High concordance of 70-gene recurrence risk signature and 80-gene molecular subtyping signature between core needle biopsy and surgical resection specimens in early-stage breast cancer
Abstract
Background and Objectives
With increased neoadjuvant therapy recommendations for early-stage breast cancer patients due to the COVID-19 pandemic, it is imperative that molecular diagnostic assays provide reliable results from preoperative core needle biopsies (CNB). The study objective was to determine the concordance of MammaPrint and BluePrint results between matched CNB and surgical resection (SR) specimens.
Methods
Matched tumor specimens (n = 121) were prospectively collected from women enrolled in the FLEX trial (NCT03053193). Concordance is reported using overall percentage agreement and Cohen's kappa coefficient. Correlation is reported using Pearson correlation coefficient.
Results
We found good concordance for MammaPrint results between matched tumor samples (90.9%, κ = 0.817), and a very strong correlation of MammaPrint indices (r = 0.94). The concordance of BluePrint subtyping in matched samples was also excellent (98.3%).
Conclusions
CNB samples demonstrated high concordance with paired SR samples for MammaPrint risk classification and BluePrint molecular subtyping, suggesting that physicians are provided with accurate prognostic information that can be used to guide therapy decisions.
Abbreviations
-
- CNB
-
- core needle biopsy
-
- EBC
-
- early-stage breast cancer
-
- ER
-
- estrogen receptor
-
- FFPE
-
- formaldehyde-fixed paraffin-embedded
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HR
-
- hormone receptor
-
- IHC
-
- immunohistochemistry
-
- NPV
-
- negative predictive value
-
- PPV
-
- positive predictive value
-
- PR
-
- progesterone receptor
-
- SR
-
- surgical resection
-
- TN
-
- triple negative
1 INTRODUCTION
Neoadjuvant systemic therapy has been increasingly used as clinical trial results have demonstrated no difference in disease-free survival or overall survival compared with patients who received adjuvant therapy.1-3 In light of the extraordinary circumstances that the COVID-19 pandemic presented last year, use of neoadjuvant therapy has accelerated further. Health experts and professional societies, including the American College of Surgeons, published treatment guidelines to conserve supplies, triage cases, and reduce risk without compromising patient care.4-7 As a result, neoadjuvant therapy became even more prevalent in breast cancer treatment plans which requires prognostic information to be obtained from a core needle biopsy (CNB) before preoperative therapy.
Multigene assays, like MammaPrint, provide prognostic information that can guide physicians on therapy decisions for patients. However, multigene assays have typically been developed and tested using primary surgical specimens in an adjuvant setting. Only a few studies have determined the performance of multigene assays using CNB in recent years.8-11 In a large unpaired study, Jakubowski et al. found a similar range of Oncotype Dx Recurrence Scores between CNB and surgical resection (SR) samples (10–22 vs. 11–22, respectively).8 In three smaller studies using matched CNB and SR samples, researchers reported Pearson correlation coefficients ranging from 0.20 to 0.99 and overall concordance ranging from 72.0% to 95.0% using either EndoPredict, GenesWell, or Oncotype Dx multigene assays.9-11
MammaPrint is a 70-gene assay that can be used to predict the likelihood of recurrence and response to chemotherapy.12, 13 Patients identified as MammaPrint Low Risk can safely forego chemotherapy without compromising outcome, compared to those identified as High Risk for whom chemotherapy is recommended.13 MammaPrint has demonstrated precision (99.0%) and reproducibility (98.9%) in fresh frozen tissue, with 95% agreement between two sample sites from the same tumor.14 Use of formalin-fixed paraffin-embedded (FFPE) tissue, which make up most CNB samples, demonstrated very strong correlation (r = 0.92) and a 91.5% concordance of MammaPrint classification results with fresh tissue.15
In addition to risk classification, multigene assays, such as BluePrint, can be utilized to reliably determine the molecular subtype of women with early-stage breast cancer (EBC).16 We have previously tested the performance of BluePrint molecular subtyping in comparison with immunohistochemistry (IHC) using multiple cohorts and have demonstrated overall subtype reclassification of up to 30%.17-19 Additionally, tumors reclassified by MammaPrint and BluePrint exhibited more accurate pathological complete response (pCR) rates compared to pCR rates based on their respective clinical subtype.16, 18 These results support the importance of multigene assays in treatment decisions.
Though CNB samples have been used for MammaPrint and BluePrint in development and validation studies, as well as in clinical trials I-SPY2 (NCT01042379) and NBRST (NCT01479101), no study has directly compared CNB and SR specimens. In our current study, we have prospectively collected 139 matched CNB and SR specimens from women with confirmed EBC enrolled in the ongoing FLEX study (NCT03053193). The objective of our study is to determine the concordance of MammaPrint and BluePrint results between CNB and SR, to ensure reliable prognostic information can be captured from a CNB.
2 MATERIALS AND METHODS
2.1 Patient cohort
FLEX is an ongoing, multiinstitutional prospective study of patients with Stage I–III EBC. This study was conducted in accordance with the ethical standards established by the Declaration of Helsinki. The protocol was approved by Institutional Review Boards at all participating sites and registered with ClinicalTrials.gov (NCT03053193). Enrolled patients receive a MammaPrint test with or without BluePrint molecular subtyping and consent to clinically annotated gene expression data collection. Patients within the FLEX study (n = 139) with matched CNB and SR specimens were prospectively collected from six institutions. We excluded 18 patients due to neoadjuvant treatment, which resulted in 121 eligible matched CNB and SR tissues for this study.
2.2 Molecular classification
For this study, patients were identified in our FLEX patient database by their CNB MammaPrint result, and corresponding SR tissue was requested from their respective local institution. Upon receipt of SR FFPE tissue blocks at Agendia (Irvine, CA), sections were prepared. In accordance with diagnostic quality controls and standards, one section per sample was reviewed internally to verify >30% tumor cellularity. RNA was isolated with the RNeasy FFPE kit (Qiagen), and concentration measured by NanoDrop 2000 (Thermo Fisher Scientific). Complementary DNA was labeled and hybridized to 44k arrays and scanned using a dual laser scanner (Agilent Technologies) as previously described.15, 20, 21
MammaPrint Low Risk tumors have a MammaPrint index of >0.000; High Risk tumors have a MammaPrint Index of ≤0.000. BluePrint classifies tumors as Luminal-type, Basal-type, or human epidermal growth factor receptor 2 (HER2)-type. Each tumor sample is scored for all three subtypes, with the highest index representing the respective categorical tumor subtype.20 Together, MammaPrint and BluePrint stratify Luminal-type tumors into Luminal A (MammaPrint Low Risk) or Luminal B (MammaPrint High Risk). For this study, borderline samples (MammaPrint index between −0.05 and 0.05) were excluded due to a higher probability of technical inaccuracy, as reported in the FDA 510k summary (#K070675). It should be noted that borderline results occur in less than 5% of the total cases, and the likelihood of a borderline result is independent of sample type (i.e., CNB or SR).
2.3 Statistical analysis
A power analysis was conducted using Lin's Concordance Correlation Coefficient to evaluate the reproducibility of the two methods. The two one-sided option was chosen to test equivalence, assuming equivalence limit range of 90.0%–99.9% and expected correlation to be 0.90; 121 samples provide a power of 93.2%. Descriptive statistics were used to summarize patient clinicopathological characteristics. The primary objective of this study was to evaluate the concordance of MammaPrint results between CNB and SR, measured using overall percentage agreement, positive predictive value (PPV, High Risk), negative predictive value (NPV, Low Risk), and Cohen's kappa coefficient (k). The secondary objective was to determine the concordance of BluePrint molecular subtypes between matched CNB and SR samples. Pearson correlation coefficient (r) and Pearson correlation test were calculated using the MammaPrint index or BluePrint indices for CNB and SR specimens. Pearson Correlation values were interpreted as outlined by Schober, et. al.,22 where r = 0.40–0.69 is considered a “moderate correlation”, r = 0.70–0.89 is a “strong correlation”, and 0.90–1.00 is a “very strong correlation”. A p value less than 0.05 is considered statistically significant. Statistical analyses were performed using GraphPad Prism (version 9.0.2) and R (version 4.0.5).
3 RESULTS
3.1 Patient characteristics
A total of 121 patients from the FLEX study database with diagnostic MammaPrint and BluePrint results with matched CNB and SR specimens were included in this study. Table 1 summarizes clinicopathological characteristics of this cohort. Out of the 119 patients with documented age, the majority of patients were over the age of 50 (97/119; 81.5%). Of the 114 patients with clinical subtyping data, most patients (97/114; 85.1%) had hormone receptor (HR) positive/HER2 negative tumors, 10 (8.8%) had HR positive/HER2 positive tumors, and 7 (6.1%) had triple negative (TN) tumors. No patients in this cohort had HR negative/HER2 positive tumors. Out of 119 patient tumors, 36 (30.3%) were low grade (G1), 53 (44.5%) were intermediate grade (G2), and 30 (25.2%) were high grade (G3).
| Patient number (%) Total n = 121 | |
|---|---|
| Agea | |
| >50 | 97 (81.5%) |
| ≤50 | 22 (18.5%) |
| Clinical subtypeb* | |
| HR positive/HER2 negative | 97 (85.1%) |
| HR positive/HER2 positive | 10 (8.8%) |
| Triple negative | 7 (6.1%) |
| Tumor gradec | |
| G1 low grade | 36 (30.3%) |
| G2 intermediate grade | 53 (44.5%) |
| G3 high grade | 30 (25.2%) |
| Tumor staged | |
| T1 | 76 (66.7%) |
| T2 | 33 (28.9%) |
| T3 | 5 (4.4%) |
| Nodal statuse | |
| Negative | 53 (76.8%) |
| Positive | 16 (23.2%) |
| Surgery typef | |
| Lumpectomy | 30 (62.5%) |
| Mastectomy | 18 (37.5%) |
| Method of detectiong | |
| Clinical palpation/finding | 3 (2.5%) |
| Screening mammogram | 90 (75.6%) |
| Self-exam/patient discovered | 26 (21.8%) |
- Note: a,c,gn = 119, 1.7% unknown clinical data; b,dn = 114, 5.8% unknown clinical data; *HER2 equivocal patients (n = 3) counted as HER2 negative; en = 69, 41.3% unknown clinical data; fn = 48, 60.3% unknown clinical data.
- Abbreviation: HR, hormone receptor.
3.2 Concordance of MammaPrint results between CNB and SR tumor specimens
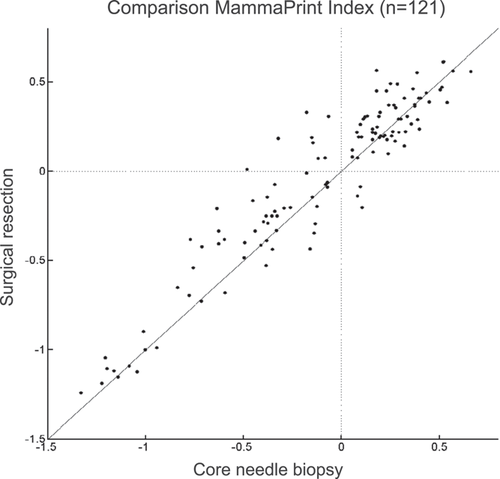
Overall, 50 patients had High Risk CNB and SR specimens and 60 had Low Risk CNB and SR specimens, resulting in 90.9% overall agreement (κ = 0.817), 95.2% NPV, and 86.2% PPV (Table 2). Out of the discordant samples, eight were High Risk on CNB and Low Risk on SR, whereas three were Low Risk on CNB and High Risk on SR. A Pearson correlation test of MammaPrint indices between 121 CNB and SR specimens was performed and resulted in a very strong correlation of r = 0.94 (p < 0.0001) (Figure 1).
| SR | |||
|---|---|---|---|
| MammaPrint result | High risk | Low risk | Total |
| CNB | |||
| High risk | 50 | 8 | 58 |
| Low risk | 3 | 60 | 63 |
| Total | 53 | 68 | 121 |
- Abbreviations: CNB, core needle biopsy; SR, surgical resection.
3.3 Concordance of BluePrint molecular subtyping between CNB and SR tumor specimens
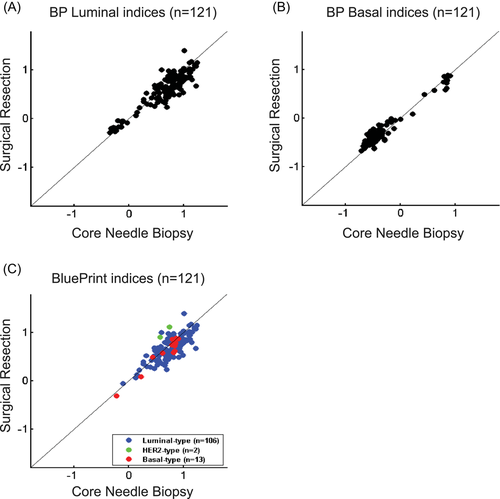
CNB and SR tumors were in agreement for 105/106 Luminal-type (99.1%), 2/2 HER2-type (100%), and 12/13 Basal-type (92.3%) tumors (Table 3). Overall, we determined the concordance of BluePrint between CNB and SR to be 98.3%. In addition, we observed very strong correlations of BluePrint index scores between CNB and SR specimens for Luminal-type (r = 0.92; p < 0.0001) and Basal-type (r = 0.97; p < 0.0001) tumors (Figure 2). We did not determine the Pearson correlation coefficient for HER2-type patients due to too few by BluePrint (n = 2).
| SR | ||||
|---|---|---|---|---|
| BluePrint result | Luminal-type | HER2-type | Basal-type | Total |
| CNB | ||||
| Luminal-type | 105 | 0 | 1 | 106 |
| HER2-type | 0 | 2 | 0 | 2 |
| Basal-type | 1 | 0 | 12 | 13 |
| Total | 106 | 2 | 13 | 121 |
- Abbreviations: CNB, core needle biopsy; SR, surgical resection.
4 DISCUSSION
In recent years, neoadjuvant therapy has been increasingly used for tumor and/or nodal downstaging, monitoring treatment response, and to allow time for genomic testing, which has expanded the number of patients receiving genomic testing and helped streamline care.23 With an overall increase in neoadjuvant therapy and where triaging patients for neoadjuvant therapy is recommended (e.g., during the COVID-19 pandemic4-7), physicians can use molecular subtyping and risk classification information in addition to clinical pathology on preoperative core biopsies, which can guide therapy decision making. The standard of care method for breast cancer subtype classification and treatment decisions are based on clinical features (e.g., tumor grade, lymph node status, receptor status, and Ki-67) as determined by IHC/FISH. Multiple studies have analyzed the concordance of CNB and SR specimens in tumor grade, with overall agreement ranging from 64% to 77%.24-27 In addition, numerous reports comparing SR and CNB samples in estrogen receptor, progesterone receptor, HER2, and Ki-67 IHC staining have been performed with overall agreement ranging from 84.0% to 99.1%, 77.9% to 94.3%, 80.0% to 98.8%, and 79.5% to 87.0% respectively, and a wide range of overall concordance (75%–97%).26, 28-35
Based on our previous performance and concordance studies using MammaPrint and BluePrint,20, 36, 37 we anticipated the overall agreement of MammaPrint results between CNB and SR tumor samples to be similar. As expected, we observed an overall agreement of 90.9%. In the current study, 11/121 samples were discordant between CNB and SR, with 8 samples having High Risk CNB results and Low Risk SR results, and 3 samples with Low Risk CNB results and High Risk SR results. It is important to note that discordance between sample type reflects the complex tumor heterogeneity, where sample size and sample location within the tumor can lead to differences in risk assessment, but both are accurate results. Therefore, High Risk CNB results reflect a portion of the tumor that was High Risk, even if the SR specimen was Low Risk, and recommended therapeutic options would follow guidelines for High Risk tumors. Importantly, clinically relevant discordant cases that affect treatment decisions are cases in which CNB is Low Risk and SR is High Risk, which account for approximately 2.5% of patients in the current analysis. In these few cases, the High Risk tumor biology not captured on the preoperative CNB would potentially underestimate treatment decisions. Overall, these results confirm that MammaPrint risk classification from CNB are in high agreement with SR and provide consistent and accurate results. Thus, for more than 97% of patients in this study, treatment decisions and potential outcome are precise and consistent based on MammaPrint testing of the CNB.
In addition to our primary analysis, we determined the concordance of BluePrint genomic subtyping between the matched samples. Out of 121 tumors, 119 were in agreement, resulting in an overall concordance of 98.3%; superior to concordance rates of 75.0%–87.5% among intrinsic biological subtypes based on IHC assessment.26-28 Molecular subtyping results have demonstrated more accurate prediction of a pCR and outcome in comparison with IHC assessment.17, 38, 39 Although reclassification by BluePrint was not an objective of this study, it was notable that 8 of the 10 patients clinically identified as HER2+ were reclassified as Luminal-type by BluePrint. Additionally, a CNB provides accurate and fast results that can be used for treatment decisions, and shortens time-to-treat, which can ultimately improve patient outcomes.40 A limitation of our paired study is data maturity, in which patient follow-up data is currently unavailable to correlate outcome with MammaPrint and BluePrint results from CNB and SR samples. However, several studies, including the NBRST trial (NCT01479101), ISPY1 (NCT00033397), and ISPY2 (NCT01042379), have demonstrated accurate prediction in neoadjuvant treatment response and long-term outcome by MammaPrint and BluePrint on core needle biopsies.17, 39, 41-43
5 CONCLUSIONS
In summary, this analysis represents the largest powered study using prospective real-world data to evaluate the concordance of a genomic assay on matched CNB and SR samples. The high concordance rates of MammaPrint and BluePrint results between paired samples strongly support the utility of these assays to obtain reliable prognostic information on core biopsy tissue, which can guide timely and appropriate treatment decisions.
ACKNOWLEDGMENTS
We would like to thank all the patients who volunteered to participate in FLEX, as well as the FLEX site coordinators and investigators. We would also like to thank the following people for their contribution to this manuscript: Jeffrey Falk, Jia-Perng Wei, Sammy Mee, Jake Ruby, Suoyi Yang, Yen Huynh, Anke Witteveen, Christine Finn, Kate Corcoran, Christa Dreezen, Andrea Menicucci, Annie Tran, Erin Yoder, and Bastiaan van der Baan. Research was supported by Agendia Inc.
CONFLICT OF INTERESTS
Jennifer A. Crozier, Julie Barone, Pat Whitworth, Abraham Cheong, Robert Maganini, and Jose Perez Tamayo are FLEX principal investigators and have contracted research with Agendia Inc. Jennifer A. Crozier and Robert Maganini received honoraria as part of Speaker's Bureau for Agendia Inc. Patricia Dauer, Shiyu Wang, and William Audeh, are noncommercial employees of Agendia Inc, Irvine, CA. Annuska Glas is a noncommercial employee of Agendia NV, Amsterdam, The Netherlands. Annuska Glas is a coinventor of the BluePrint 80-gene signature and is a full-time employee of Agendia, NV (Patent Numbers: 9175351, 10072301). No other conflict of interests were reported.
AUTHOR CONTRIBUTIONS
Patricia Dauer, Shiyu Wang, William Audeh, and Annuska Glas: contributed to data analysis and interpretation. Jennifer A. Crozier, Patricia Dauer, and Shiyu Wang: contributed to manuscript preparation. All authors participated in the review and editing of the manuscript for publication. All authors contributed to the study design and conceptualization. All authors contributed to collection and assembly of data.
Open Research
DATA AVAILABILITY STATEMENT
The clinical datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Raw array data have not been made publicly available as part of a collaboration agreement with the diagnostic company Agendia Inc.
REFERENCES
SYNOPSIS
This study is the largest and only powered analysis of paired breast cancer core needle biopsy and surgical resection specimens. We found high concordance between paired samples using MammaPrint and BluePrint, which indicates that MammaPrint and BluePrint assays can be performed on core needle biopsies for reliable prognostic information.