Surgical efficacy and learning curves of laparoscopic complete mesocolic excision with intracorporeal anastomosis for right-sided colon cancer: A retrospective two-center cohort study
Abstract
Background
There is a potential benefit on long-term outcomes following complete mesocolic excision (CME) for right-sided colon cancer when compared to conventional colectomy. This study aims to analyze the learning curve and short-term outcomes of laparoscopic CME with intracorporeal anastomosis (ICA) for right-sided colon cancer in the hands of experienced colorectal surgeons.
Methods
A two-center cohort study of consecutive patients undergoing right-sided colectomy from September 2021 to May 2022 at two tertiary colorectal centers in Denmark. Learning curves of surgical time were estimated using a cumulative sum analysis (CUSUM).
Results
A total of 61 patients were included. According to the CUSUM analysis, 32 cases were needed to obtain a peak in operative time, resulting in a decrease in time consumption (group 1/learning phase: 217.2 min [SD 53.6] and group 2/plateau phase 191.6 min [SD 45.1], p = 0.05). There was a nonsignificant reduction in the rates of severe surgical complications (Clavien–Dindo > 3) (13% vs. 7%, p = 0.67) between the two groups, while the length of hospital stay remained constant (median 3.0 days, interquartile range, IQR [2.0; 4.0]).
Conclusion
The learning curve of laparoscopic CME with ICA for right-sided colon cancer demonstrated that 32 cases were needed to obtain a plateau phase expressed by operative time.
1 INTRODUCTION
The complete mesocolic excision (CME) technique for right-sided colon cancer was initially described by Hohenberger in 2009 and is still cautiously practiced due to studies suggesting a possible improvement in overall- and disease-free survival rates.1, 2 It has been associated with an increased risk of intraoperative injuries and postoperative morbidity rates, but it is still questioned whether it should be applied to all tumor stages.3 Recently published data from meta-analyses suggests that CME surgery has equal postoperative morbidity rates compared to conventional colectomy.4, 5 CME is a challenging surgical procedure that requires an extensive vascular skeletonizing of all right-sided colon branches originating from the superior mesenteric vessels. The blood supply to the right colon is likewise characterized by a high degree of anatomical variations placing a greater demand on the surgeon.6 The possible benefits of CME on long-term survival are still under debate, as there is no clear evidence of applying an extensive mesocolic excision in all Union for International Cancer Control (UICC) stages.7 In addition, long-term results of two prospective randomized controlled trials (RCT) (RELARC and COLD) comparing CME and non-CME surgery for right-sided colon cancer are still awaited.8, 9
Minimally invasive surgery leads to a faster recovery due to a reduction in postoperative pain and a faster mobilization and resumption of bowel function compared to open surgery. In addition, the intracorporeal anastomosis (ICA) technique is steadily implemented, being associated with an improvement in overall morbidity rates and a reduction in hospitalization time compared to the extracorporeal method.10-13 However, more recently published prospective trials only confirmed a reduction in postoperative pain, not resulting in a shortened length of hospital stay or morbidity rates when comparing ICA versus extracorporeal anastomosis technique.14
Some studies analyzing learning curves for CME in robot-assisted compared to laparoscopic surgery for right-sided colon cancer suggested a mean of 16 cases for the robot-assisted method and 25 for the laparoscopic to obtain a proficiency phase measured in operative time (OT).15, 16 However, the learning curve of laparoscopic CME surgery with ICA is not well examined.
This study aims to analyze the learning curve and short-term outcomes of laparoscopic CME with ICA for right-sided colon cancer.
2 METHODS
2.1 Study setting and study population
A two-center retrospective cohort study of consecutively collected data since the implementation of laparoscopic CME surgery with ICA for right-sided colon cancer. Patients were identified by unique social security numbers and International Classification of Diseases-10 cancer- and procedure codes if undergoing right-sided and extended right-sided laparoscopic hemicolectomy. We included patients from two tertiary colorectal centers (Odense University Hospital—Svendborg and Hospital of South West Jutland, Denmark) from September 2021 until May 2022. All surgical procedures were performed by certified colorectal and experienced laparoscopic surgeons, with 149 newly diagnosed cases of colon cancers per institution.17 We only included senior colorectal surgeons who had completed a training program in CME surgery to minimize selection bias. The surgeons were preoperatively supervised and examined by an experienced surgeon mastering the CME technique, who approved their achieved competencies to perform CME surgical procedures independently. Exclusion criteria for patients were as follows: (i) American Society of Anesthesiologists (ASA) > 3, (ii) open or robot-assisted surgical procedures, (iii) extracorporeal anastomosis, (iv) emergency surgery, (v) palliative resections, (vi) multivisceral resections, (vii) non-CME/conventional resection, (viii) in case of conversion to open surgery due to the inclusion of patients only having an ICA, and (ix) patients with an ostomy formed. The study was approved by the regional ethical committee/legal secretariat in the Region of Southern Denmark (ID number 22/26728) and conducted following the ethical guidelines of the Helsinki declaration.
2.2 Surgical procedure
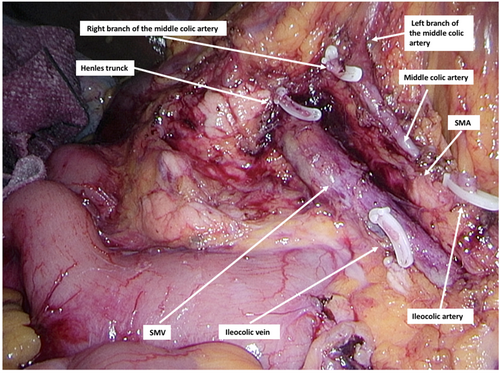
After establishing pneumoperitoneum and trocar placement, the ileocolic vessels were identified following a cranial retraction of the cecum and ligated at the root of the superior mesenteric vein (SMV) and superior mesenteric artery (SMA). The dissection continued along the SMV's anterior surface to identify the descending part of the duodenum and the pancreatic head in a medial-to-lateral direction. Right-sided colon vessels were centrally divided if present. The right branch of the middle colic artery was also isolated and transected at the root of superior mesenteric vessels (Figure 1). In case an extended right colectomy was performed, the dissection included a central ligation of the middle colic vessels at the root of the SMV and SMA. The dissection continued by incising the lesser sac and lateral peritoneal attachments of the ascending colon until the specimen could be transected at the terminal ileum and proximally corresponding to the transverse colon. An intracorporeal isoperistaltic side-to-side anastomosis was performed by incising the intestinal ends, followed by the insertion of EndoGIA 60 mm Tri-Staple™ Technology loaded with a gold cartridge. The enterotomy was closed with a continuous multifilament suture. No mechanical bowel preparation was administered. Patients had single-dose antibiotic prophylaxis routinely. All surgical procedures were performed according to the enhanced recovery after surgery (ERAS) principles, which in the perioperative course included carbohydrate administration and prophylaxis for postoperative nausea and vomiting (PONV). The patients were mobilized early and resumed oral nutrition, including fluids and solid intake; likewise, multimodal opioid-sparing treatment and venous thromboembolism prophylaxis were administered postoperatively.18
2.3 Outcomes of interest
We recorded the following baseline demographics: age, sex, body mass index (BMI), ASA score, World Health Organization performance status, Charlson Comorbidity Index, tumor location, UICC staging, and alcohol/tobacco consumption. Data on intraoperative morbidity and surgical efficacy were reviewed for the following outcomes: surgical time consumption, intraoperative injuries, intraoperative blood loss, length of hospital stay, time of first stool, history of prior abdominal surgery, 30 days readmission, and reoperation rates. We also evaluated the postoperative complication rates according to the Clavien–Dindo classification (≤30 days).19 The surgical specimens were reviewed for tumor- (T) and nodal (N) staging. Histopathological outcomes of interest included: histological type, the plane of surgical dissection (mesocolic, intramesocolic, or muscularis propria in case of disruption/defects in the mesocolon), microradicality of the procedure classified as either R0 (margin negative resection) or R1 (margin positive resection), and the amount of harvested lymph nodes. A diagnostic laparoscopy was performed if anastomotic leakage was clinically suspected or diagnosed by computed tomography.20
2.4 Statistics
| Case 1–31 | Case 32–61 | p | |
|---|---|---|---|
| Age (mean, SD) | 73.7 (10.4) | 75.4 (8.6) | 0.485 |
| Female/male | 17/14 (55%/45%) | 16/14 (53%/47%) | 1.000 |
| BMI (mean, SD) | 26.8 (4.6) | 27.1 (4.6) | 0.800 |
| WHO performance | 18/8/4 (58%/26%/13%) | 18/10/2 (60%/33%/7%) | 0.718 |
| WHO missing | 1 (3%) | 0 (0%) | |
| Active smoker | 3 (10%) | 3 (10%) | 1.000 |
| Alcohol > 14 items/week | 3 (10%) | 3 (10%) | 1.000 |
| ASA-score 1/2/3 | 2/20/9 (6%/65%/29%) | 0/25/5 (0%/83%/17%) | 0.175 |
| Prior abdominal surgery | 11 (37%) | 11 (37%) | 1.000 |
| CCI total score (mean, SD) | 3.6 (1.5) | 3.7 (2.1) | 0.963 |
| Tumor location | |||
|
12 (39%) | 11 (37%) | 0.726 |
|
5 (16%) | 8 (27%) | |
|
8 (26%) | 5 (17%) | |
|
6 (19%) | 6 (20%) | |
| UICC stage | |||
|
8 (26%) | 5 (17%) | 0.361 |
|
5 (16%) | 7 (23%) | |
|
18 (58%) | 18 (60%) | |
- Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CCI, Charlson Comorbidity Index; UICC, Union for International Cancer Control; WHO, World Health Organization.
2.5 Results
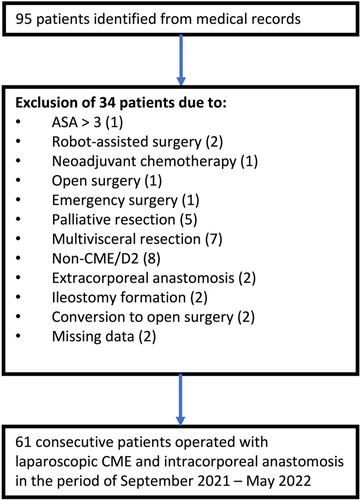
We identified 95 patients undergoing right-sided colectomy between September 2021 and May 2022. We excluded 34 patients who did not meet the inclusion criteria. Sixty-one patients were included in the final analysis (Figure 2). The cohort consisted of 28 (46%) men and 33 (54%) women with an overall mean age of 74.6 years (±9.5 SD). The preoperative baseline characteristics did not differ between the two groups, according to Table 1. The CUSUM estimated a peak in surgical time after 32 procedures, dividing the patient population into two groups (group 1—initial/learning phase: case 1–31 and group 2—plateau phase: case 32–61) (Figure 3).
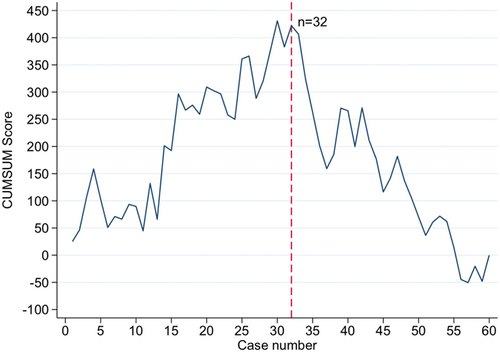
The intraoperative blood loss increased during the study period (group 1: 25 mL vs. group 2: 50 mL, p = 0.254). The length of hospitalization (3.0 days [IQR 2.0; 4.0]) and time to first stool (2.0 days [IQR 2.0; 3.0]) were equal in both groups (Table 2). No significant reduction in the thirty-day readmission rate (group 1: 16% and group 2: 14%, p = 1.000) or reoperation rate (group 1: 16% and group 2: 7%, p = 0.425) was demonstrated during the study period. A reduction in OT could be demonstrated between the two groups [group 1: 217.2 min (SD 53.6) vs. group 2: 191.6 min (SD 45.1), p = 0.051]. In total, nine patients (15%) had surgical complications, of which six (10%) were classified as major (>Clavien–Dindo II). A total of four patients (13%) had a major surgical complication in the learning phase, while this accounted for two patients (7%) in the plateau phase. The anastomotic leakage rate was 3% in group 1 and 0% in group 2 (Table 3).
| 1–31 cases | 32–61 cases | p | |
|---|---|---|---|
| Surgical duration, min (mean, SD) | 217.2 (53.6) | 191.6 (45.1) | 0.051 |
| Intraoperative blood loss, mL (mean, SD) | 25.0 (0.0; 100.0) | 50.0 (25.0; 100.0) | 0.254 |
| Blood transfusion | 1 (3%) | 0 (0%) | 1.000 |
| Intraoperative lesions | 1 (3%) | 1 (3%) | 1.000 |
|
0 (0%) | 1 (100%) | 1.000 |
|
1 (100%) | 0 (0%) | 1.000 |
| Length of hospital stay (median, IQR) | 3.0 [2.0; 4.0] | 3.0 [2.0; 4.0] | 0.607 |
| Time to first stool (median, IQR) | 2.0 [2.0; 3.0] | 2.0 [2.0; 3.0] | 0.590 |
| Readmission rate < 30 days | 5 (16%) | 4 (14%) | 1.000 |
| Readmission medical complications | 3 (10%) | 0 (0%) | 0.167 |
| Readmission surgical complications | 2 (6%) | 4 (14%) | 0.167 |
| Reoperation rate < 30 days | 5 (16%) | 2 (7%) | 0.425 |
| 30-days mortality | 0 (0%) | 1 (3%) | 0.492 |
- Abbreviation: IQR, interquartile range; SD, standard deviation.
| 1–31 cases | 32–61 cases | p | |
|---|---|---|---|
| Surgical complications < 30 days | 5 (16%) | 4 (13%) | 0.588 |
|
1 (3%) | 2 (7%) | 0.612 |
|
4 (13%) | 2 (7%) | 0.671 |
| Bleeding | 1 (3%) | 2 (7%) | 0.742 |
| Fascial dehiscence | 1 (3%) | 1 (3%) | 1.000 |
| Bowel obstruction | 1 (3%) | 1 (3%) | 1.000 |
| Intraabdominal abscess | 1 (3%) | 0 (0%) | 1.000 |
| Anastomotic leak | 1 (3%) | 0 (0%) | 1.000 |
| Medical complications < 30 days | 5 (16%) | 4 (13%) | 0.588 |
|
5 (16%) | 3 (10%) | 0.707 |
|
0 (0%) | 1 (3%) | 0.492 |
| Aspiration | 0 (0%) | 1 (3%) | 0.492 |
| Heart failure | 1 (3%) | 0 (0%) | 1.000 |
| Pulmonary embolism | 1 (3%) | 0 (0%) | 1.000 |
| Respiratory failure | 1 (0%) | 1 (3%) | 1.000 |
| Sepsis | 0 (0%) | 1 (3%) | 0.492 |
| Other medical complications | 2 (6%) | 1 (3%) | 1.000 |
Pathological specimen quality demonstrated a microradical resection rate of 98%, with 90% of cases performed in the mesocolic resection plane [group 1: 29 patients (94%), vs. group 2: 26 patients (87%), p = 0.425]. There was a slight but not significant increase in the amount of harvested lymph nodes between the two groups (group 1: 34.0 [IQR 27.0; 39.0], vs. group 2: 35.5 [IQR 27.0; 39.0], p = 0.425) (Table 4).
| 1–31 cases | 32–61 cases | p | |
|---|---|---|---|
| Histopathology | |||
|
25 (81%) | 22 (73%) | 0.563 |
|
2 (6%) | 4 (13%) | |
|
2 (6%) | 0 (0%) | |
|
2 (6%) | 4 (13%) | |
| pT-stage | |||
|
2 (6%) | 1 (3%) | 0.584 |
|
3 (10%) | 7 (23%) | |
|
22 (71%) | 18 (60%) | |
|
4 (13%) | 4 (13%) | |
| pN-stage | |||
|
21 (68%) | 22 (73%) | 0.719 |
|
6 (19%) | 3 (10%) | |
|
4 (13%) | 5 (17%) | |
| Microradical resection rate | 30 (97%) | 30 (100%) | 1.000 |
| Mesocolic plane of resection | 29 (94%) | 26 (87%) | 0.425 |
| Intramesocolic plane of resection | 2 (6%) | 4 (13%) | |
| Harvested lymph nodes (median, IQR) | 34.0 [27.0; 39.0] | 35.5 [31.0; 39.0] | 0.597 |
- Abbreviation: IQR, interquartile range.
2.6 Discussion
The current study demonstrated by the learning curve of OT that 32 procedures were required to obtain a plateau phase following the introduction of laparoscopic CME with an ICA technique for right-sided colon cancer.
CME is a principle including resection of the specimen in the mesocolic plane, respecting the embryological planes, and a central vascular ligation of the tumor supplying vascular structures. There has been a concern for increased morbidity rates due to intraoperative vascular injuries following the introduction of the CME technique. According to a newly published large scaled RCT study of patients undergoing CME versus non-CME for right-sided colon cancer, no significant increase in the amount of blood transfusion was noticed despite a higher rate of vascular injuries in the CME group.8 Recently, short-term results of D2 versus CME surgery for primary right-sided colon cancer have been published in randomized clinical trials suggesting comparable leakage and morbidity and mortality rates.8, 9, 25 These results were also reproduced in meta-analyses, including predominantly cohort studies.5, 26 However, we are still awaiting the long-term results of prospective studies, which may be able to clarify a possible benefit of applying the CME principle on cancer recurrence- and overall survival rates.9
Conducting an ICA using laparoscopy requires some surgical experience and technical skills in laparoscopic suturing compared to the extracorporeal technique. The two mentioned anastomotic techniques have recently been compared in several study designs. While some cohort studies suggest an enhanced recovery rate in favor of ICA, more newly published randomized controlled trials only confirmed a reduction in postoperative pain, not resulting in a decrease in time of hospitalization or morbidity rates.13, 14, 27 From our results, the learning curve of CME with ICA did not result in a reduced length of hospital stay or a faster time to bowel function when comparing the learning and plateau phase. It should be mentioned that the overall length of hospital stay in our study was short compared to other studies reporting the length of hospital stay varying from 5 to 9 days. Part of the explanation may be institutional and regional differences, patient demography, and strict adherence to ERAS protocol.8, 9, 28, 29 The rate of nonsevere complications (Clavien–Dindo I–II) represented the majority of cases in our study (18%). In comparison, the proportion of severe complications (>Clavien–Dindo III) was 11%, which is acceptable compared to previously published data.9, 25, 28, 29 Compared to other RCTs of CME versus non-CME resections for colon cancer, the overall morbidity rates of patients undergoing CME surgery are reported to be between 20.0% and 48%.8, 9, 25
Studies reporting learning curves within laparoscopic colorectal surgery have been published within the last decade. The general trend is a decreasing number of procedures to achieve the necessary surgical skills, most often demonstrated by CUSUM analyses. While some studies indicated learning curves varying from 55 to 150 cases to acquire necessary competencies dependent on the performed colorectal procedure, more recent studies have reported a need for significantly fewer cases (20–40 cases).16, 30, 31 Learning curves of technical competencies in laparoscopic colorectal surgery are often assessed by reporting the OT or conversion rates to open surgery measured as a surrogate marker for a successful surgical procedure. However, a decrease in surgical time solely does not equal competence. Most often, when conducting CUSUM analyses on operating time, a decline is seen in the initial phase. Following an implementation phase, where less complex cases are included, comes a maintenance phase. During the maintenance phase, the surgeons include more difficult cases entailing longer OT and more complications until the curve again decreases.32 Therefore, the procedure's complexity and surgeons’ experience should be considered essential factors influencing the learning curve, not OT as the only success parameter.
We use CUSUM analyses of the operation time as a surrogate measure of proficiency, but we also include patient characteristics and operational outcomes. All of these, combined, could aid in assessing the quality of the surgical methods when introducing a new procedure. There is no significant discrepancy in the baseline characteristics of the first 31 patients and the last 31 patients (ASA-score, BMI, UICC tumor stage, pTNM staging, or the amount of prior abdominal surgery), nor do we see an increase in operational time or an increase of complications from the first to the second group with no evidence of selection bias.
Little is published on objective proficiency standards or requirements in already certified surgeons implementing new operative methods. Using CUSUM analyses of operating time does not, as mentioned, specifically tell anything about the specific surgeon's proficiency but is often used as a surrogate marker of general proficiency or surgical method quality.33 Hence, structured and evidence-based assessments do not exist for all new surgical methods; the surgeons must rely on an apprenticeship model with critical and constructive feedback from the surgeons already experienced in the new procedure. However, this method suffers from subjective assessments that could be biased. Subsequently, dyadic procedures, where two experienced surgeons operate together and assist and discuss the course of the operation continuously, could increase the learning and quality during the introduction of a new surgical procedure.34
This study has some limitations, mainly due to the retrospective design causing a risk of residual confounding and selection bias. Multiple factors often bias studies exploring learning curves using the CUSUM analysis. These usually include a selection of previous well-experienced surgeons and more simple cases in the early cases when introducing new surgical techniques. It may positively influence the learning curve and possibly overestimate the results, which may raise questions about the external validity of data. The CUSUM analysis of OT is expressed as a deviation between the consumption of OT per case and the average mean OT of the total cohort. It is, therefore, an expression of the progression in OT per institutional level rather than individual level, thereby reducing the risk of heterogeneity. However, this study reflects real-time data of laparoscopic CME with ICA, including patients not selected preoperatively who were operated on up-front. All procedures (CME and ICA) were carried out systematically and uniformly based on a common consensus. Furthermore, the study was predesigned with clear inclusion and exclusion criteria to minimize the risk of selection bias.
2.7 Conclusion
In conclusion, some 32 cases of laparoscopic CME with ICA for right-sided colon cancer are required to achieve a plateau phase in the learning curve measured by the OT when operated by experienced colorectal surgeons.
AUTHOR CONTRIBUTIONS
Study conception and design: Pedja Cuk, Mark Bremholm Ellebæk, Issam Al-Najami, and Per Vadgaard Andersen. Data collection: Randi Maria Simonsen and Selab Sherzai. Analysis and interpretation of results: Pedja Cuk, Mark Bremholm Ellebæk, Issam Al-Najami, and Sören Möller. Drafting manuscript preparation: Pedja Cuk and Pia Iben Pietersen. All authors reviewed the results and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
SYNOPSIS
Learning curves of laparoscopic complete mesocolic excision (CME) for colon cancer with intracorporeal anastomosis have not been previously well examined. This study demonstrated that CME surgery could be safely implemented. In the case of previous experience with malignant laparoscopic colon cancer surgery, 32 cases were needed to achieve a plateau phase in the learning curve measured in operative time consumption.




