Heterogeneity in survival within age groups of early-onset colorectal cancer patients: A National Cancer Database analysis
Abstract
Background
We aimed to identify predictors of and heterogeneity in survival among different age groups of patients with early-onset colorectal cancer (EOCRC).
Methods
This retrospective cohort study used National Cancer Database data from 2004 to 2019. Differences in survival among CRC patients <50 years, subcategorized into age groups (<20, 20–29, 30–39, 40–49 years) were compared for demographic, clinical, and histologic features by univariate and multivariate analyses. Cox hazard regression and Kaplan Meier survival analysis were performed.
Results
134 219 of the 1 240 787 individuals with CRC (10.8%) were <50 years old; 46 639 (34.8%) had rectal and 87 580 (65.3%) had colon cancer. Within the colon cancer cohort, individuals aged between 30 and 39 years had the highest overall survival rate (66.7%) during a median follow-up of 47.6 months (interquartile range IQR 23.1–89.7). The same age group in the rectal cancer cohort had the lowest survival rate (31%) over a median follow-up of 54.5 (IQR 28.24–97.31) months. Leading factors affecting survival included tumor stage (HR 8.23 [4.64–14.6]; p < 0.0001), lymphovascular invasion (HR 1.88 [1.70–2.06]; p < 0.0001) and perineural invasion (HR 1.08 [1.02–1.15]; p = 0.001).
Conclusion
Survival trends vary within age groups of patients affected with early onset colon cancer compared to rectal cancer. Tumor stage and unfavorable pathological characteristics are the strongest factors predicting survival.
1 INTRODUCTION
There are global epidemiological changes in colorectal cancer with a stable or declining incidence among individuals >50 years of age in high index countries in North America, Europe, and Oceania.1 This change may be at least in part due to screening targeting the detection and removal of colorectal polyps or early-stage cancers. In contrast, a rising incidence is observed among individuals <50 years of age—early-onset colorectal cancer (EOCRC).1 A cursory explanation of increased detection of genetic-influenced CRC is countered by the report of the low rate of EOCRC, approximately 20%, attributable to hereditary causes.2 Gaps in the knowledge of EOCRC exist largely due to the lack of comprehensive epidemiological data. Potential risk factors of CRC include a westernized diet, obesity, cigarette smoking, sedentary lifestyle, antibiotic use, and the gut microbiome.3
A recent comparative survival study between individuals with EOCRC and those with CRC diagnosed between ages 51–55 years showed a higher stage-adjusted survival in early age disease.4, 5 Notably, individuals diagnosed from age 35–39 years had the greatest mortality reduction (adjusted HR, 0.88 [95% CI, 0.84–0.92]; p < 0.001).6-8 This finding was attributed by authors to a more common diagnosis of hereditary nonpolyposis colorectal cancer (HNPCC), which arises from mismatch repair deficiency. HNPCC is more commonly diagnosed in individuals from ages 35 to 45 years old and is reported to have better survival.9 In contrast, familial adenomatous polyposis (FAP) is more commony diagnosed at ages <20 years and is associated with a worse prognosis.10 Further insight for an assumption of heterogeneity in survival within age groups of individuals with early onset CRC is needed. Thus, this study aimed to identify the predictors of survival and assess for heterogeneity in demographic, clinical, and histologic factors influencing survival among the different age groups of individuals diagnosed with EOCRC.
2 METHODS
This retrospective cohort study used the National Cancer Database (NCDB), a clinical oncology registry jointly developed by the American College of Surgeons and the American Cancer Society.11 These robust data from >1500 hospitals in the United States and accredited by the Commission on Cancer have an expected 90% follow-up of diagnosed cases over a 5-year period.12 Deidentified data were extracted on individuals diagnosed with primary colorectal cancer in the study period from 2004 to 2019. The NCDB and participating hospitals are not involved in the validation of data analysis or the conclusions from this study report.
2.1 Ethics
Due to the deidentified nature of personal details in the data used, Institutional Review Board approval or patients’ informed consent were not required.
2.2 Eligibility criteria
All cases of histologically proven colorectal adenocarcinoma in patients diagnosed at <50 years of age were included. Individuals <18 years and patients having other histologic types of CSRC such as neuroendocrine tumor, squamous cell carcinoma, and sarcoma were excluded.
2.3 Study variables
Sociodemographic variables studied were age at diagnosis, sex, race/ethnicity, type of insurance, and residence setting (rural/urban/metro). Other variables included the modified Charlson-Deyo comorbidity score, American Joint Commission on Cancer (AJCC) staging (clinical and pathological), type of treatment (surgery, radiation, chemotherapy, and immunotherapy), as well as 30- and 90-day mortality and overall survival (OS) by number of months to last contact if alive/dead.
2.4 Outcomes
The primary outcome measure was OS and the second outcome measure was the rank order of risk factors of mortality in rectal and colon cancer cohorts.
2.5 Definition of terms
Early-onset colorectal cancer: A malignant tumor of the colon and rectum diagnosed in a patient at <50 years of age. The detection bias from the significantly increased number of individuals undergoing screening for CRC at the age of 50 years was avoided by the exclusion of this age in the categorization.
Overall survival: The time from diagnosis of colorectal cancer until death from any cause or date of last contact.
2.6 Statistical analysis
Statistical analysis was performed using EZRTM (version 1.55) software.13 Continuous variables were assessed for normality of data by the Kolmogorov-Smirnov test and summarized as means and standard deviations or median (interquartile range, IQR) as appropriate. Categorical variables were summarized as frequencies and percentages. Age group comparison of rectal and colon cancer cohorts was performed using the x2 test and Fisher exact test. Variables with p-value < 0.1 were included in the multivariate analysis. Kaplan-Meier survival analysis was performed, and survival differences were assessed by a log-rank test. Cox hazard regression analysis was performed to evaluate for predictive demographic, clinical, and histologic factors for survival based on significance of p-value. Statistical significance was set at p < 0.05.
3 RESULTS
3.1 Baseline characteristics
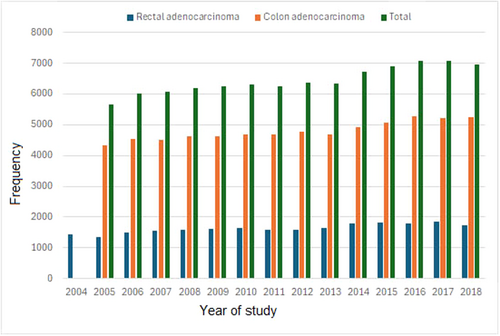
In all, 134 219 (10.8%) of 1 240 787 individuals with colorectal adenocarcinoma were <50 years of age. The primary site of cancer was the rectum in 46 639 (34.8%) and the colon in 87 580 (65.3%) individuals (Figure 1). A near-progressive increase in the frequency of EOCRC cases was recorded during the study years (Figure 2). 49.5% of the patient population survived during the median follow-up period of 37.3 months (IQR 10.7–60.3) for the rectal cancer cohort and 72.9 months (IQR 41.89–119.85) for the colon cancer cohort. Patient characteristics are shown in Table S1. There were 71 887 (53.6%) males and 62 332 (46.4%) females. A racial disparity was noted with Whites (104 300 (77.7%)) predominantly affected, then distantly followed by Blacks (19 543 (14.7%)), Asians (5.616 (4.2%)) and American Indians (710 (0.5%)). Private health insurance status (95 484 (71.1%)) was predominant among the EOCRC cohort compared to non-private health insurance status including Medicaid (17 929 (13.4%)), no health insurance status, (10 019 (7.5%)) and Medicare (5940 (4.4%)).

Two-thirds of cancer cases (67.0%) were left-sided (splenic flexure 2.4%, descending colon 5.3%, sigmoid colon 24.7%, and rectum 34.8%). Advanced tumor stage III was recorded in 17 120 (12.8%) and stage IV disease in 27 076 (20.2%). In terms of histology, classical adenocarcinoma was recorded in 88.9% of patients, mucinous adenocarcinoma (MUC) in 9.0%, and signet-ring cell carcinoma in 2.1%. Minimally invasive approaches to definitive surgery (endoscopic/laparoscopic and robotic) were more frequently performed than was laparotomy (26.4% vs. 20.2%). Overall, the percentage administration frequency of chemotherapy, radiotherapy, and immunotherapy was 49.7%, 24.5%, and 4.5%, respectively.
3.2 Age group-specific characteristics for rectal cancer cohort
Demographic trends revealed three quarters (35 126/87 580) of this cohort as individuals aged between 40 and 49 years (Table 1). A male predominance was observed in all age groups but for a marginal difference in patients aged <20 years. Racial disparity was noted with an increasing percentage frequency of EOCRC diagnosis in Whites as the age group advanced (77.2% for 20–29 age group rose to 83.7% for the 40–49 age group). A reversal of this trend was observed with a lower percentage involvement of Asians and Blacks in the same age groups (5.3%–4.2% and 13.1%–10.0% respectively; p < 0.0001). The diagnosis of EOCRC was predominantly recorded among metro residents. The percentage frequency of diagnoses increased for urban residents as age advanced (8.6%–14.9%). Stage IV disease decreased as the age group advanced (<20 years: 36.4%), 20–29 years: 26.7%, 30–39 years: 23.4%, and 40–49 years: 22.1%). The proportion of patients with clinical stage III disease in the age group 30-39 years was the highest at 40.7% (2496/6130). This age group was also marginally associated with the highest proportion of tumors with lymphovascular invasion characteristics at 25.4%. Patients in age groups <20 yrs, 20–29 years, 30–39 years, and 40–49 years were associated with poor tumor differentiation (G3/4) in decreasing frequency (32.6%, 23.4%, 18.1%, and 15.0%, respectively; p < 0.0001). The age group <20 years had more markers of genetic influence (microsatellite instability (MSI) : 33.4%). More systemic cancer therapy was administered in patients <30 years in contrast to patients ≥30 years (chemotherapy: 43.1%–46.0% vs. 40.9%–44.0% (0 < 0.0001); immunotherapy: 5.6%–6.6% vs. 4.4%–5.4% (p = 0.0003), respectively). Overall, minimally invasive surgical approaches were more commonly performed with no significant differences among the age groups (p = 0.404).
| <20 years | 20–29 years | 30–39 years | 40–49 years | ||
|---|---|---|---|---|---|
| Variables | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p value |
| Months from initial diagnosis to last contact or death | 37.29 (10.69–60.25) | 48.39 (24.85– 90.05) | 54.47 (28.24– 97.31) | 57.07 (29.83– 101.95) | <0.001 |
| n (%) | n (%) | n (%) | n (%) | ||
|---|---|---|---|---|---|
| Survival | |||||
| Dead | 24 (42.9) | 1123 (64.0) | 6050 (69.0) | 22 166 (67.9) | <0.00001 |
| Alive | 32 (57.1) | 631 (36.0) | 2714 (31.0) | 10 494 (32.1) | |
| Sex | |||||
| Female | 31 (50.8) | 862 (45.8) | 4229 (44.2) | 14 458 (41.2) | < 0.00001 |
| Male | 30 (49.2) | 1020 (54.2) | 5341 (55.8) | 20 668 (58.8) | |
| Race | |||||
| American Indian | 0 (0.0) | 17 (0.9) | 57 (0.6) | 197 (0.6) | <0.00001 |
| Asian | 2 (3.4) | 99 (5.3) | 450 (4.8) | 1453 (4.2) | |
| Black | 8 (13.6) | 243 (13.1) | 955 (10.1) | 3469 (10.0) | |
| White | 49 (83.1) | 1436 (77.2) | 7756 (82.2) | 29,071 (83.7) | |
| Other | 0 (0.0) | 64 (3.4) | 223 (2.3) | 532 (1.6) | |
| Residence area | |||||
| Metro | 53 (91.4) | 1535 (85.2) | 7738 (84.6) | 28,154 (83.3) | 0.01386 |
| Rural | 0 (0.0) | 25 (1.4) | 161 (1.8) | 606 (1.8) | |
| Urban | 5 (8.6) | 241 (13.4) | 1252 (13.7) | 5042 (14.9) | |
| Health insurance | |||||
| Medicaid | 20 (32.8) | 390 (21.5) | 1417 (15.2) | 4108 (12.0) | <0.00001 |
| Medicare | 2 (3.3) | 43 (2.4) | 295 (3.2) | 1492 (4.4) | |
| Not insured | 3 (4.9) | 159 (8.8) | 700 (7.5) | 2343 (6.8) | |
| Other government | 0 (0.0) | 31 (1.7) | 146 (1.6) | 526 (1.5) | |
| Private | 36 (59.0) | 1194 (65.7) | 6772 (72.6) | 25 819 (75.3) | |
| Charlson-Deyo Score | |||||
| 0 | 58 (95.1) | 1786 (94.9) | 8842 (92.4) | 31 076 (88.5) | <0.00001 |
| 1 | 3 (4.9) | 86 (4.6) | 642 (6.7) | 3243 (9.2) | |
| 2 | 0 (0.0) | 5 (0.3) | 60 (0.6) | 505 (1.4) | |
| 3 | 0 (0.0) | 5 (0.3) | 26 (0.3) | 302 (0.9) | |
| Clinical Stage | |||||
| 0 | 2 (4.5) | 42 (3.3) | 230 (3.8) | 903 (3.8) | <0.00001 |
| 1 | 3 (6.8) | 162 (12.8) | 807 (13.2) | 3831 (16.3) | |
| 2 | 6 (13.6) | 208 (16.5) | 1160 (18.9) | 5099 (21.7) | |
| 3 | 17 (38.6) | 513 (40.6) | 2496 (40.7) | 8451 (36.0) | |
| 4 | 16 (36.4) | 337 (26.7) | 1437 (23.4) | 5191 (22.1) | |
| Surgical approach | |||||
| Laparoscopic | 9 (42.9) | 285 (37.6) | 1533 (37.6) | 5614 (37.6) | 0.4044 |
| Open or not specified | 8 (38.1) | 312 (41.2) | 1606 (39.4) | 6127 (41.0) | |
| Robotic | 4 (19.0) | 160 (21.1) | 937 (23.0) | 3198 (21.4) | |
| Surgical margin | |||||
| R+ | 5 (13.9) | 138 (10.5) | 617 (8.6) | 1949 (7.4) | <0.0001 |
| R0 | 31 (86.1) | 1179 (89.5) | 6518 (91.4) | 24 716 (92.7) | |
| 30-day mortality | |||||
| No | 37 (97.4) | 1295 (99.9) | 6800 (99.8) | 25 853 (99.8) | 0.01464 |
| Yes | 1 (2.6) | 1(0.1) | 17 (0.2) | 63 (0.2) | |
| Chemotherapy | |||||
| Chemotherapy administered | 25 (43.1) | 848 (46.0) | 4119 (44.0) | 14 031 (40.9) | <0.00001 |
| No chemotherapy | 33 (56.9) | 995 (54.0) | 5245 (56.0) | 20 314 (59.1) | |
| Radiotherapy | |||||
| Radiotherapy administered | 35 (57.4) | 1031 (62.6) | 6195 (66.9) | 22 461 (65.8) | 0.1913 |
| No radiotherapy | 26 (42.6) | 678 (37.5) | 3063 (33.2) | 11 664 (34.2) | |
| Immunotherapy | |||||
| Immunotherapy administered | 4 (6.6) | 105 (5.6) | 526 (5.5) | 1547 (4.4) | 0.00026 |
| No immunotherapy | 57 (93.4) | 1764 (94.5) | 8955 (94.4) | 33 282 (95.5) | |
| Histology | |||||
| Adenocarcinoma | 45 (73.8) | 1671 (88.8) | 8874 (92.7) | 33 263 (94.7) | <0.00001 |
| Mucinous adenocarcinoma | 3 (4.9) | 127 (6.7) | 511 (5.3) | 1529 (4.4) | |
| Signet-ring cell carcinoma | 13 (21.3) | 84 (4.5) | 185 (1.9) | 334 (1.0) | |
| Tumor grade | |||||
| 1 | 3 (7.0) | 100 (7.7) | 583 (8.9) | 2190 (9.7) | <0.00001 |
| 2 | 26 (60.5) | 900 (68.9) | 4804 (73.0) | 16 980 (75.3) | |
| 3 | 11 (25.6) | 280 (21.4) | 1083 (16.5) | 3108 (13.8) | |
| 4 | 3 (7.0) | 26 (2.0) | 108 (1.6) | 269 (1.2) | |
| MSI status | |||||
| Stable | 4 (66.7) | 228 (78.4) | 1315 (85.7) | 5820 (88.8) | <0.00001 |
| Unstable high | 1 (16.7) | 21 (7.2) | 42 (2.7) | 214 (3.3) | |
| Unstable low | 0 (0.0) | 14 (4.8) | 71 (4.6) | 268 (4.1) | |
| Unstable NOS | 1 (16.7) | 28 (9.6) | 106 (6.9) | 250 (3.8) | |
| Lympho-vascular invasion | |||||
| Yes | 18 (14.3) | 557 (25.2) | 2928 (25.4) | 11 102 (22.3) | 0.00022 |
| No | 3 (85.7) | 188 (74.8) | 996 (74.6) | 3186 (77.7) | |
| Perineural invasion | |||||
| Yes | 3 (25) | 111 (24) | 720 (20.1) | 2383 (17.7) | 0.1171 |
| No | 1 (75) | 35 (76) | 181 (79.9) | 511 (82.3) | |
- Note: Bold text in p value column indicates statistical significance.
- Abbreviations: MSI, microsatellite instability; IQR, interquartile range.
3.3 Age group-specific characteristics of colon cancer cohort
Racial disparity was also noted with an increased proportion of cancer patients observed among Whites as the age groups progressed, with a reversal of this trend in Asians (Table 2). The proportion of colon cancer patients with clinical stages III and IV disease was highest in the age group <20 years (15.7% and 52.8, respectively). A striking difference in the proportion of signet-ring cell carcinoma was noted in the age group <20 years (14.6%) compared to the other age groups (30–39 years: 2.8% and 40–49 years: 2.1%). Mucinous adenocarcinoma was more frequently recorded in age groups <20 years (17.3%) and 20–29 years (14.8%) compared to age groups of 30–39 years (13.0%) and 40–49 years (10.5%). A higher proportion of patients <30 years received multiagent chemotherapy (38.4%–41.0%) in contrast to patients aged 30 years (32.2%–35.8%). More MSI unstable tumors and perineural invasion were noted in the younger age groups.
| <20 years | 20–29 years | 30–39 years | 40–49 years | ||
|---|---|---|---|---|---|
| Variables | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p value |
| Follow-up (months) | 31.4 (12.6–82.7) | 42.3 (19.5– 80.4) | 47.6 (23.1–89.7) | 49.7 (23.7–93.7) | <0.001 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
|---|---|---|---|---|---|
| Survival | |||||
| Dead | 70 (40.9) | 1467 (37.4) | 5996 (33.3) | 21 037 (35.7) | <0.0001 |
| Alive | 101 (59.1) | 2453 (62.6) | 11 996 (66.7) | 37 972 (64.3) | |
| Sex | |||||
| Female | 87 (47) | 2056 (48.8) | 9609 (49.2) | 31 000 (48.7) | 0.6951 |
| Male | 98 (53) | 2158 (51.2) | 9936 (50.8) | 32 636 (51.3) | |
| Race | |||||
| American Indian | 2 (1.1) | 23 (0.5) | 111 (0.6) | 303 (0.5) | <0.0001 |
| Asian | 12 (6.5) | 202 (4.8) | 880 (4.5) | 2518 (4.0) | |
| Black | 35 (18.9) | 682 (16.2) | 3214 (16.4) | 10 937 (17.2) | |
| White | 125 (67.6) | 3122 (74.1) | 14 708 (75.3) | 48 033 (75.5) | |
| Other | 8 (4.3) | 129 (3.1) | 410 (2.1) | 1150 (1.8) | |
| Residence area | |||||
| Metro | 151 (84.4) | 3571 (88.2) | 16 514 (87.5) | 53 628 (87.1) | 0.1258 |
| Rural | 2 (1.1) | 57 (1.4) | 279 (11.0) | 858 (1.4) | |
| Urban | 26 (14.5) | 420 (10.4) | 2079 (11.0) | 7087 (11.5) | |
| Health insurance | |||||
| Medicaid | 55 (29.7) | 927 (22.0) | 3115 (15.9) | 7897 (12.4) | <0.0001 |
| Medicare | 3 (1.6) | 119 (2.8) | 659 (3.4) | 3327 (5.2) | |
| Not insured | 21 (11.4) | 390 (9.3) | 1,705 (8.7) | 4698 (7.4) | |
| Other government | 5 (2.7) | 69 (1.6) | 259 (1.3) | 801 (1.3) | |
| Private | 97 (52.4) | 2587 (61.4) | 13 394 (68.5) | 45 585 (71.6) | |
| Charlson-Deyo Score | |||||
| 0 | 175 (94.6) | 3910 (92.8) | 17 753 (90.8) | 54 336 (85.4) | <0.0001 |
| 1 | 8 (4.3) | 254 (6.0) | 1451 (7.4) | 7354 (11.6) | |
| 2 | 0 (0) | 29 (0.7) | 207 (1.1) | 1177 (1.8) | |
| 3 | 2(1.1) | 21 (0.5) | 134 (0.7) | 769 (1.2) | |
| Primary site | |||||
| Right colon | 95 (51.3) | 1895 (45.0) | 8744 (44.8) | 28 466 (44.7) | <0.0001 |
| Left colon | 70 (37.9) | 1984 (47.0) | 9606 (49.1) | 31 713 (49.9) | |
| Overlapping | 1 (0.5) | 84 (2.0) | 315 (1.6) | 864 (1.4) | |
| Not specified | 19 (10.3) | 251 (6.0) | 880 (4.5) | 2593 (4.1) | |
| Clinical Stage | |||||
| 0 | 3 (3.4) | 105 (5.4) | 525 (5.7) | 2074 (6.8) | <0.0001 |
| 1 | 16 (18.0) | 276 (14.2) | 1421 (15.5) | 5455 (18.0) | |
| 2 | 9 (10.1) | 274 (14.1) | 1349 (14.7) | 4302 (14.2) | |
| 3 | 14 (15.7) | 301 (15.5) | 1327 (14.5) | 4001 (13.2) | |
| 4 | 47 (52.8) | 981 (50.6) | 4548 (49.6) | 14 519 (47.8) | |
| Surgical approach | |||||
| Endoscopic or laparoscopic | 31 (37.8) | 1051 (47.3) | 4711 (46.1) | 15 844 (48.2) | 0.0001 |
| Open or not specified | 45 (54.9) | 1003 (45.2) | 4680 (45.8) | 14 131 (43.0) | |
| Robotic | 6 (7.3) | 164 (7.4) | 823 (8.1) | 2848 (8.7) | |
| Surgical margin | |||||
| R0 | 132 (71.4) | 3065 (72.7) | 14 678 (75.1) | 48 861 (76.8) | <0.0001 |
| R+ | 18 (9.15) | 433 (10.2) | 1714 (8.8) | 4940 (8.8) | |
| 30-day mortality | |||||
| No | 144 (100) | 3354 (98.4) | 15 564 (98.3) | 51 045 (98.3) | 0.0027 |
| Yes | 0 (0) | 45 (1.3) | 173 (1.1) | 506 (1.0) | |
| Unknown | 0 (0) | 11 (0.3) | 89 (0.6) | 386 (0.7) | |
| Chemotherapy | |||||
| Chemotherapy administered | 79 (44.4) | 1788 (44.3) | 7756 (41.4) | 23 200 (37.7) | <0.0001 |
| No chemotherapy | 99 (55.6) | 2246 (55.7) | 10 966 (58.6) | 37 723 (62.1) | |
| Radiotherapy | |||||
| Radiotherapy administered | 7(3.8) | 148 (3.5) | 707 (3.6) | 2080 (3.3) | 0.3046 |
| No radiotherapy | 169 (91.4) | 3961 (94.0) | 18 381 (94.0) | 60 095 (94.4) | |
| Immunotherapy | |||||
| Immunotherapy administered | 7 (3.8) | 297 (7.0) | 1394 (7.1) | 4349 (6.8) | 0.1654 |
| No immunotherapy | 177 (95.7) | 3879 (92.1) | 17 993 (92.0) | 58 739 (92.3) | |
| Histology | |||||
| Adenocarcinoma | 126 (68.1) | 3325 (78.9) | 16 442 (84.1) | 55 609 (87.4) | <0.0001 |
| Mucinous adenocarcinoma | 32 (17.3) | 622 (14.8) | 2547 (13.0) | 6659 (10.5) | |
| Signet-ring cell carcinoma | 27 (14.6) | 267 (6.3) | 556 (2.8) | 1368 (2.1) | |
| Tumor grade | |||||
| 1 | 18 (9.8) | 344 (8.2) | 1729 (8.9) | 5852 (9.2) | <0.0001 |
| 2 | 77 (41.8) | 2067 (49.3) | 10 792 (55.5) | 36 915 (58.3) | |
| 3 | 51 (27.7) | 1022 (24.4) | 3558 (18.3) | 9896 (15.6) | |
| 4 | 6 (3.3) | 136 (3.2) | 533 (2.7) | 1225 (1.9) | |
| MSI status | |||||
| Stable | 28 (65.1) | 662 (69.5) | 2957 (72.6) | 9264 (79.2) | <0.0001 |
| Unstable high | 7 (16.3) | 115 (12.1) | 374 (9.2) | 751 (6.4) | |
| Unstable low | 1 (2.3) | 38 (4.0) | 209 (5.1) | 571 (4.9) | |
| Unstable NOS | 7 (16.3) | 138 (14.5) | 533 (13.1) | 109(9.5) | |
| KRAS mutation | |||||
| Mutated | 9 (31) | 185 (33.6) | 998 (40.5) | 3053 (42.8) | 0.0001 |
| Wild | 20 (69) | 366 (66.4) | 1465 (59.5) | 4085 (57.2) | |
| Lymphovascular invasion | |||||
| Yes | 36 (27.7) | 957 (31.5) | 4062 (29.1) | 12 169 (27.7) | <0.0001 |
| No | 63 (48.5) | 1371 (45.2) | 6668 (47.8) | 21 597 (49.2) | |
| Perineural invasion | |||||
| Yes | 17 (23.3) | 391 (22.2) | 1637 (20.2) | 4590 (17.4) | <0.0001 |
| No | 56 (76.7) | 1370 (77.8) | 6459 (79.8) | 21 826 (82.6) | |
- Note: Bold text in p value column indicates statistical significance.
- Abbreviations: MSI, microsatellite instability; IQR, interquartile range; NOS, not otherwise specified.
3.4 Survival
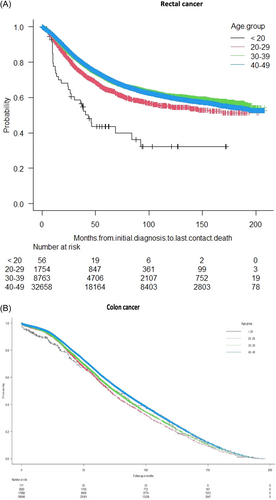
In this EOCRC cohort, after different treatment strategies, 57 975 (43.2%) patients had died at the current time of this study analysis. Patients in the age group <20 years had the lowest survival curve (Figure 3). The impact of tumor stage was more pronounced in the rectal cancer cohort (Figure 4).
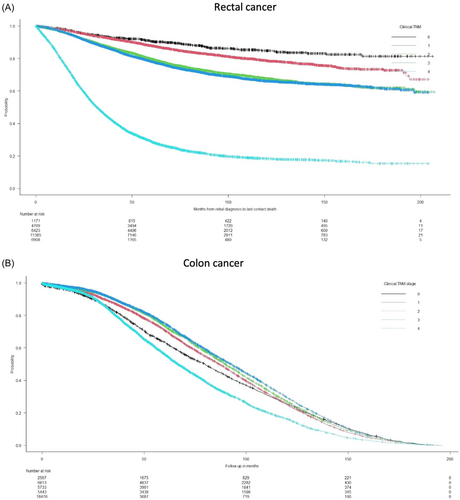
In the rectal cancer cohort, significant predictive clinical and histologic factors of OS in decreasing rank order were stage IV tumor (HR 8.23 (95% CI 4.64–14.6); p < 0.0001), lymphovascular invasion (HR 1.88 (95% CI 1.70–2.06); p < 0.0001) and male sex (HR 1.21 (95% CI 1.10–1.33); p < 0.0001) (Table 3).
| Factors | HR (95% CI Lower- Upper) | p-value | Adjusted HR (95% CI Lower-Upper) | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Age group | ||||
| 20–29 years | 0.53 (0.37–0.77) | <0.001 | 0.41 (0.13–1.30) | 0.129 |
| 30–39 years | 0.44 (0.31–0.64) | <0.0001 | 0.41 (0.13–1.27) | 0.121 |
| 40–49 years | 0.45 (0.31–0.64) | <0.0001 | 0.45 (0.14–1.40) | 0.167 |
| Sex | ||||
| Male | 1.29 (1.24–1.33) | <0.0001 | 1.21 (1.10–1.33) | <0.0001 |
| Race | ||||
| Asian | 0.77 (0.61–0.96) | 0.018 | 1.20 (1.07–1.50) | 0.690 |
| Black | 1.04 (0.85–1.28) | 0.690 | ||
| White | 0.82 (0.67-1.00) | 0.052 | ||
| Other | 1.03 (0.74-1.44) | 0.845 | ||
| Residence area | ||||
| Rural | 1.08 (0.96–1.23) | 0.214 | ||
| Urban | 1.09 (1.04–1.15) | <0.001 | 1.20 (1.07–1.36) | 0.0021 |
| Insurance status | ||||
| Medicare | 1.13 (1.04–1.15) | 0.032 | ||
| Not insured | 1.03 (0.97–1.11) | 0.330 | ||
| Other government | 0.65 (0.56–0.76) | <0.0001 | ||
| Private | 0.54 (0.51–0.56) | <0.0001 | ||
| Clinical | ||||
| Charlson Deyo Score | 1.26 (1.21–1.32) | <0.0001 | ||
| Clinical stage | ||||
| 1 | 1.32 (1.10–1.59) | 0.0027 | 1.20 (0.67–2.14) | 0.551 |
| 2 | 2.09 (1.75–2.49) | <0.0001 | 2.21 (1.25–3.94) | <0.0001 |
| 3 | 2.20 (1.85–2.61) | <0.0001 | 2.61 (1.47–4.61) | <0.0001 |
| 4 | 10.38 (8.74–12.33) | <0.0001 | 8.23 (4.64–14.6) | <0.0001 |
| Chemotherapy | ||||
| No chemotherapy | 0.82 (0.79–0.86) | <0.0001 | ||
| Single-agent | 0.95 (0.89–1.00) | 0.090 | ||
| Type not documented | 0.84 (0.73–0.96) | 0.011 | ||
| Immunotherapy | ||||
| Immunotherapy administered | 0.45 (0.29–0.70) | <0.0001 | 0.54 (0.22–1.33) | 0.182 |
| No immunotherapy | 0.39 (0.02–0.60) | <0.0001 | 0.44 (0.18–1.07) | 0.071 |
| Histologic | ||||
| Adenocarcinoma | 0.20 (0.13–0.32) | <0.0001 | 0.34 (0.25–0.46) | <0.0001 |
| Mucinous carcinoma | 0.31 (0.19–0.51) | <0.0001 | 0.53 (0.38–0.73) | <0.0001 |
| Grade | 1.27 (1.13–1.44) | <0.0001 | 1.36 (1.25–1.47) | <0.0001 |
| R0 | 0.34 (0.20–0.44) | <0.0001 | 0.50 (0.42–0.60) | <0.0001 |
| R1 | 0.88 (0.64–1.21) | 0.419 | 1.00 (0.81–1.25) | 0.947 |
| R2 | 1.71 (0.88–3.35) | 0.116 | 0.48 (0.27–0.85) | 0.0124 |
| MSI | ||||
| High | 0.75 (0.47–1.17) | 0.205 | ||
| Low | 0.96 (0.69–1.71) | 0.799 | ||
| Unstable NOS | 1.35 (1.06–1.71) | 0.015 | ||
| Lymphovascular invasion | ||||
| Yes | 2.26 (1.97–2.60) | <0.0001 | 1.88 (1.70–2.06) | <0.0001 |
- Note: Bold text in the p-value column indicates statistical significance.
- Abbreviations: HR, hazard ratio; MSI, microsatellite instability; IQR, interquartile range.
In the colon cancer cohort, perineural tumor invasion was the leading risk factor negatively associated with OS (HR 1.08 (95% CI 1.02–1.15); p = 0.001) (Table 4).
| Factors | HR (95% CI Lower- Upper) | p-value | Adjusted HR (95% CI Lower- Upper) | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Age group | ||||
| 20–29 years | 1.06 (0.87–1.29) | 0.586 | ||
| 30–39 years | 1.00 (0.82–1.22) | 0.972 | ||
| 40–49 years | 0.93 (0.77–1.13) | 0.479 | ||
| Sex | ||||
| Male | 1.29 (1.24–1.33) | <0.0001 | ||
| Race | ||||
| Asian | 0.96 (0.84–1.10) | 0.549 | 1.04 (0.78–1.39) | 0.792 |
| Black | 0.81 (0.71–0.92) | 0.001 | 0.96 (0.72–1.27) | 0.777 |
| Other | 1.17 (1.02–1.34) | 0.028 | 1.36 (1.00–1.86) | 0.0511 |
| White | 0.81 (0.71–0.91) | <0.001 | 0.96 (0.72–1.27) | 0.765 |
| Insurance status | ||||
| Medicare | 0.82 (1.04–1.15) | 0.032 | 0.78 (0.70–0.87) | <0.0001 |
| Not insured | 1.03 (0.97–1.11) | 0.330 | 0.86 (0.79–0.94) | <0.001 |
| Other government | 0.83 (0.78–0.86) | <0.0001 | 0.83 (0.70–0.99) | 0.0394 |
| Private | 0.78 (0.76–0.80) | <0.0001 | 0.86 (0.81–0.91) | <0.0001 |
| Clinical | ||||
| Clinical stage | ||||
| 1 | 0.97 (0.93–1.02) | 0.0287 | 0.82 (0.76–0.87) | <0.0001 |
| 2 | 0.91 (0.86–0.96) | <0.001 | 0.77 (0.72–0.83) | <0.0001 |
| 3 | 0.87 (0.82–0.92) | <0.0001 | 0.74 (0.69–0.80) | <0.0001 |
| 4 | 1.39 (1.32–1.47) | <0.0001 | 0.99 (0.91–1.06) | 0.711 |
| Primary Site | ||||
| Cecum | 1.01 (0.94–1.09) | 0.781 | ||
| Ascending colon | 1.06 (0.98–1.15) | 0.154 | ||
| Hepatic flexure | 1.03 (0.93–1.14) | 0.596 | ||
| Transverse colon | 1.04 (0.95–1.13) | 0.382 | ||
| Splenic flexure | 1.03 (0.93–1.14) | 0.533 | ||
| Descending colon | 1.08 (0.99–1.17) | 0.076 | ||
| Sigmoid colon | 1.04 (0.97–1.12) | 0.235 | ||
| Chemotherapy | ||||
| No chemotherapy | 1.00 (0.97–1.03) | 0.919 | ||
| Single agent | 1.01 (0.94–1.09) | 0.786 | ||
| Type not documented | 0.72 (0.64–0.81) | <0.0001 | ||
| Histologic | ||||
| Grade 2 | 1.02 (0.85–1.21) | 0.8611 | ||
| Grade 3 | 0.90 (0.74–1.09) | 0.288 | ||
| Grade 4 | 0.78 (0.59–1.03) | 0.076 | ||
| Perineural invasion | 1.22 (1.10–1.36) | <0.001 | 1.08 (1.02–1.15) | 0.001 |
- Note: Bold text in the p-value column indicates statistical significance.
- Abbreviations: HR, hazard ratio; CI, confidence interval.
4 DISCUSSION
This nationwide study examined the demographic, clinical, and histologic characteristics of patients of different age groups diagnosed with EOCRC. The majority of CRCs are adenocarcinomas, but less frequently mucinous adenocarcinomas and signet-ring cell carcinomas are seen.14 Both subtypes are diagnosed in more advanced tumor stages than are classical adenocarcinomas.15 The definition of mucinous adenocarcinoma is that >50% of the lesion is composed of mucin, typically in extracellular pools that contain malignant epithelium as acinar structures, strips of cells, or single cells.16 To qualify as a signet-ring cell cancer, >50% of the lesion must be composed of tumor cells with prominent intracytoplasmic mucin, typically characterized by large mucin vacuoles that fill the cytoplasm and displace the nucleus.16 The poor prognosis of signet-ring cell carcinoma is more evident, mainly due to high rates of synchronous and metachronous distant organ metastasis associated with this histological subtype.17, 18 Tumor stage was the strongest predictor of survival outcome identified. In this present study, approximately one-fifth of EOCRC patients had stage IV disease. The next most frequent tumor stage recorded was stage III disease in 12.2% (Table S1). The group aged <20 years was associated with the lowest survival curve (Figure 3). The commonly presumed delayed diagnosis for EOCRC in general may not explain the observed advanced tumor stages. The more aggressive tumor biology of mucinous adenocarcinoma and signet-ring cell carcinoma, including pathologic features such as perineural and lymphovascular invasion, are more likely the reason.
The majority of EOCRC diagnoses were recorded in individuals aged 40–49 years. Reports show the largest absolute increases in incidence occurred among individuals 40–49 years from 18.2 per 100 000 in 1992 to 26.5 per 100 000 in 2015.19 Mortality rates have slightly increased during the same period from 5.4 to 6.5 per 100 000 in this age group.19 The average annual percent change of 1.6% was reported in those aged 40–49 years during the period 2004–2016.20 This trend has facilitated the updated screening guidelines of the American Cancer Society and US Preventive Services Task Force recommendation that reduced the start-off screening age on average-risk individuals to 45 years.21, 22 The greater use of screening or diagnostic technologies to detect cancer earlier is often associated with increasing incidence and stable mortality.22 This strategy may have affected the numbers in this age group in this more recent study. A prior NCDB study that reported on EOCRC with a smaller population suggested a survival advantage among individuals diagnosed from 35 to 39 years.4 In this study, no age group was statistically predictive of OS from EOCRC. However, individuals aged between 30 and 39 years with colon cancer had the highest OS rate (66.7%) in comparison to the different age groups during a median follow-up duration of 47.6 months (IQR 23.1–89.7). An opposite trend was noted in rectal cancer as the same age group recorded the lowest survival rate (31%) over a median follow-up duration of 54.47 months (IQR 28.24–97.31). Male sex was identified as a risk factor for rectal cancer. This may be related to the challenge of surgery with tumor-free margins in a narrower male pelvis with advanced-stage disease.
Overall, two-thirds of EOCRC patients (67%) were left-sided (rectum 34.8%, sigmoid colon 24.7%, splenic flexure 5.3%, and descending colon 2.4%). A similar international observation of left-sided predominance of EOCRC has been reported.23 This study however recorded more colon cancers (65.3%) than rectal cancers (34.7%). The proportion of MSI unstable colonic tumors steadily decreased from 34.9% in advancing age groups to 20.8% in those aged 40-49%. Inherited syndromes associated with CRC including HNPCC and FAP are common in younger individuals. Lynch syndrome is associated with germline mutation in a DNA mismatch repair (MMR) gene (MLH1, MSH2, MSH6 or PMS2) and often presents as EOCRC with high-level MSI.24 FAP is characterized by a mutation in the APC gene and individuals with this pre-malignant entity have nearly 100% at risk of developing EOCRC by the age of 40.25 The adoption of preventative strategies of genetic testing and prophylactic colectomy among FAP families is recommended to decrease the incidence of EOCRC.26
4.1 Limitations
This study has some limitations including its retrospective design and an inherent weakness of non-exclusion of some confounding factors. The effect of temporal trends on survival with regard to the rising incidence of EOCRC was not ascertained from the dataset in the absence of the value for the normal population under the age of 50 years. Another limitation is the absence of data on family history, which is needed to fulfill the Amsterdam criteria for a diagnosis of HNPCC. Incomplete data, especially on molecular features, was yet another limitation of this study. More extensive studies on molecular differences in age groups affecting survival outcomes, including optimizing biomarker-directed therapy in metastatic CRC, are needed to improve the survival of EOCRC associated with the large proportion of advanced tumor-stage disease.
5 CONCLUSION
The majority of individuals with EOCRC had advanced-stage disease. Survival trends within the age groups of EOCRC patients varied between colon and rectal cancer. Tumor stage and unfavorable pathological characteristics are the strongest predictors of OS in EOCRC.
CONFLICT OF INTEREST STATEMENT
None of the authors report any relevant financial disclosures. Dr. Wexner is a consultant for Baxter, Becton, Dickinson and Co, Glaxo Smith Kline, Intuitive Surgical, Livsmed, Medtronic, OstomyCure, Stryker, Takeda, Virtual Ports, is a member of the Data Safety Monitoring Board of JSR/WCG/ACI (chair), Polypoid (chair), and Boomerang and receives royalties from Intuitive Surgical, Karl Storz Endoscopy America Inc., and Unique Surgical Solutions, LLC.
Open Research
DATA AVAILABILITY STATEMENT
Upon reasonable request to first author.
REFERENCES
6 SYNOPSIS
Survival trends vary within age groups of patients affected with early onset colon cancer compared to rectal cancer. Tumor stage and unfavorable pathological characteristics are the strongest factors predicting survival.




