Cefuroxime hydrolysis kinetics and stability predictions in aqueous solution
Abstract
Cefuroxime hydrolysis rate constants (k) were determined to predict the degradation rate of cefuroxime in aqueous solution as a function of pH, temperature, and buffer. At constant temperature, the pH-rate expression was: k = kH(aH+) + ks1f1 + ks2f2 + kOH(a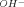 ), where f1 is the anionic fraction of cefuroxime in the undissociated form and f2 is the anionic fraction, kH and kOH are the catalytic rate constants for hydrogen activity (aH+) and hydroxyl ion activity (a
), where f1 is the anionic fraction of cefuroxime in the undissociated form and f2 is the anionic fraction, kH and kOH are the catalytic rate constants for hydrogen activity (aH+) and hydroxyl ion activity (a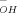 ), and ks1 and kS2 are first-order rate constants for spontaneous hydrolysis. Formate, acetate, phosphate, and borate buffers did not catalyze degradation. Temperature dependencies for kH, kS1, kS2, and kOH were described with values for A (pre-exponential term) and E (energy of activation) calculated from k = Ae−EIRT (where R is 1.987 cal/mol-deg and T is absolute temperature). Combining the pH and temperature equations allowed predictions for cefuroxime hydrolysis rates in aqueous solutions at any pH and temperature. Results were validated by predicting the observed rate constants for every experimental condition and also for a reconstituted commercial product stored at 30 °C. Maximum stability was observed in the pH-independent region from pH 4 to 7, where the time during which cefuroxime concentration exceeded 90% of its initial concentration at 25 °C was 1.2 days. Rate constants employed in predictions were based on stability-indicating HPLC assays. For selected conditions, additional rate constants were calculated from changes in cefuroxime UV absorbance. These UV constants did not consistently agree with the k values obtained with HPLC assays or with either of the rate constants determined for loss of cefuroxime to descarbamoyl cefuroxime or to the competing route, which is primarily β-lactam hydrolysis.
), and ks1 and kS2 are first-order rate constants for spontaneous hydrolysis. Formate, acetate, phosphate, and borate buffers did not catalyze degradation. Temperature dependencies for kH, kS1, kS2, and kOH were described with values for A (pre-exponential term) and E (energy of activation) calculated from k = Ae−EIRT (where R is 1.987 cal/mol-deg and T is absolute temperature). Combining the pH and temperature equations allowed predictions for cefuroxime hydrolysis rates in aqueous solutions at any pH and temperature. Results were validated by predicting the observed rate constants for every experimental condition and also for a reconstituted commercial product stored at 30 °C. Maximum stability was observed in the pH-independent region from pH 4 to 7, where the time during which cefuroxime concentration exceeded 90% of its initial concentration at 25 °C was 1.2 days. Rate constants employed in predictions were based on stability-indicating HPLC assays. For selected conditions, additional rate constants were calculated from changes in cefuroxime UV absorbance. These UV constants did not consistently agree with the k values obtained with HPLC assays or with either of the rate constants determined for loss of cefuroxime to descarbamoyl cefuroxime or to the competing route, which is primarily β-lactam hydrolysis.




