
Canakinumab was reported to be effective for PAPA syndrome in one patient.7 The dose used was 150 mg subcutaneously every 8 weeks. Treatment was associated with complete clearing up of acne lesions.
2 MATERIALS AND METHODS
We conducted a real-life retrospective study on the effectiveness and safety of IL-1 inhibitors in PAPA and PASH syndromes. Our study included all PASH and PAPA treated with anti-IL-1 between 2012 and today in Toulouse, Montpellier and Brest, France. Clinical information was collected from patients' records and through interviews with the four patients treated. In this paper, we report on the patients' clinical response to treatment notably the effects on skin lesions and on the others treatments.
3 RESULTS
Patients were mostly male (3/4). They were between 21 and 44 years old when treatment was introduced.
All four patients had a clinical diagnosis of PAPA,2 or PASH2 syndrome, and only one patient had an identified genetic mutation (FMF gene mutation). On average, patients received four different lines of therapy including antibiotics, colchicine, isotretinoin, oral corticosteroids and intravenous immunoglobulins. All the patients had previously received TNF inhibitors and experienced limited improvement in skin lesions. One patient received Canakinumab, one received Anakinra and two received first Anakinra and then Canakinumab to decrease the frequency of injections (one patient) or due to poor tolerability of anakinra (painful inflammatory nodules at the injection site in one patient). For anakinra the doses used varied between 100 mg/day and 200 mg/day and for canakinumab they varied between 150 mg every 2 weeks and 300 mg every 8 weeks.
Treatment was administered in association with at least one concomitant systemic agent or biological treatment (Table 1). Treatment with IL-1 antagonists was associated with an improvement in skin lesions in all patients (Figure 1). The effectiveness of the treatments was rapid for all patients with an improvement in the lesions after 1–2 months of treatment. The majority of patients had long-lasting efficacy except for the patient who presented with recurrences of lesions at 6 months.
| Subject | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Age | 37 years old (yo) | 48 yo | 46 yo | 25 yo | |
| Disease | PASH syndrome | PAPA syndrome | PASH syndrome | PAPA syndrome | |
| Medical background | Bipolar disorder, acromegaly | 0 | 0 | 0 | |
| Gender | Female | Male | Male | Male | |
| Onset | 18 yo | 38 yo | 36 yo | 20 yo | |
| Age at introduction of anti-IL-1 | 37 yo | 38 yo | 44 yo | 21 yo | |
| Duration of treatment with anti-IL-1 | 6 Months and continued (still ongoing at the time of the report) | 10 Years and continued (still ongoing at the time of the report) | 2 Years and continued (still ongoing at the time of the report) | 6 Months | |
| IL-1-targeting treatment | Canakinumab | Anakinra 7 years and then Canakinumab 3 years | Anakinra for a few months and then Canakinumab | Anakinra for 6 months in 2017 | |
| Dose and frequency of administration | 300 mg/month and then 150 mg every 15 days | Anakinra 100 mg/day and then Canakinumab 300 mg/month | Canakinumab 300 mg every 8 weeks and then every 6 weeks | Anakinra 100 mg/day and then 200 mg/day | |
| Associated treatments | Antibiotics | Etanercept | Ciclosporin, oral steroids, Mycophenolate mofetil, Dapsone | oral corticosteroids | |
| Number of treatments previously received | 2 | 7 | 4 | 4 | |
| Antibiotics | 0 | 0 | Yes | Yes | |
| Colchicine | 0 | Yes | 0 | 0 | |
| Retinoids | 0 | Isotretinoin | Isotretinoin | Acitretin | |
| Oral steroids | 0 | Yes | 0 | Yes | |
| Immunosuppressants | Ciclosporin | Ciclosporin | 0 | 0 | |
| Intravenous Immunoglobulins | 0 | 0 | Yes | 0 | |
| TNF inhibitor | Infliximab | Infliximab, Etanercept and Adalimumab | Infliximab | Adalimumab | |
| Associated inflammatory disease | 0 | Familial Mediterranean fever | 0 | 0 | |
| Mutation | 0 | Gene FMF | 0 | 0 | |
| Clinical characteristics | Acne | Yes | Yes | Yes | Yes |
| Hidradenitis suppurativa | Yes | Yes | Yes | Yes | |
| Pyoderma gangrenosum (PG) | 0 | Yes | Yes | Yes | |
| Abscesses | Yes | 0 | Yes | Yes | |
| Pyogenic arthritis | 0 | Yes | 0 | 0 | |
| Spondyloarthopathy | 0 | Yes | 0 | Yes | |
| Family history | Father and sister have HS | 0 | 0 | 0 | |
| Clinical benefits provided by anti-IL-1 on: | |||||
| Acne | 0 | 0 | 0 | Yes | |
| SH | Yes | Yes | Yes | Moderate | |
| PG | / | Yes | Yes | Yes | |
| Arthralgia | / | 0 | / | Yes | |
| Change of therapy with anti-IL-1 | 0 | Oral steroid withdrawal | 0 | 0 | |
| Side effects | 0 | 0 | 0 | 0 | |
- Abbreviations: IL-1, Interleukin-1; PAPA, Pyogenic arthritis, pyoderma gangrenosum and acne; PASH, pyoderma gangrenosum, acne and hidradenitis suppurativa; TNF, tumor necrosis factor.
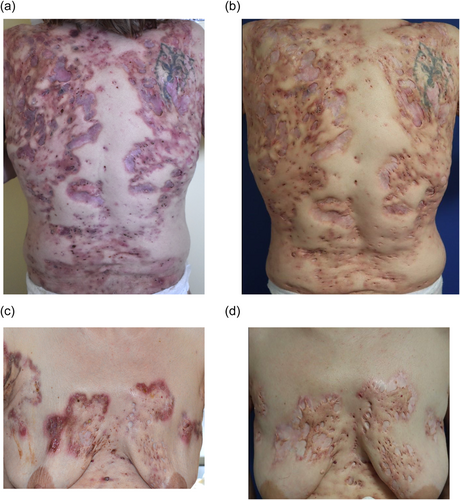
Complete clearance and an absence of new hidradenitis suppurativa lesions were observed in three patients. An absence of new flares with a persistence of new lesions was observed in one patient. Treatment led to complete disappearance of acne lesions (full resolution) in one patient.
Three patients showed complete clearance of pyoderma gangrenosum lesions with an absence of new lesions. Finally, one patient experienced concomitant improvement in spondyloarthropathy with complete pain relief (Table 1). Treatment with IL-1 antagonists also led to therapeutic de-escalation. One patient was able to stop oral corticosteroids after 16 months of treatment with Canakinumab.
One patient who was treated continuously with Anakinra relapsed at 6 months and Anakinra was stopped. For the three other patients, the duration of treatment varied between 6 months and 10 years and the treatment was still ongoing.
Treatment with IL-1 antagonists was well tolerated by all the patients.
4 DISCUSSION
Treatment of PAPA and PASH is difficult and conventional immunosuppressants such as oral corticosteroids, methotrexate and cyclosporine are of limited value to control skin lesions.1, 3, 7, 11, 13 Frequently, patients develop a dependence on oral corticosteroids resulting in adverse events in the long run. Some case series have reported TNF-α antagonist effectiveness. There is limited data regarding the efficacy of IL-1 antagonists in PAPA/PASH syndrome with a total of seven patients described in the literature7, 11, 15-18). In most patients, Anakinra was used with variable results (absence of complete remission and a need for combination with other treatments).
Our study is in line with previous reports showing a short time to onset of efficacy with anti-IL-1 antagonists which provide benefits after 1–2 months of treatment.7, 11, 15 We confirm the usefulness of IL-1-antagonists in PAPA and PASH syndromes and provide additional data on canakinumab which appears to be effective in patients resistant or intolerant to anakinra.7 We also show that the responses are dose-dependent and that it may be of use to increase the dose and the frequency of administration in some patients.
It is important to note that complete response was obtained through a combination with other agents such as oral corticosteroids, antibiotics, ciclosporin or TNF inhibitors in all patients. Therefore, the effectiveness of anti-IL-1 as a monotherapy cannot be fully established.
Canakinumab appears to be a useful alternative to Anakinra with a longer half-life that enables dosing every 1–2 months and has been associated with a more stable and sustained response than anakinra.
The study is limited by the small sample size due to the rarity of the diseases.
To conclude, IL-1 targeted therapies, especially Canakinumab, appear to be a useful therapeutic alternative for PAPA and PASH syndrome patients who are nonresponders to conventional systemic immunosuppressants and TNF antagonists.
CONFLICTS OF INTEREST
Challamel C: none. Dr. Girard: Abbvie, Janssen, Leo Pharma, Novartis, UCB, Citog, Lilly. Prof Misery: Novartis. Dr. Touhouche: none. Dr. Fradet: none. Dr. Severino: none. Dr. Livideanu: none. Dr. Konstantinou: none. Dr. Jendoubi: Janssen, Sanofi. Prof Paul: Abbvie, Boehringer, Amgen, Eli Lilly, Almirall, Janssen, BMS, Pierre Fabre, Sanofi, UCB, Leo Pharma, Mylan, Novartis, Pfizer, IQVIA.
ETHICS STATEMENT
The patients in this manuscript have given written informed consent to publication of their case details.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.