A Novel Technology for Resolution of CEUS Imaging Problems in Patients With High BMI and Fatty Liver
Equipment support—A Siemens Acuson Sequoia ultrasound machine is available in our tertiary ultrasound facility as part of a research agreement between Siemens-Healthineers, Issaquah Washington, and Foothills Medical Center (FMC)/the University of Calgary (UofC).
Thanks to Dr. Paul Freiburger and Dr. Gerald Moran for their support and information on the technical design of the SDP, which was integrated into our article. Christina Merrill – none; Anna Samuel – none; Saransh Gupta – none; Stephane R. Wilson; Philips, Speakers Bureau; Lantheus Medical Imaging, Advisory Board, Definity; Equipment support, Siemens; Equipment support, Samsung; Equipment support, Philips.
Abstract
Objectives
In high-BMI patients with and without fatty liver, we evaluate performance of a commercially available specially designed ultrasound probe (SDP) for scanning at depth. Greyscale and contrast-enhanced ultrasound (CEUS) capability of SDP for parenchymal assessment and liver mass characterization, emphasizing HCC, is compared with standard curvilinear probes.
Methods
This retrospective study included 60 patients. Fifty-five with measured BMI included 46/55 (84%) overweight or obese, and 9/55(16%) in the normal range with severe fatty liver. Fifty-six patients with focal liver abnormality included 37 with a mass and 19 with post-ablative treatment site. Masses included 23 confirmed malignancies, 15 HCC, 4 ICC, and 4 metastases. SDP followed suboptimal ultrasound using a standard probe. Images with varying fat content were compared for depth of penetration on greyscale and ability of CEUS to diagnose tumors.
Results
SDP showed statistically significant improvement P = <.05 in CEUS penetration for all degrees of fatty liver (mild, moderate, and severe). In malignant tumors, SDP improved detection of lesion washout in the portal venous/late phase (PVP/LP) at depth >10 cm, and in all malignant masses (P < .05).
Fifteen confirmed deep HCC showed arterial phase hyperenhancement on standard probe in 10/15 (67%) and 15/15 (100%) on SDP. PVP/LP washout on standard probe was shown in 4/15 (26%) and on SDP, 14/15, (93%). Therefore, 93% of LR-5 tumors were diagnosed with SDP. Removing necessity for biopsy.
Conclusions
Metabolic syndrome and obesity challenge ultrasound, especially CEUS. SDP overcame limitations of standard probes for CEUS penetration especially in fatty liver. SDP was optimal for the liver mass characterization by detecting washout.
Abbreviations
-
- ALD
-
- alcoholic liver disease
-
- AP
-
- arterial phase
-
- APHE
-
- arterial phase hyperenhancement
-
- CEUS
-
- contrast-enhanced ultrasound
-
- LP
-
- late phase
-
- MBCA
-
- microbubble contrast agents
-
- NAFLD
-
- non-alcoholic fatty liver disease
-
- NASH
-
- non-alcoholic steatohepatitis
-
- PVP
-
- portal venous phase
-
- SDP
-
- specially designed ultrasound probe
-
- WO
-
- washout
Radiology departments must provide high-quality abdominal imaging to all patients in the health care system regardless of their size or weight. Obesity, always present within the North American population, has rapidly escalated in incidence in recent decades to a situation now referred to as an obesity epidemic. The estimated prevalence of obesity at pre-pandemic levels in the United States was 41.9% from 2017 to 2020.1 Obesity is projected to increase in response to stress levels during the pandemic stimulated by overconsumption of alcohol and food. Fatty Liver, including Non-Alcoholic Fatty Liver disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) has a strongly recognized association with obesity.2, 3 Together, they present a significant obstacle to successful imaging, including all modalities such as MRI and CT but especially Ultrasound. It has become essential to develop solutions for the medical management of these patients. Although historically considered a benign inconsequential event, fat in the liver is now recognized to have a substantial relationship with the development of chronic liver disease and its associated risk for HCC.4-7 Therefore, in the presence of body and liver fat, liver imaging requires new and innovative methods to assess chronic liver disease and to detect and characterize tumors.
Ultrasound, including contrast-enhanced ultrasound (CEUS), plays a critical role in liver mass identification and characterization. AASLD guidelines include greyscale ultrasound for surveillance of high-risk patients to detect liver masses potentially representing HCC.8
After identifying a liver lesion on surveillance ultrasound, characterization of that lesion is recommended using a contrast imaging modality. Traditionally, this characterization on imaging has been performed with contrast-enhanced CT and MR scan, with an emphasis on the selection of MRI.
More recently, CEUS has played a successful role in this pursuit, although there is little mention of the use of CEUS in international guidelines for the same application. Although MRI has been considered the gold standard, CEUS is highly competitive with MRI and can play an essential and critical role in characterizing detected liver masses.9-11
CEUS, performed with microbubble contrast agents (MBCA), is safe, readily available, and widely accepted by patients. Its performance has no nephrotoxicity and no requirement for ionizing radiation. Despite the clear evidence of the efficacy of CEUS,10, 12 there is always a concern for its suboptimal performance, especially in the frequently encountered obese patient population. The biggest threat to the advancement of CEUS is not related to dangers from either the equipment or the contrast agents, but to skepticism regarding its performance, especially in obese patients.
Equipment vendors are challenged to develop unique imaging solutions to increase the sensitivity and penetration of ultrasound systems. When faced with the problem of imaging an obese patient, attention has been given to re-designing ultrasound probes. Probe frequencies can be decreased to achieve maximal penetration, but continually reducing the frequency is not unlimited.
Here, we address a specially designed, commercially available, internationally approved ultrasound probe for imaging patients with a high BMI, fatty liver disease, and obesity.
This specially designed probe (SDP) is the Siemens ACUSON DAX transducer (Siemens-Healthineers, Issaquah, WA) with a multi-D curved transducer with multiple rows in the elevation dimension and a high element count. Every element in the transducer array is individually controlled to provide a uniform narrow elevation slice thickness. Additionally, the lower frequency transducer in the 1.0–3.5 MHz range allows for a depth of field up to 55 cm.13 Henceforth, in this manuscript, we will refer to this probe as a SDP.
To test the success of the probe design, we compare the performance of the SDP with standard curvilinear probes. We address the challenge of characterizing a focal liver abnormality within a fatty liver or positioned at >10 cm depth. We hypothesize that the SDP will improve greyscale and CEUS performance at increased depth in obese patients, showing better detection and characterization of liver masses and liver parenchymal perfusion information necessary for the diagnosis of both focal and diffuse disease.
Methods/Materials
Our institutional research ethics board (IRB), IRISS (Institutional Research Information Services Solution), approved this retrospective study. Informed consent is waived.
Patient Cohort
From July 2019 to September 2020, 200 patients with liver pathology were scanned at our facility using the SDP. Motivation to use the SDP related to technically challenging image acquisition on ultrasound/CEUS, using any of the four machines in our department, attributed to patient BMI and/or fatty liver. From these, 60 non-consecutive patients scanned on the SDP were chosen retrospectively, and images were extracted from a secure ultrasound storage system.
There were 46 males and 14 females. 52/60 patients (87%) had existing liver risk factors, which include a combination of cirrhosis, NAFLD, alcoholic liver disease (ALD), hepatitis B/C, and prior hepatocellular malignancies. A total of 41/60 (68%) patients have cirrhosis. Nineteen patients had a prior history of ablative therapy for HCC treatment.
Obesity was dominant within our cohort. BMI calculation was available on 55/60 (91%) patients. Five patients had no data. BMI ranges were determined using the world obesity classification system.14 In the normal BMI range of 18.5–24.9, there were 9/55 (16%) patients. In the overweight BMI range of 25–29.9, there were 11/55 (20%) patients. In the obese BMI range of 30–39.9, there was 25/55 (45%). In The severely obese BMI range of 40 and above, there were 10/55 (18%) patients. Of the 9/55 (16%) patients in the normal BMI range, all had severe fatty liver on subjective analysis. Therefore, 46/55 (84%) of patients in our study cohort were overweight or obese.
Further, we routinely comment on the quality of all scans. In the entire cohort, we noted on all patients that the responsible radiologist commented on a “technically challenging scan related to patient body habitus.”
Diffuse Liver Status
Subjective evaluation showed 18/60 (30%) patients had mild fatty liver, 16/60 (27%) had moderate fatty liver, 21/60 (35%) had severe fatty liver, and 5/60 (0.08%) patients had no signs of fatty liver disease.
Liver Masses and Depth
A total of 56/60 patients had a focal liver abnormality evaluated with the SDP, including a liver mass in 37 patients and a post-ablative treatment site in 19 and 4 patients with no focal liver abnormality, which were excluded. Our objective is to demonstrate the ability of US/CEUS probes to show the essential enhancement feature of focal liver abnormalities including arterial phase hyperenhancement (APHE) and washout (WO). We used CEUS LI-RADS features for the diagnosis of LR-5 tumors or HCC and the description of LR-4 tumors.15
Final diagnoses were available for all patients. A total of 23/56 (41%) malignant lesions are confirmed by LR-5 categorization in 15 HCC and biopsy in 4 ICC and 4 Mets. Two hemangioma, 2 FNH, 2 focal fat sparing, and 1 adenoma are confirmed by concordant CEUS and MR and 1 adenoma is confirmed by biopsy at liver resection. Six regenerative and dysplastic nodules are in routine surveillance. Nineteen treatment sites include 8 with recurrent tumor and 11 negative for recurrent tumor, in agreement with MR.
Thirty-one focal liver abnormalities (lesions and treatment sites) were located at a depth >10 cm from the skin surface, and 25 were <10 cm. Of all sites examined, 23/56 (41%) lesions are malignant.
Inclusion Criteria
- CEUS exam of the liver at our facility
-
Ultrasound performed utilizing the SDP and a standard US probe from any one of four US machines in our department.
Evidence that a comparison could be made between the performance of the SDP and the standard probe.
Exclusion Criteria
- Any cases where the SDP was not utilized.
Within the study population of 60 patients, 50 comprise group 1, comparing the SDP with the standard abdominal probe (5C-1) for the same machine.
Group 2 includes the remaining 10 patients comparing the SDP with the standard abdominal probe from a different US machine.
US/CEUS Technique
The ultrasound scans were performed by four sonographers with more than 10 years of experience in image acquisition using CEUS, and a Radiologist with more than 25 years of experience in CEUS. Routine liver ultrasound with CEUS for liver abnormality evaluation in our ultrasound department may involve one of four high-end ultrasound machines from four different vendors. The patient's scan was initiated on one of the four machines, and we complete our routine abdominal ultrasound evaluation of the liver and mass by following our departmental protocols.
Our standard assessment for fat quantification included a subjective fat assessment with the grading of fat using the brightness of the liver and its attenuation, liver size, and the hepatorenal index.16 Cirrhosis assessment included gross morphological features as well as elastography.
When a mass was detected on routine greyscale evaluation, its size, morphology, and location were documented; we performed a CEUS with the standard ultrasound probe on the original machine selected. Frequency ranges for our standard abdominal ultrasound probes are between 5 and 8 MHz. Scanning parameters including the mechanical index were preselected and not adjusted during the scan. Standard MI was between 0.6 and 0.8.
The contrast agent, Definity (Lantheus Medical Imaging, Billerica, MA), is used routinely throughout the study, administered to a maximum dose of 10 μL/kg. Multiple injections, between 2 and 6, are routinely used; for each injection, the patient is given a dose of 0.2 mL, followed by a 10 mL standard saline flush as a bolus injection.
If the initial contrast exam performed with a standard probe failed to penetrate or was suboptimal during the first injection, the patient underwent the same examination with the SDP.
Subjective and objective comparisons were made between the SDP images with those from the original standard probe. The analysis of the images of the study was performed as a collaboration by three of the authors, AS a research student, CDM lead sonographer, and SRW department head. To the best of our ability, we reproduced the initial parameters, including patient positioning and probe placement, to perform the comparisons.
Image Acquisition and Mass Characterization
CEUS for nodule characterization includes images acquired at the peak of arterial phase (AP) enhancement and intervals in the portal and late phase. Mass enhancement was assessed at each interval relative to the adjacent enhanced liver parenchyma. For those with a focal liver mass (n = 37), we analyzed the ability of the probe to characterize the AP enhancement pattern and the PVP enhancement characteristics, including the presence or absence of washout and, if present, also its timing and intensity.17
Imaging for the characterization of liver masses must show blood flow dynamics. Specific to CEUS are observations of bubble hyperenhancement in the arterial phase (APHE) 10–45 seconds, and especially washout in the portal venous phase (PVP) 30–120 seconds and late phase (LP) >120 seconds, all in accordance with the CEUS algorithm.18, 19 Washout describes decreased bubble enhancement relative to the background liver following initial enhancement.20 At-risk lesions are assigned a LI-RADS category based on the algorithm.15
Data Analysis
-
Penetration in three sub-categories
-
Greyscale and CEUS liver parenchyma
Penetration is the ability to scan and provide diagnostic information as the distance from the transducer increases. On Greyscale ultrasound, the depth of visualization of structures in the far field measured in centimeters from the skin surface was recorded. On CEUS, the depth of penetration of microbubble enhancement was assessed in the liver parenchyma, recorded as the depth from the skin surface to the depth of the last visualized microbubble. To incorporate the time intervals specific to the CEUS algorithm, following the injection of a MBCA, the analysis included the depth of penetration in the AP from 0 to 45 seconds, PVP from 45 seconds to 2 minutes, and LP at 2, 3, and 4 minutes.17, 18 The percent difference in CEUS penetration between the SDP and the standard probe was calculated. Statistical significance was evaluated using a two-tailed paired t-test where ***P < .001.
-
Fatty liver
CEUS examination assessed the ability of the SDP and standard probes to see microbubble enhancement in the liver parenchyma in livers classified with mild, moderate, and severe fat. Microbubble enhancement was measured from the skin surface to the greatest distance in which microbubbles are observed. A one-tailed paired t-test was done to compare the depth of CEUS penetration performance of the SDP versus the standard probe.
-
Depth ≥ than 10 cm and ≤10 cm
The ability to visualize enhancement parameters in focal liver abnormalities at >10 cm and <10 cm depth in the AP/PVP and LP was recorded.
-
-
Characterization of liver masses is assessed in three sub-categories
-
LR-5 masses
With high specificity for HCC, require APHE and late weak washout, identified at later than 1 minute. They were assessed independently.15
-
Malignant masses
Including HCC were assessed for detection of washout as the most reliable indicator of malignancy.21 This requires determining its presence or not, its timing, and intensity.
-
Post-ablative treatment sites
Were assessed for enhancing foci in the AP within or adjacent to treatment sites suggesting recurrence or residual tumor, and for washout, LR-TR viable. In their absence, they were assessed as LR-TR nonviable.
-
Results
-
Penetration
-
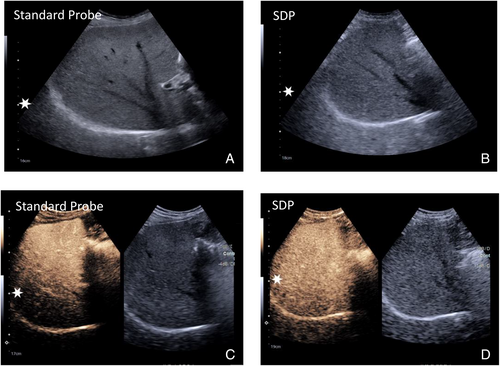
Greyscale liver parenchyma—The standard probe and the SDP functioned well for the greyscale evaluation of normal liver parenchyma and pathology at depth. In 57 patients, there was a 10.3% increase in penetration on greyscale between the SDP and the standard probe.
CEUS liver parenchyma—Compared with the standard probe, the SDP probe achieved a better depth of CEUS penetration at all critical time points 1, 2, 3, and 4 minutes. All differences in penetration depth were statistically significant, evaluated using a two-tailed t-test, P < .001. A low standard error indicated that the data presented accurately represent population characteristics (Figure 1).
Note on Statistical Analysis: Patient body habitus, especially in a high BMI population, can weaken our ability to directly compare observations such as depth of greyscale visualization and bubble penetration on CEUS. To account for this, standard probes use percent differences to normalize quantitative observations against baseline measurements.
Significance: The ability of SDP to image at depth is most realized on CEUS, with an improvement in depth penetration of the SDP relative to the standard probe between 25 and 38% at up to 6 minutes (Figures 1 and 2).
-
NAFLD-specific CEUS penetration
The SDP provided a statistically significant increase in CEUS penetration depth compared with the standard probe in mild, moderate, and severe fatty livers. At most CEUS time points, the crucial difference in CEUS penetration was observed in severe fatty livers (Table 1).
Significance: When liver fat content increased, the difference in penetration between the SDP and standard probe became greater. However, when assessing penetration for the SDP alone, there were no significant differences in penetration as fat content increased.
Notably, the data suggested that increasing fat content in the liver does not compromise the penetration ability of the SDP.
- Penetration affecting visualization of CEUS enhancement of liver masses (n = 37) and treatment sites (n = 19) related to their depth.
-
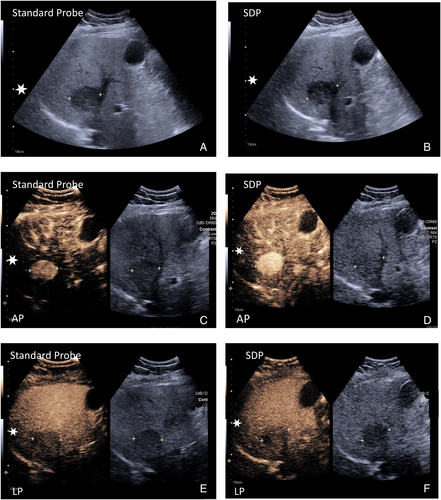
At <10 cm depth: 25 focal liver abnormalities.
In the AP, enhancement parameters were demonstrated on the standard probe in 16/25 (64%) of focal liver abnormalities and on the SDP in 25/25 (100%).
In the PVP/LP enhancement parameters on the standard probe, 21/25 (84%) of liver abnormalities were accurately visualized, and 25/25 (100%) on the SPD probe.
-
At >10 cm depth: 31 focal liver abnormalities.
In the AP, the standard probe visualized enhancement parameters in 13/31 (41%) focal liver abnormalities, and the SDP accurately visualized 18/31 (58%).
In the PVP/LP, the standard probe accurately visualized the enhancement parameters in 7/31 (22%) focal liver abnormalities, and the SPD probe visualized the same in 24/31 (77%) lesions (Figure 3).
-
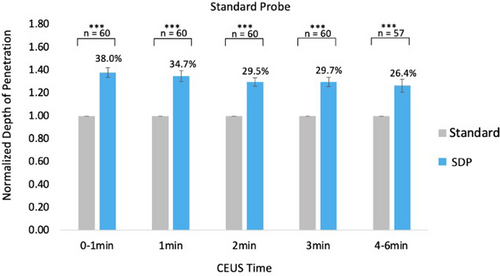
| Fat Content | 0–1 minutes | 1–2 minutes | 2–3 minutes | 3–4 minutes | 4–6 minutes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δcm | P | Δcm | P | Δcm | P | Δ cm | P | Δcm | P | |
| Mild | 3.2 | **8.8 × 10−5 | 2.9 | **2.6 × 10−4 | 2.5 | **2.3 × 10−4 | 2.8 | **1.4 × 10−4 | 3.1 | **5.6 × 10−5 |
| Moderate | 4.0 | **5.0 × 10−8 | 3.5 | **2.1 × 10−6 | 2.7 | **2.0 × 10−5 | 2.5 | **1.3 × 10−5 | 2.3 | *1.9 × 10−3 |
| Severe | 3.8 | **2.3 × 10−5 | 3.5 | **4.8 × 10−5 | 3.7 | **2.0 × 10−5 | 3.5 | **8.8 × 10−5 | 3.9 | **1.2 × 10−5 |
- Note: P values from one-tailed, paired t-test. Compares depth of CEUS penetration on Standard Probe vs the SDP in livers of varying fat content. **P < .001, *P < .05.
- Characterization of liver masses
-
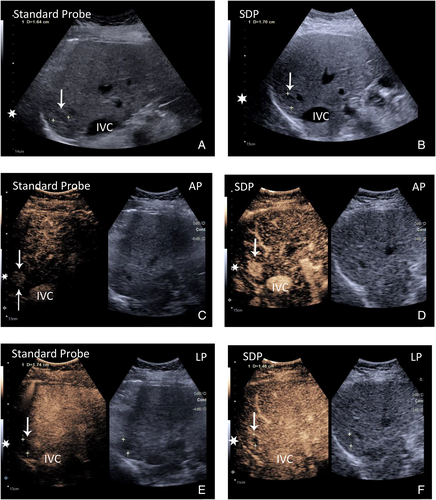
HCC, 15 LR-5 lesions. Assessment of essential APHE and washout
In the AP, (APHE) was shown on the standard probe in 10/15 (40%) masses, and on the SDP, 15/15 (100%) masses accurately displayed APHE.
In the PVP/LP (Washout) on the standard probe, 4/15 (26%) masses accurately showed washout. On the SDP 14/15, (93%) did the same.
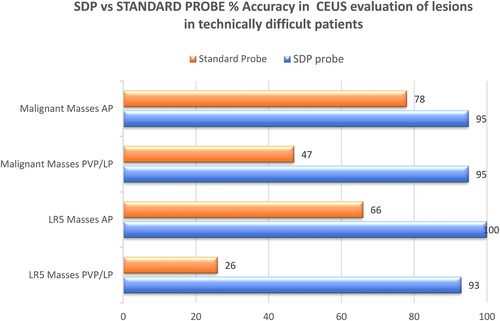
Significance: There was a statistically significant difference that showed an improved ability of SDP to see enhancement in both the AP for APHE and PVP/LP for the detection of washout. The difference was most marked for the ability to detect washout (Table 2; Figures 4 and 5).
-
Malignant masses, 23 evaluated for assessment of APHE and washout.
In all malignant masses, including HCC, cholangiocarcinoma (ICC), and metastasis, AP scans correctly demonstrated APHE on the standard probe in 18/23 (78%) of masses and on the SDP in 22/23 (95%) masses (Figure 4).
PVP/LP demonstration of washout was shown on the standard probe in 11/23 (47%) of masses and on the SDP 22/23 (95%).
Significance: The SDP probe offered an improved diagnostic accuracy and a statistically significant improvement in the detection of enhancement features for the characterization of malignant liver masses, most notable in the PVP/LP, (Table 2 and Figure 5).
-
Treatment sites, 19 areas assessed with prior ablative therapy.
LR-TR viable positive for HCC recurrence was observed in 8/19 masses with the demonstration of foci of APHE within or adjacent to an avascular treatment site in the AP.
In the AP, 6/8 (75%) of treatment sites on the standard probe, and 8/8 100% on the SDP showed enhancing foci positive for recurrence.
In the PVP/LP, Washout was shown related to the enhancing foci on the standard probe in 4/8 (50%) of treatment sites and 8/8 (100%) on the SDP.
LR-TR nonviable negative recurrence 11/19 masses.
In the AP, the Standard probe 4/11 (36%) and the SDP 11/11 (100%) accurately showed an avascular treatment site.
The PVP/LP on the standard probe 5/11 (45%) and the SDP 11/11 (100%) continued to accurately show the avascular treatment site.
Significance: Both probes performed well. A small sample of eight lesions in LR-TR viable prevented accurate statistical analysis. However, for LR-TR non-viable, the SDP showed a better ability to penetrate, in both the AP and PVP/LP (Figure 6).
| Parameters | AP Enhancement, P Value | PVP/LP Washout, P Value |
|---|---|---|
| Depth > 10 cm | .3907 | .0001 |
| Depth < 10 cm | .16 | .1099 |
| HCC | .0421 | .0005 |
| Malignant masses | .1968 | .0003 |
Discussion
In routine liver sonography, a probe optimized for depth would not be chosen in preference to a standard curvilinear probe as it has a slightly inferior resolution. However, imaging, challenges for ultrasound/CEUS arise when a patient has a high BMI, the fat content in the liver is increased or pathology is positioned >10 cm from the transducer crystal. Although greyscale and CEUS scans are compromised when challenged with fatty liver and increased BMI, our study shows that these factors have the greatest impact on CEUS.
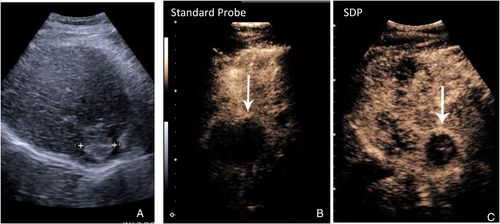
In our evaluations of probe contribution to the characterization of focal liver abnormalities at a depth >10 cm, with the standard probe and SDP, we were not surprised to find that a hyper-enhancing mass can often be identified in the Arterial Phase with both probes (Figure 3). They each perform reasonably well at depth. Most benign and malignant tumors are avidly enhancing/vascular, and their enhancement can be seen independently of the enhancement of background liver tissue. If the lesion has APHE, it is possible to determine this without the supportive comparison to the background liver parenchyma. However, without enhancement of the liver parenchyma surrounding a mass (Figure and 5), washout cannot be appreciated. The relative enhancement of the mass to the background liver parenchyma is an essential comparison. It is, therefore, the improved ability to show the liver parenchyma deep into the liver in the PVP and LP with the SDP probe, which allows for the appreciation that the washout region is different than the remainder of the parenchyma.
LR-5 classification, allowing for confident diagnosis of HCC without biopsy, has two criteria: APHE and late weak washout. If either is missing, the nodule is classified as LR-4, requiring a biopsy before treatment.15 If the SDP were unavailable, 11/15 patients evaluated in our study would not have been classified as showing washout based on the PVP/LP Imaging, and 9 patients would not have shown APHE. HCC within LR-5 would have been under-represented. This significance for HCC diagnosis is important, allowing for an earlier confident diagnosis of this tumor (Figure 4).
In our considerable evaluation of post-ablative therapy treatment sites, the SDP outperformed the standard probe in LR-TR nonviable sites. However, the small number of tumors in our cohort with LR-TR viable prevented us from performing a satisfactory statistical analysis. Nonetheless, we believe that at all depths, the SDP is superior. Consequently, today, the SDP is a regular choice in our department for evaluating post-ablation treatment sites. Our evaluation of post-ablative therapy was not compelling at this time, related to the small and insufficient cohort.
Strengths
This study addresses a critical healthcare issue: liver imaging within the recognized obesity epidemic.
Our study, although retrospective, includes a good sample of malignant liver masses where the demonstration of washout is shown to be a critical component of their non-invasive diagnosis. The clear benefit of using the SDP for this purpose is the most robust result of this study.
Limitations
This study's most significant negative factor was a retrospective design that took all comers in our liver-focused department. However, we included a reasonable number of patients with a high rate of confirmed malignancy. Further, our tertiary facility has a high level of performance in CEUS exams.
A limitation of this article relates to the dominant selection of one particular ultrasound machine, the SDP's source. Although we considered eliminating other vendors, we thought all scans were of good quality and reproduced evidence that high BMI compromises diagnostic outcomes.
Further, the SDP was always selected because of a proven lack of performance in the ability of another probe to penetrate.
Conclusion
This study has addressed the imaging problems concerning patients with high BMI, obesity, and fatty liver. As patient factors are likely to stay the same in the short term, unique imaging solutions based on novel technological developments are required to provide imaging methods to meet the needs of our current populations. The SDP increases penetration and improves the ability to see lesions on CEUS above the generally accepted depth of 10 cm in patients with high BMI and fatty liver. It also improves the ability to detect the presence of washout, recognized as the most reliable indicator of malignancy.21
Due to patient weight limitations, options for CT or MRI may not exist. CEUS must remain competitive with MR and CT. The SDP improves ultrasound quality, specifically in CEUS, and offers the opportunity for improved clinical outcomes. We must continue to develop technological tools that can help the performance of CEUS now and into the future.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




