Successful treatment of recalcitrant follicular lichen planus (lichen planopilaris) with the topical JAK 1/2 inhibitor ruxolitinib
Abstract
We report the successful treatment of a patient with refractory follicular lichen planus (LP) with topical ruxolitinib, a Janus kinase (JAK) 1/2 inhibitor, which resulted in significant improvement of lesional skin within 1 month of daily use.
INTRODUCTION
Lichen planopilaris is the follicular variant of lichen planus (LP), characterized by an inflammatory, primary cicatricial alopecia that results in hair loss. The exact pathogenesis of the disease is still unclear, but is widely regarded as a hair-specific autoimmune disorder in which cytotoxic T-lymphocytes target follicular antigens, driven by interferon (IFN)-associated proinflammatory cytokines.1, 2 Topical and systemic corticosteroids, antimalarials and other immunosuppressive/immunomodulatory drugs are commonly used therapeutically, with variable success.1 However, to date no specific therapy has been approved for the treatment of the this difficult condition.3
CASE REPORT
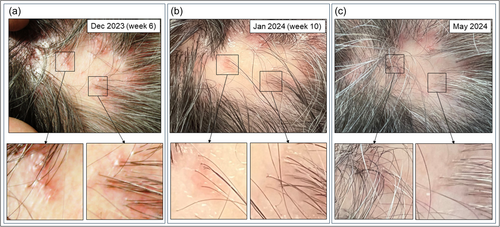
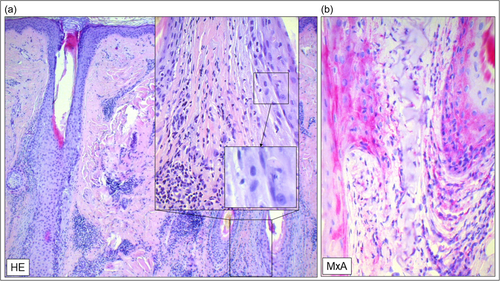
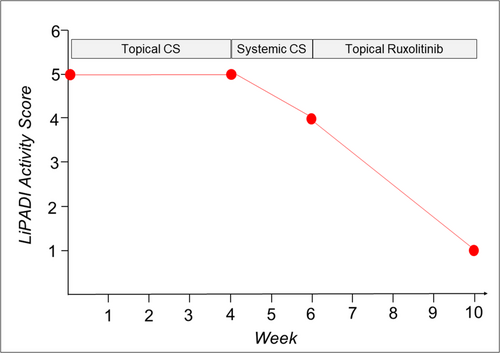
A 62-year-old man presented to our department with a one-and-a-half-year history of inflammatory hair loss and itch in the occipital region of the scalp, which did not respond to treatment with topical corticosteroids (0.1% betamethasone valerate cream). Clinical examination revealed multifocal asymmetric patches of scarring alopecia with perifollicular erythema and scaling (Figure 1a). A scalp biopsy showed a lichenoid inflammatory infiltrate around the upper part of the hair follicle, vacuolar degeneration, necroptotic keratinocytes, as well as a strong lesional expression of type I/III IFN surrogate marker Myxovirus Resistance A (MxA) (Figure 2a,b), consistent with the diagnosis of follicular LP with a LiPADI (Lichen Planus Activity and Damage Index).4 Activity score of 5. We initiated topical treatment with Mometasone cream for 4 weeks, followed by adding oral Prednisolone (40 mg/day in tapering dosage to zero) for an additional 2 weeks, which had to be discontinued due to drug-related side effects (hypertension, dizziness). Unfortunately, these therapies did not improve his skin lesions which remained in an active state with follicular hyperkeratosis and lesional inflammation (LiPADI = 4, Figure 3). Therefore, we decided to initiate a topical treatment with the Janus kinase (JAK1/2) inhibitor Ruxolitinib (Opzelura Cream, 2x/d), which led to a significant improvement of the follicular LP lesions within 4 weeks accompanied by a decline of the LiPADI activity score from 4 to 1 (Figures 1b and 3). This therapy was then reduced to a single dose every 1−2 days, resulting in a well-controlled disease to date (Figure 1c).
DISCUSSION
LP is considered an inflammatory autoimmune skin disease driven by IFN-associated proinflammatory cytokines, including CXCL9 and CXCL10, and is characterized by a cytotoxic interface dermatitis resulting in keratinocytic cell death and loss of hair follicle stem cells.5, 6 The spectrum of clinical LP manifestations is broad, reaching from mucosal lesions to cutaneous LP featuring characteristic skin papules, as well as LP with destructive nail and hair disease (follicular LP). Among those, the treatment of follicular LP is particularly challenging due to the poor response to available therapies.3 Topical corticosteroids, which are widely used as first choice treatment, have a limited effect on follicular LP due to the limited penetration of these drugs into the deeper dermis and hair follicle.7 Topical JAK inhibitors such as Ruxolitinib are small molecules and might, therefore, be more effective in this condition. With their low molecular weight of approximately 300 g/mol, they are smaller than the theoretical epidermal penetration limit of 500 g/mol and may penetrate deeper into the skin than corticosteroids (Mometasonfuroate: g/mol 521.4 g/mol, Betamethasone valerate: 476.58 g/mol).8 Mechanistically, they are well-suited to treat the inflammatory pathways in LP: Ruxolitinib strongly inhibits the expression of LP-typical IFN-regulated cytokines, including CXCL10 and MxA, and might therefore help to break the inflammatory vicious circle of this disease.9, 10
This notion is supported by recent clinical observations: Topical ruxolitinib has been shown to be effective in a small case series of 12 patients with cutaneous LP as well as in one patient with recalcitrant LP pigmentosus.11, 12 In addition, oral JAK inhibitors, including tofacitinib, baricitinib, ruxolitinib and upadacitinib, have been shown to reduce LP disease activity in recalcitrant LP.13
Currently, several studies are underway on topical JAK inhibitors. It will be interesting to learn how effective these substances are in treating dermatological diseases compared to established therapeutic options, particularly those with extensive inflammatory infiltrates in deeper dermal areas.
AUTHOR CONTRIBUTIONS
Tanja Fetter drafted the manuscript and designed the figures. Joerg Wenzel contributed in writing and editing of the manuscript as well as designing the figures. Natalija Novak, Dagmar Wilsmann-Theis and Luka de Vos-Hillebrand revised the manuscript. All authors approved the final manuscript for publication.
ACKNOWLEDGEMENTS
We thank the patient for granting permission to publish this information. T. F. is supported by a grant from the University of Bonn BONFOR program (Gerok position, grant number 2023-1A-17). T. F. is supported by a grant of the University Hospital Bonn (BONFOR). Open Access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTEREST STATEMENT
The authors have been an advisor and/or received speakers' honoraria or travel expense reimbursements and/or received grants and/or participated in clinical trials of the following companies: Tanja Fetter: Biogen, AstraZeneca. Dagmar Wilsmann-Theis: Abbvie, Almirall, Amgen, Hexal, Boehringer Ingelheim, BMS, Eli Lilly, Janssen-Cilag, Leo Pharma, Medac, Novartis, UCB Pharma, Klinge Pharma. Luka de Vos-Hillebrand: Pierre Fabre. Natalija Novak: Abbvie, Leo Pharma, Eli Lilly, Novartis, Pfizer, Regeneron, Sanofi Genzyme. Joerg Wenzel: Biogen, Janssen, Bayer, GSK, Incyte, BMS, Actelion, AstraZeneca, Merck, Medac, Novartis, LEO, Kyowa Kirin.
ETHICS STATEMENT
The patient in this manuscript has given written informed consent for use of his deidentified, anonymized, aggregated data and their case details (including photographs) for publication. Ethical Approval: not applicable.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.