Vena cava leiomyosarcoma surgery results in a retrospective cohort of 41 patients from two centers
This study was presented as a poster at the annual meeting of the European Society of Medical Oncology (ESMO) 2023.
Abstract
Background
Leiomyosarcoma of the vena cava (LMS-VC) is a rare entity with poor oncological outcomes and a lack of histological staging prognostic factors.
Methods
Outcomes of consecutive patients operated on LMS-VC between March 2003 and May 2022, in two specialized sarcoma centers were reported.
Result
Forty-one patients were identified. Median size of LMS-VC was 9 cm with 68% of complete obstruction. After surgery, severe complication rate was 30%. No postoperative mortality was reported. Microscopic complete excision was obtained for 71% of patients, R1 for 27% and one patient presented an R2 resection. Grade 3 was found in 24%. After a median follow-up of 70 months, 3 years disease-free survival (DFS) and 5 years DFS were 34% and 17%, and 3 years overall survival (OS) and 5 years OS were 74% and 50%. Distant metastasis concerned 54% of recurrences, local 7% and local and distant 5%. Multivariate analysis showed that FNCLCC grade (p < 0.001) and perioperative chemotherapy (p = 0.026) were significant factors for DFS. In multivariate analysis, FNCLCC grade was a significant factor for OS (p = 0.004).
Discussion
Perioperative chemotherapy may have a role to play in lowering the risk of recurrence for LMS-VC, particularly in high-grade tumor.
1 INTRODUCTION
Leiomyosarcomas (LMS) are rare malignancy of mesenchymal origin that can arise anywhere on the body, including within the endothelial smooth muscle of the intima of great vessels.1, 2 To date, approximately 450 cases of vascular LMS have been reported worldwide.3 Primary tumor site within the vascular system most frequently observed is the inferior vena cava (IVC), followed by renal vein, great saphenous vein, pulmonary vein, and femoral vein.4 This condition predominantly affects women, with a sex ratio favoring females at 3.5:1, and is typically diagnosed during the sixth decade of life.5-7 Traditionally, three tumor growth patterns have been described: extraluminal (62%), intraluminal (5%), and a combination of extraluminal and intraluminal (33%).7 According to the Kieffer classification, based on the morphology, Leiomyosarcoma of the vena cava (LMS-VC) can be classified into Type I (K1), located below the renal veins, Type II (K2), situated below the hepatic veins up to the renal veins, and Type III (K3), located above the hepatic veins.8 The role of perioperative chemotherapy and/or radiotherapy in the management of this condition remains unclear3, 9 and is not formally recommended by current guidelines.10 Factors such as tumor size, absence of metastatic disease at the time of surgery, K3 tumor classification, intraluminal growth pattern, extensive IVC involvement, IVC occlusion and its associated clinical consequences (lower limb edema, Budd-Chiari syndrome), completeness of resection, margin status, and the necessity for adjuvant therapy are all intuitive factors influencing survival and are supported by extensive data sets.11-14 Nevertheless, there is a lack of conclusive evidence regarding histological staging and its significant prognostic implications.11, 13
This retrospective study aims to report our experience with LMS-VC surgery and its associated perioperative chemotherapy.
2 PATIENTS AND METHODS
2.1 Patients
All consecutive patients treated at Gustave Roussy and Marie-Lannelongue Hospital between 2000 and 2022 with a pathologically confirmed LMS-IVC were retrospectively identified from our prospectively maintained histological database of patients treated for soft tissue sarcoma (STS). Cases of LMS not originating from the IVC (or renal vein) were excluded. All cases underwent review at our multidisciplinary tumor boards specialized in sarcoma and member of the NetSarc+ network.15 An extensive chart review was performed from June to September 2022, based on the Saikia et al. reporting checklist.12 French law does not warrant informed consent for retrospective study and all data were treated anonymously. This study adheres to the Strengthening the Reporting of Cohort, cross-sectional and case-control studies in Surgery (STROCSS 2021) guidelines.16
2.2 Perioperative management
All patients underwent a contrast-enhanced CT scan to assess LMS-VC extension17 and the presence of pulmonary metastases. In cases where a locally advanced tumor was suspected, an MRI was performed to evaluate adjacent invasion and tumor thrombosis.18
Definitive diagnosis through biopsies was ideally made before initiating treatment.15 They were ideally performed via a retroperitoneal way with a co-axial system. Some patients referred to our centers after diagnosis may have had other modalities (e.g., echo-endoscopy, surgical biopsy…).
Preoperative chemotherapy (CT) and radiotherapy (RT) were discussed in multidisciplinary tumor boards for borderline resectable tumors (tumors abutting 180° circumferentially around the abdominal aorta and superior mesenteric artery, with significant contact with the duodenum, pancreas, or liver) and high grade to enhance resectability rates. Adjuvant CT or RT was proposed to patient with incomplete microscopic resection margins or higher grade tumors.
2.3 Intervention
The primary goal was to achieve a complete en-bloc excision of the mass.12 The surgical strategy was determined based on the Kieffer level and the presence of intraluminal extent or free-floating thrombus. Cardiopulmonary bypass was typically required for K3 patients, with a sterno-laparotomy approach being preferred. For K2 and K1 patients, a median laparotomy approach with or without a transverse splint was favored. Regarding the IVC, exposure was achieved via Kocher, Cattel-Braasch, or even right liver mobilization to obtain proximal and distal control of the IVC and sufficient margins.19 Systemic heparin was delivered before the proximal IVC was clamped while monitoring blood pressure. The distal IVC was secured. Thrombectomy was carried out if needed. Four options were then considered: ligation (if cardiac input monitoring remained stable and venous collaterals were identified on preoperative CT scans); primary suture (if more than 75% of vena cava circumference remained after tumor excision); autologous or biologic patch (if more than 25% of vena cava circumference remained after tumor excision); and ringed Gortex graft in other cases (Figure 1).20 Large arteries were resected if any tumoral rupture risk was suspected and reconstruction performed by dacron graft, venous bypass, or primary suture. In terms of major organ resection: the duodenum-pancreas was preserved when not directly invaded; minor liver resections were performed for minimal tumoral contact; and major liver resection (usually right hepatectomy) was carried out only for involvement of the hepatic veins, with reimplantation of the middle and left hepatic vein into the right atrium. Partial right atrium resection was accepted. The operative site was then covered with an epiplooplasty, and a drain was left in place. All procedures were conducted by thoracic-vascular and surgical oncologist with expertise in locally advanced tumors and sarcomas.
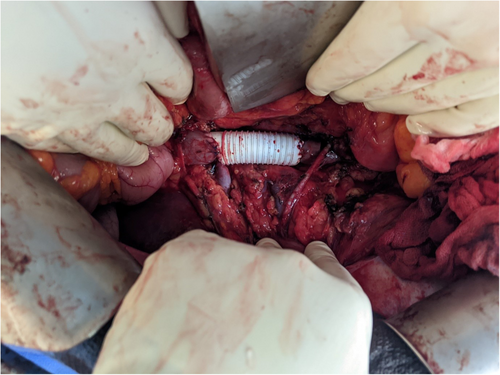
2.4 Outcomes
Mortality was defined as death occurring in the hospital or within 90 days of the surgery. Postoperative morbidity was classified according to the Clavien-Dindo (CD) Classification.21 Tumors were graded according to the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grade.22 A positive margin was defined as the presence of tumor cells less than 1 mm from the resection margin. Overall survival (OS) was calculated from the date of diagnosis to the date of death and censored at the date of the last follow-up for patients alive at that time. Disease-free survival (DFS) was calculated from the date of diagnosis to the date of first radiographic evidence of recurrence, metastatic disease, or death, or censored at the date of the last follow-up.
2.5 Statistics
Quantitative variables were presented as median, IQR. Qualitative data were presented as count (percentage). Survival curves were calculated according to Kaplan–Meier. Median follow-up was estimated by reversed Kaplan–Meier. Univariate and multivariable Cox model was used to identify variables associated with OS and DFS. Given the number of patients, no method of variable selection was used for the multivariable analysis. Tumor size and grade, margin, and Kieffer level which were previously identified in the literature were used to control for confounding on the chemotherapy effect.23
All tests were bilateral and a p value of less than 0.05 was considered significant. Statistical analysis was performed using SPSS 26.0 for Windows software (SPSS Inc.).
3 RESULTS
3.1 Patients' selection
Ninety-seven patients were identified from the histological database. Among them, 41 were included in the cohort (Figure 2). The median age was 58 years (Table 1). Males were accounting for 37% of the cohort. Abdominal pain was the most common symptom in 44% of the patients followed by palpable mass (10%). Only one patient presented an omentum metastasis at presentation.
No CT N = 29 |
CT N = 12 |
All N = 41 |
|
|---|---|---|---|
| Patients' characteristics | |||
| Age at diagnosis (years, median, IQR) | 60 (54–67) | 52 (42–58) | 58 (52–67) |
| Male (n, %) | 11 (38) | 4 (33) | 15 (37) |
| BMI (kg/m2, median, IQR) | 25 (23–28) | 25 (21–26) | 25 (23–28) |
| Symptomatic lesion (n, %) | 19 (66) | 11 (91.7) | 30 (73) |
| Palpable mass | 2 (7) | 2 (17) | 4 (10) |
| Abdominal pain | 15 (41) | 6 (50) | 18 (44) |
| Morphine use | 2 (7) | 4 (33) | 6 (15) |
| ASA score (n, %) | |||
| ASA I | 1 (3) | 4 (33) | 5 (12) |
| ASA II | 19 (66) | 7 (58) | 26 (63) |
| ASA III | 9 (31) | 1 (8) | 10 (24) |
| Disease's characteristics | |||
| Tumor size (cm, median, IQR) | 8 (6–10) | 10 (8–15) | 9 (6–11) |
| Kieffer levels (n, %) | |||
| K1 | 6 (21) | 3 (25) | 9 (22) |
| K2 | 17 (59) | 6 (50) | 23 (56) |
| K3 | 6 (20) | 3 (25) | 9 (22) |
| Primary tumor site (n, %) | |||
| Right renal vein | 3 (10) | 1 (8) | 4 (10) |
| IVC | 24 (83) | 11 (92) | 35 (85) |
| SVC | 2 (7) | 0 | 2 (5) |
| Tumor pattern (n, %) | |||
| Vena cava complete obstruction | 20 (69) | 8 (67) | 28 (68) |
| Intraluminal extent | 6 (21) | 2 (17) | 8 (20) |
| Free-floating thrombus | 3 (10) | 2 (17) | 5 (12) |
| Preoperative organ involvement (n, %) | |||
| Adrenal extension | 5 (17) | 1 (8) | 6 (15) |
| Kidney extension | 14 (48) | 4 (33) | 18 (44) |
| Duodenum extension | 1 (3) | 5 (42) | 6 (15) |
| Pancreas extension | 1 (3) | 4 (33) | 5 (12) |
| Liver extension | 2 (7) | 3 (25) | 5 (12) |
| Atrial extension | 3 (10) | 0 | 3 (7) |
| Aortic extension | 2 (7) | 2 (17) | 4 (10) |
| Iliac vessels extension | 4 (14) | 2 (17) | 6 (15) |
| Metastasis at diagnosis (omentum) | 0 | 1 (8) | 1 (2) |
- Abbreviations: ASA, American Society of Anesthesia; BMI, body mass index; CT, perioperative chemotherapy; IVC, inferior vena cava; SVC, superior vena cava.
The radiologic evaluation found IVC as the most common primary site, with a K2 presentation and complete obstruction in 68% of cases. The main organ involved was the right kidney (44%).
3.2 Perioperative chemotherapy
A total of 12 patients received perioperative chemotherapy. Ten patients were given as neoadjuvant and two as adjuvant treatment. They tended to have larger tumors with a median size of 12 cm, more K3 presentation (30%), and heavier organ involvement: duodenum and pancreas each at 40% and liver at 30%. Neoadjuvant CT regimens were Doxorubicin plus Dacarbazin for 50% of patients, Doxorubicin plus Ifosfamid for 30% and Gemcitabin for 20%. The median number of cycles was 5. Neoadjuvant RT was delivered to one patient (50.4 Gy). Stable disease was seen in five patients and partial response in the five others. The median time between neoadjuvant treatment initiation and surgery was 146 days. Two patients needed adjuvant CT with Doxorubicin plus Dacarbazine for six cycles: one R0 with grade 3 tumor and one R1 with grade 2 tumor. Four patients needed ajuvant RT with 50,4 Gy: three R1 with two grade 2, one grade 3 and one R0 with grade 1 but aortic and iliac vessels contact. The median time between surgery and adjuvant treatment onset was 71 days.
3.3 Surgical management, early and late postoperative complications
All but one patient had complete excision of the tumor (Table 2). Median laparotomy was the most frequent approach (54%). The two main options for VC management were ringed Gortex graft (42%) and ligation (no reconstruction) (32%). Five patients needed associated aortic (1 dacron graft, one suture) or iliac vessels (2 ligations, 1 bypass) resections. The right kidney was the most frequently resected organ (54%) followed by minor liver resection (20%). Median preoperative bleeding was 600 mL, and median operative time was 270 min. Cardiopulmonary bypass was required for 12% of patients.
All N = 41 |
|
|---|---|
| Surgical approaches (n, %) | |
|
22 (54) |
|
8 (20) |
|
2 (5) |
|
1 (2) |
|
6 (15) |
|
2 (5) |
| Excision strategy (n, %) | |
|
3 (7) |
|
37 (93) |
| Thrombectomy (n, %) | 8 (20) |
| Vena cava management (n, %) | |
|
13 (32) |
|
5 (12) |
|
6 (15) |
|
17 (42) |
| Associated organ resections (n, %) | |
|
22 (54) |
|
8 (20) |
|
2 (5) |
|
3 (7) |
|
1 (2) |
|
2 (5) |
|
2 (5) |
| Perioperative bleeding (mL, median, IQR) | 600 (200–2000) |
| Perioperative transfusion (units, median, IQR) | 0 (0–4) |
| Cardiopulmonary bypass (n, %) | 5 (12) |
| Operative time (min, median, IQR) | 270 (180–360) |
| Early postoperative course (<90 POD) (n, %) | |
|
26 (63) |
|
12 (30) |
|
6 (15) |
|
17 (10–25) |
| Late postoperative course (>90 POD) | |
|
6 (15) |
|
2 (5) |
|
2 (5) |
|
2 (5) |
- Abbreviations: CT, perioperative chemotherapy; POD, postoperative days.
Sixty-three percent of patients presented a complication. CD ≥ 3 complications concerned 30% of patients and 15% needed reinterventions. The most frequent complications were acute kidney injury (AKI) with 30% of patients, thrombotic events (13%), pleural effusion (10%), and hemorrhage (8%). Reconstructed IVC thrombosis accounted for two of the thrombotic events. No postoperative death was reported. Late reintervention was needed for 5% of patients.
After POD90, 15% of patients presented a complication (5% of CD ≥ 3). Two patients needed a reintervention and two others needed a new hospitalization. Thrombo-embolic events occurred in 7.5% of patients and two others presented chronic kidney disease (CKD).
3.4 Pathology
Surgical specimens showed complete tumoral excision (R0) in 71% of patients (Table 3). Concerning no CT patients, R0 accounted for 83% of the surgical specimen, R1 14%, and R2 3%. Concerning CT patients, R0 accounted for 42% of surgical specimens, R1 58%, and no incomplete macroscopic margin was observed. The median residual cell rate after NACT or NACT-NART was 70%. A majority of patients presented FNCLCC Grade 2 LMS-VC.
No CT N = 29 |
CT N = 12 |
All N = 41 |
|
|---|---|---|---|
| Surgical specimens (n, %) | |||
|
24 (83) | 5 (42) | 29 (71) |
|
4 (14) | 7 (58) | 11 (27%) |
|
1 (3) | 0 | 1 (2%) |
|
9 (6–12) | 12 (9–13) | 10 (7–13) |
|
70 (61–80) | ||
| FNCLCC grade | |||
|
5 (17) | 0 | 5 (12) |
|
18 (62) | 8 (67) | 26 (63) |
|
6 (21) | 4 (33) | 10 (24) |
| Recurrence (n, %) | 18 (60) | 9 (90) | 27 (67.5) |
|
2 (7) | 1 (8) | 3 (7) |
|
14 (48) | 8 (67) | 22 (54) |
|
0 | 2 (17) | 2 (5) |
| Cause of death (n, %) | 14 (46.7) | 8 (80) | 22 (55) |
|
3 (10) | 3 (25) | 6 (15) |
|
1 (3) | 1 (8) | 2 (5) |
|
0 | 1 (8) | 1 (2) |
|
3 (10) | 0 | 3 (7) |
|
2 (7) | 1 (8) | 3 (7) |
|
5 (17) | 2 (17) | 7 (17) |
- Abbreviation: CT, perioperative chemotherapy.
3.5 Oncological outcome
After a median follow-up of 70 months, there were 27 recurrences and 22 deaths. The median DFS was 22 months and OS was 55 months. Respectively, the 3 and 5-year DFS rates were 34% and 17%. OS, at the same time points were 74% and 50% (Figure 3). Seven patients were lost to follow-up. The median follow-up time was 70 months (95% CI 66 - Not Reached).
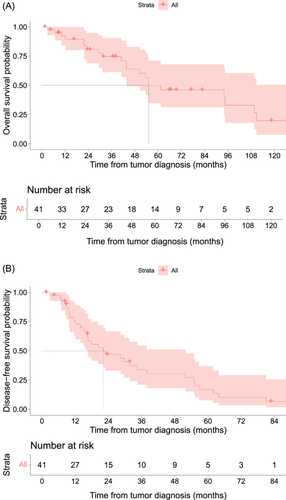
Distant metastasis was the main site of recurrence and occurred in 54% of the patients. On the contrary, local recurrence only occurred in 7% of patients, and local and distant recurrence was scarce (5%). First distant metastasis was found in the lung (25%), liver 15(%), bone (15%), peritoneum (2.5%), and mammary tissue (2.5%).
Recurrence treatment was generally multimodal but CT was the first-line therapy (27.5%).
3.6 Prognostic analysis
Univariate analysis showed that microscopic margins, tumor size on surgical specimens, and FNCLCC grade were associated with DFS (Table 4). Multivariable analysis showed that FNCLCC grade (p < 0.001), and perioperative chemotherapy (p = 0.026) were independently associated with DFS.
| Categories | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Disease-free survival | |||||||
| Microscopic Margin | R0 | 1 | 0.025 | ||||
| R1 | 1.95 | 0.9–4.1 | |||||
| R2 | 47.9 | 2.9–780 | |||||
| Kieffer levels | K1 | 1 | 0.5 | ||||
| K2 | 1.1 | 0.5–2.7 | |||||
| K3 | 1.75 | 0.6–5. | |||||
| CT | No CT | 1 | >0.9 | 1 | 0.026 | ||
| CT | 1.04 | 0.5–2.2 | 0.30 | 0.38 | 0.2–0.92 | ||
| FNCLCC grade | Grade 1 | <0.001 | 1 | <0.001 | |||
| Grade 2 | 10.4 | 1.3–81.2 | 20 | 2.3–175 | |||
| Grade 3 | 26. | 3.1–227 | 65.6 | 6.3–683 | |||
| Tumor size on the surgical specimen (cm) | 1.12 | 1–1.2 | 0.006 | ||||
| Overall survival | |||||||
| Microscopic margin | R0 | 1 | 0.055 | ||||
| R1 | 1.65 | 0.7–4 | |||||
| R2 | 44.8 | 2.7–731 | |||||
| Tumor size on the surgical specimen (cm) | 1.07 | 1–1.2 | 0.2 | ||||
| FNCLCC grade | Grade 1 | 1 | 0.004 | 1 | 0.004 | ||
| Grade 2 | 4.50 | 0.6–35 | 4.12 | 0.43–39.2 | |||
| Grade 3 | 16.8 | 2–149 | 19.7 | 1.7–222 | |||
| Kieffer levels | K1 | 1 | 0.054 | 1 | 0.1 | ||
| K2 | 1.58 | 0.4–5.7 | 1.21 | 0.3–5 | |||
| K3 | 4.41 | 1.1–18 | 3.48 | 0.8–15 | |||
| CT | No CT | 1 | >0.9 | 1 | 0.3 | ||
| CT | 0.96 | 0.4–2.31 | 0.63 | 0.2–1.7 | |||
- Abbreviations: CI, confidence interval; CT, perioperative chemotherapy; DFS, disease-free survival; OS, overall survival.
Considering OS, univariate analysis showed microscopic margins and FNCLCC grade were prognostic factors. Multivariable analysis showed that FNCLCC grade was the only independent predictor of OS (p = 0.004).
4 DISCUSSION
In this 20-year experience from two specialized centers, we analyzed 41 patients who underwent surgical treatment for LMS-VC. This series stands as one of the largest available, and it yields noteworthy findings from both surgical and oncological perspectives.
From a surgical standpoint, three major aspects warrant discussion: the extent of resection, the IVC reconstruction, and surgical complications. In the last decade, compartmental resection has become the standard of care for retroperitoneal sarcomas. However, existing evidence primarily focuses on well-differentiated or de-differentiated liposarcomas rather than leiomyosarcomas. It's important to note that while liposarcomas can have tumor boundaries that are challenging to discern intraoperatively, leiomyosarcomas typically have more easily identifiable boundaries. Moreover, liposarcomas tend to recur locally, whereas leiomyosarcomas tend to recur at distant sites. Considering these distinctions, we advocate for tailoring surgical resection plans to the specific characteristics of leiomyosarcomas, without necessarily extending resections to uninvolved organs, such as the colon. Fairweather et al. have reported that while 26% and 33% of resected adjacent kidneys and colons showed histopathological organ involvement in dedifferentiated liposarcoma and well-differentiated liposarcoma, respectively, the figure for LMS was only 9%.24, 25 Nonetheless, over 55% of our patients required organ resection, with the kidney and liver being the most commonly affected. For liver involvement, most resections were minor hepatectomies, with major hepatectomies (required when the hepatic vein was involved) being relatively infrequent. Similarly, duodenal or pancreatic head resection was infrequent, which is advantageous given the poor outcomes associated with these procedures.26, 27 In our series, we achieved a complete R0 resection in 71% of patients, a rate higher than that reported by Jeong et al. and Blair et al.14, 28 The second important surgical aspect is the necessity of IVC reconstruction. While it is often assumed that the IVC should always be reconstructed, it's worth noting that since LMS is a slow-growing tumor, many patients have the time to develop collateral vessels before surgery. Consequently, 32% of our patients did not require IVC reconstruction. Clinical examination and CT scan analysis can provide valuable evidence for the presence of collaterals. In cases of good tolerance, we routinely opted for no reconstruction. When reconstruction was necessary, the most commonly used technique was a Ringed Goretex graft, allowing for the reconstruction of even extensive IVC defects. However, this approach has two drawbacks: an increased risk of infection, particularly when combined with digestive resection, and the need for lifelong anticoagulation. In our sample, tumor size tended to be larger with a median of 9 cm and a range from 2.4 to 23 cm. Sulpice et al. and Teixeira et al. did not observed such size.29, 30 Notably, our sample had a larger proportion of K3 LMS-VC cases, explaining our higher rate of cardiopulmonary bypass utilization14, 28-31 and simpler resections (patch or direct suture) were less frequent. In general, it's essential to strike a balance between systematic reconstruction and the associated need for anticoagulation and ligation, considering potential complications such as lower limb edema. Currently, no preoperative interventional procedures have been described to promote collateral venous pathway development before excision surgery for K1 and K2 LMS-VC. One potential option could be adapted from coil and cover techniques developed for venous hemorrhage or endoleak transcaval embolization.32, 33 The rate of CD ≥ 3 complications was 30%, with no postoperative deaths observed. While these figures are within the range typically observed for major procedures, it underscores the importance of careful patient selection and the use of prehabilitation techniques to reduce risks. Nevertheless, we did observe a high rate of postoperative AKI, affecting 30% of patients, with two patients developing CKD after surgery. This can impact access to certain adjuvant treatment modalities. In particular, radical nephrectomy with vena cava thrombectomy, high BMI, prolonged perioperative hypotension, and postoperative anemia are known risk factors for postoperative AKI, while selective renal artery embolization offers protection.34, 35 Prehabilitation and liberal postoperative transfusion could potentially mitigate the most common surgical morbidities after LMS-VC excision.
From an oncological perspective, three key points merit discussion: the aggressiveness of the disease, patterns of recurrence, and the use of neo/adjuvant therapies. In our series, LMS-VC exhibited marked aggressiveness, with a median DFS of less than 2 years and a 5-year OS of 50%. However, these outcomes compare favorably to other studies; for instance, Wachtel et al. reported a median DFS of 12 months and an OS of 23 months, while Saikia et al. found 28 and 60 months, respectively.11, 12 Among the 27 patients experiencing recurrence, 24 (89%) had distant or combined distant and local recurrences, with local-only recurrence observed in only 3 (11%) patients. This pattern underscores that LMS-VC is a systemic disease, and despite tumor size and limited surgery extent, local recurrence is rarely a primary concern. Given this high risk of early recurrence, it is imperative to establish rigorous follow-up protocols and consider neo/adjuvant therapies. Recent ESMO guidelines recognize the scarcity of available studies guiding optimal surveillance for these patients but suggest differential follow-up approaches for high and low-risk patients. In our view, LMS-VC should be classified as a high-risk sarcoma, warranting shorter intervals for follow-up, adhering to guidelines such as every 3–4 months in the initial 2–3 years, followed by semiannual check-ups for up to 5 years, and then annual assessments for up to 10 years. The question of whether neo/adjuvant treatments can reduce recurrence risk is also essential. Our data supports the use of neo/adjuvant therapies, particularly for FNCLCC grade 2 and 3 lesions. Unfortunately, we had insufficient data on grade 1 sarcoma patients to evaluate the potential benefits for this subgroup. However, after adjusting for grade, we observed that patients who received neo/adjuvant chemotherapy had a lower hazard for DFS (HR = 0.38 [95% CI 0.2–0.92], p = 0.026). Due to our limited sample size, we could not compare the timing of chemotherapy administration (neo vs. adjuvant). Neo-adjuvant treatment offers potential advantages, including the possibility of achieving an objective response that allows for organ-sparing surgeries, especially when dealing with tumors in contact with the liver or pancreatic head. Additionally, it ensures that treatment is administered without delays due to postoperative complications, which can be common after high-risk procedures, potentially impeding timely treatment. Although only a few patients underwent radiotherapy, considering the recurrence pattern, its substantial impact on risk reduction is unlikely. The two main CT modalities have been described by the EORTC and Gronchi et al.36, 37 Our results are not in line with SMAC analysis, EORTC 62931 trial, and Saikia and all that have not found a link between adjuvant treatment (mainly adjuvant CT) and DFS for LMS,12, 38, 39 but the particular location to the IVC may be different than other LMS. Concerning adjuvant RT, the Scandinavian Group has found a lesser risk of local and distant recurrence respectively.40 Their results are not supported by an in vitro study which found a large heterogeneity of radiation response between different LMS types.41 As well in the STRASS trial, subgroup analyses of abdominal recurrence-free survival based on sarcoma subtype and grade indicate that preoperative radiotherapy does not benefit for leiomyosarcoma and high-grade retroperitoneal sarcoma (but the individual sizes of all subgroups were relatively small). It goes against SMAC analysis, EORTC 62931 trial, and Saikia that all have not found an association between adjuvant treatment (mainly adjuvant CT) and DFS for LMS.12, 38, 39 One of the key elements of the decision could be the FNCLCC grade which was found significant for DFS and OS in our series whereas Laskin et al. concluded its absence of effect on prognosis.13
Our study has several strengths. First, it is one of the largest studies in the current literature. Second, all cases were confirmed by a specialized pathologist and patients were managed by teams trained in sarcomas. Finally, a multivariable model was used to control confounding bias. Yet, this work has several limitations which should be kept in mind. First, given the rarity of the disease, we present highly annotated data from a relatively limited number of selected patients. Second, the retrospective nature of this study does not allow us to make strong conclusions because of the risk of residual bias. Our results must be confirmed by subsequent studies.
The vena cava is a rare location for LMS that should be considered at high risk of distant recurrences. Neo/adjuvant chemotherapy may be able to lower that risk. In experienced teams, surgery can be performed with good short and long terms outcomes.
AUTHOR CONTRIBUTIONS
Thibaud Bertrand participated in the patients' screening and inclusion, data collection, and results analysis and writing of the article. Matthieu Faron participated in the study design, manuscript supervision, and corrections. Olaf Mercier, Carine Ngo, Cécile Le Pechoux, Antonin Levy, Justin Issard, Clémence Henon, and Charles Honore contributed to data collection and manuscript revision for important intellectual content. Elie Fadel and Axel Le Cesne contributed to study conception and design, project administration, manuscript drafting, and manuscript revision for important intellectual content. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of “Gustave Roussy Cancer Campus.”
Open Research
DATA AVAILABILITY STATEMENT
The data on which this study is based can be accessed on request from the corresponding author (Matthieu Faron). The data are not publicly available because they contains information that could potentially compromise the privacy of the patients.
REFERENCES
SYNOPSIS
LMS-VCs have a high risk of distant recurrence, evaluated as 59% in this cohort. Pre and/or postoperative chemotherapy and FNCLCC tumor grade were associated with improved DFS.




