Selective Oxidation of Methionine and Tryptophan Residues in a Therapeutic IgG1 Molecule
Abstract
Oxidation of methionine and tryptophan are common degradation pathways for monoclonal antibodies and present major analytical challenges in biotechnology. Generally, protein oxidation is detectable in stability and/or stressed samples (e.g., exposed to hydrogen peroxide, UV light, or metal ions). The induced chemical modifications may impact the biological activity of antibodies and may have biological consequences. However, these effects and the contribution of individual protein modifications are difficult to delineate as different amino acids are often oxidized simultaneously and accompanied by other degradants such as aggregates, especially in forced degradation studies. Here, we report a new method to obtain selective oxidation of methionine or tryptophan by using oxidation reagents combined with large excess of free tryptophan or methionine, correspondingly. More specifically, using hydrogen peroxide or tert-butyl hydroperoxide in combination with addition of free tryptophan allowed for selective oxidation of methionine. Conversely, the use of 2,2-azobis(2-amidinopropane) dihydrochloride in combination with free methionine resulted in selective tryptophan oxidation, whereas methionine oxidation was not significantly altered. This novel stress model system may prove to be valuable tool in future mechanistic studies of oxidative degradation of protein therapeutics. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2824–2831, 2015
INTRODUCTION
Oxidation is a common degradation pathway that may occur during the life cycle of proteins and peptides.1-5 The resulting chemical modifications may affect in vitro stability and in vivo biological functions.5-17 The position of oxidized amino acids in the protein sequence is crucial for their impact on protein structure, function, and biological response, especially in the case of monoclonal antibodies (mAbs) where binding properties are essential to reach target antigen. It has been previously reported that oxidation of methionine from the Fc region can reduce binding to the neonatal Fc receptor and biological half-life of the concerned mAb.8, 10, 11 Such a change in the pharmacokinetic profile is highly dependent on the position of the oxidation. A recent study reported that M252 oxidation was the dominant contributor to PK impairment observed in mice transgenic for the human FcRn receptor. In this study, an increased plasma clearance was only observed if both IgG HCs were oxidized at M252.17 Moreover, the formation of oxidized methionine or tryptophan in the complementary determining regions may induce loss of potency and antigen-binding capabilities.14, 15
During the last 30 years, cysteine, histidine, methionine, phenylalanine, tryptophan, and tyrosine residues were identified as major oxidation sites in proteins because of the high reactivity of sulfur atoms and aromatic rings toward various reactive oxygen species.5, 18 Oxidation might be induced during production, processing, or storage (e.g. because of the possible reactive impurities present or formed in excipients such as polyethylene glycol or polysorbate).19, 20 The generation of peroxides through autooxidation of these polymeric compounds might favor oxidation of these amino acid residues. The presence of transition metals may also lead to oxidation, for example, via the Fenton reaction.21, 22 One option to minimize or prevent oxidation of the active protein is the addition of antioxidants, chelating agent, or radical scavengers to the formulation buffer, if acceptable from a toxicological and regulatory perspective and thus, if considered acceptable and safe to clinical use.20, 22, 23
In order to assess the potential susceptibility of protein products to oxidation, different oxidation stress conditions have been studied, such as hydrogen peroxide (H2O2), tert-butyl hydroperoxide (t-BHP), 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH), transition metal ions, or UV exposure.15, 22-25 Such relatively harsh treatments often result in a myriad of modifications on several amino-acid residues, such as histidine, phenylalanine, and tyrosine, for example.5, 22, 26 However, methionine and tryptophan are the residues that are oxidized most commonly and to the highest extent when exposed to such conditions.23, 24, 27-29
In recent investigations, antibody samples containing both methionine and tryptophan oxidation products were used to draw conclusions regarding impact on target binding and reduced binding affinity.14-16 Still, one major impediment to the interpretation of such studies is the fact that frequently both methionine and tryptophan residues are oxidized concurrently, making the definition of the individual contribution of these residues to the overall effects difficult to delineate. Thus, the preparation of proteins in which methionine and tryptophan have been selectively oxidized would be very helpful, in order to improve the understanding of the relationship between structure and functional consequences.
In a recent report, it has been demonstrated that the addition of free L-methionine and L-tryptophan to forced oxidation reactions of parathyroid hormone can effectively limit the oxidation of methionine and tryptophan residues, respectively.22 However, so far, this observation has not been further tested and reported when mAbs are used. In the present study, we demonstrate the utility of this competition approach using a new method for selective oxidation of methionine or tryptophan residues in a model mAb from the IgG1 subclass (the experimental strategy is depicted in Fig. 1). In order to achieve this aim, a large excess of free amino acids was added to the reaction conditions, in order to protect the corresponding amino acid in the mAb when applying different oxidants (t-BHP, H2O2, AAPH). Different reaction conditions such as incubation temperature, reaction time, and oxidant concentration were evaluated. Oxidation products in the model IgG1 were characterized using size-exclusion chromatography (SEC), protein A-based affinity chromatography, reversed-phase high-pressure liquid chromatographic (RP-HPLC), and mass spectrometry.
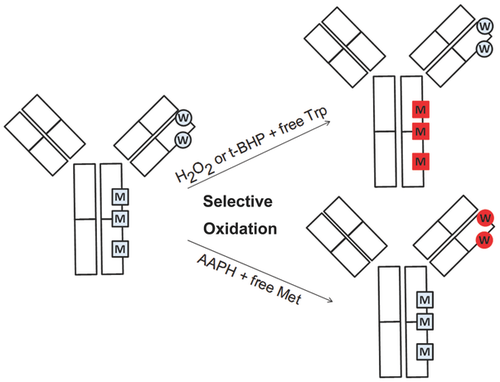
MATERIALS AND METHODS
An IgG1 therapeutic mAb was chosen as a model protein for the present study. The mAb (IgG1) solution was provided at 25 mg/mL in 51 mM sodium phosphate, 6 % trehalose, and 0.04 % polysorbate 20 (pH 6.2) by F. Hoffmann-LaRoche Ltd. (Basel, Switzerland).
Hydrogen peroxide, t-BHP, AAPH, ethylenediaminetetraacetic acid (EDTA), DTT (dithiothreitol), iodoacetic acid, and cysteine were purchased from Sigma–Aldrich (St. Louis, Missouri), and Dulbecco's phosphate-buffered saline 10× from GIBCO (Invitrogen, San Diego, California). Guanidine hydrochloride (Gdn-HCl) from AppliChem (Darmstadt, Germany) was used during the study.
Trifluoroacetic acid (TFA), formic acid (FA), acetic acid (AcOH), acetonitrile, hydrochloric acid (HCl), potassium chloride (KCl), and sodium chloride (NaCl) were purchased from Merck (Whitehouse Station, New Jersey). Tris(hydroxymethyl)-aminomethan (Tris) was obtained from Ajinomoto (Raleigh, North Carolina).
Papain from Carica papaya (10 mg/mL) was delivered by Roche Diagnostic (Indianapolis, Indiana). Trypsin Proteomics grade was provided by Roche Diagnostics GmbH (Penzberg, Germany).
L-tryptophan was purchased from Sigma–Aldrich and was dissolved directly in mAb solution to reach a final tryptophan concentration of 4 mg/mL in solution (19.6 mM). H2O2 or t-BHP were used as oxidants and added into the mAb solution with or without prior addition of free tryptophan to obtain final concentrations of oxidants between 0% and 5%.
L-methionine was purchased from Sigma–Aldrich and was dissolved directly in mAb solution to reach a final methionine concentration of 40 mg/mL in solution (0.26 M). As oxidant, AAPH was then added into the mAb solution in the presence or absence of free methionine to final oxidant concentrations between 0% and 5%.
The final mAb concentration for each prepared sample was 18.5 mg/mL. Samples were incubated under protection from direct light at 5°C, 25°C, or 40°C and aliquots were collected at 1, 6, 24, or 120 h. A control sample, without oxidant, was also incubated and analyzed for each time point and temperature. The oxidation was stopped by buffer exchange using disposable PD-10 desalting columns (GE Healthcare, Buckinghamshire, UK).
INSTRUMENTATION AND SAMPLE ANALYSIS
A Waters Alliance 2695 HPLC system equipped with a 2487 UV detector and Empower2 software (version 7) (Waters, Milford, Massachusetts) was used throughout the study. The determination of protein concentration before injection was performed on a SoloVPE spectrometer from C-Technologies (Bridgewater, New Jersey).
Size-Exclusion HPLC
Samples were analyzed by UV absorbance detection at 280 nm. A TSK G3000 SWXL column (5 μm, 250A, 7.8 × 300 mm2) (Tosoh Bioscience, Tokyo, Japan) was used for separation. The mobile phase (200 mM phosphate, 250 mM KCl, pH 7.0) was pumped at a flow rate of 0.5 mL/min. Sample size for analysis was 25 μg. The stationary phase was kept at 25 ± 2°C.
Protein A Chromatography
Analytical protein A chromatography was performed, as described previously on a POROS® A (4.6 × 50 mm2) column (Applied Biosystems, Foster City, California).12 The stationary phase was kept at 23 ± 2°C.
Reversed-Phase HPLC
Protein samples were diluted to a final concentration of 1 mg/mL in Tris buffer (0.1 M Tris, 4 mM EDTA, and 1 mM cysteine, pH 7.4). Fifty microliter of 0.1 mg/mL papain was added to 500 μL of the mAb1 solution before incubation at 37°C for 2 h. Following incubation, 11 μL of 1 M DTT was added to each tube and samples were incubated for another 30 min at 37°C. Analysis of oxidized and intact mAb was carried out using a BioBasic Phenyl column (5 μm, 300A, 2.1 × 250 mm2) from Thermo Fischer Scientific (Dreieich, Germany). Mobile phase A was 0.09% TFA in water. Mobile phase B was 0.08% TFA in acetonitrile. Flow rate was 0.45 mL/min. The chromatography was performed using a linear gradient from 35% to 40% solvent B in 16 min. Mobile phase B (40%) was maintained during 4 min and finally 35% solvent B was pumped during 10 min. Sample size for analysis was 8 μg. The fragment elution was monitored by UV absorption at 215 nm. As the method described above cannot be used to identify the tryptophan oxidation site in the protein sequence, it was utilized only as a rapid screening tool for tryptophan oxidation present in the fragment antigen-binding part of the heavy chain (HC-Fab). The tryptophan oxidation fraction was calculated by dividing the area of the prepeak by the total peak area of the HC-Fab peaks.
Proteolytic Digest of mAb
For detection and quantification of oxidized amino acids, the mAb was digested with trypsin. First, the protein was denatured in 0.4 M Tris–HCl, 8 M Gdn-HCl, at pH 8.5 by diluting 350 μg of oxidized mAb in a total volume of 300 μL. For reduction, 10 μL of 100 mg/mL DTT were added and incubated at 50°C for 1 h. After alkylation of free cysteines by adding 10 μL of 330 mg/mL iodoacetic acid and incubation at room temperature in the dark for 30 min, the buffer was exchanged to digestion buffer (0.1 M Tris–HCl, pH 7.0) by using a NAP5 gel filtration column. Subsequently, NAP5-eluate (500 μL) was mixed with 10 μL of a solution of 0.25 mg/mL trypsin in 10 mM HCl and incubated at 37°C for 18 h. The digestion reaction was stopped by adding 50 μL of a 10% TFA solution.
The tryptic peptide mixture was separated by reversed-phase UPLC (ACQUITY; Waters, Manchester, UK) on a C18 column (BEH C18 1.7 μm, 2.1 × 150 mm2; Waters) and the eluate was analyzed online on a Q-TOF SYNAPT G2 instrument (Waters). The mobile phases of RP-HPLC consisted of 0.1% FA in water (solvent A) and 0.1% FA in acetonitrile (solvent B). The chromatography was performed using a linear gradient from 1% to 35% solvent B in 45 min and finally from 35% to 80% solvent B in 3 min using a flow rate of 300 μL/min. Three microgram digested protein was applied. UPLC-system and mass spectrometer were connected by PEEK capillary tubes. Data acquisition was controlled by MassLynxTM software (Waters).
Tandem mass-spectrometry (MS/MS) experiments were performed on a LTQ Orbitrap Velos electrospray mass spectrometer (Thermo Fisher Scientific) using the chromatographic system described above in order to identify the oxidized peptides. The fragmentation was induced by low-energy CID using helium as collision gas (“top5” mode). Data acquisition was controlled by Xcalibur software (Thermo Fisher Scientific) using a manual acquisition mode. Oxidized amino acids were identified from the MS/MS data using the software Proteomics Discoverer (Thermo Fisher Scientific). The collision energy was adjusted according to stability and mass of the parent ion.
Peptides of interest were identified manually by searching for their theoretical m/z values within the experimental mass spectrum. For their quantification, specific ion current chromatograms of peptides of interest were generated on the basis of their monoisotopic mass and detected charge states using GRAMS AI software (Thermo Fisher Scientific). Relative amounts of nonoxidized and oxidized peptides were calculated by manual integration of the corresponding peaks.
Besides the summarized large number of methionine and tryptophan oxidation sites (Table 1; Tables S1 and S2), no significant levels of additional amino acid oxidations events (e.g., at histidine residues) were detected by careful inspection of the experimental mass spectra.
| Parameter | Value/Range |
|---|---|
| Oxidant | H2O2, t-BHP, AAPH |
| Oxidant concentration | 0.0%, 0.05%, 0.1%, 0.2%, 0.5%, 1.0%, 2.0%, 5.0% |
| Antioxidant | None, L-methionine (0.26 M), L-tryptophan (19.6 mM) |
| Incubation temperature | 5°C, 25°C, 40°C |
| Incubation time | 1, 6, 24, 120 h |
| Model mAb concentration | 18.5 mg/mL |
- The oxidant, oxidant concentration, antioxidant, incubation temperature, and time were varied to find optimal condition for selective oxidation of methionine or tryptophan, respectively. The mAb concentration was kept constant for the complete set of samples.
RESULTS AND DISCUSSION
In forced oxidation studies of therapeutic proteins, widely varying extents of methionine oxidation (from 0% to 100%) were reported. In these reports, the most commonly used oxidative reagents were H2O2 and t-BHP with reaction conditions spanning a broad range, including different temperatures (room temperature or 40°C), different concentrations of the oxidative agent (0.0005%–0.2% for H2O2, 0.05%–5% for t-BHP), and different reaction times (6 h to 7 days).12-14, 22 One drawback of such oxidation studies is the possible co-oxidation of tryptophan and methionine. This effect was reported for the oxidation of a recombinant antibody performed with 0.05% t-BHP at room temperature for 7 days where 65% methionine oxidation and 25% tryptophan oxidation were detected in the same sample.14 One other example of co-oxidation of tryptophan and methionine was shown during the reaction of parathyroid hormone with AAPH. Indeed, incubation of 0.1 mg/mL parathyroid hormone with 0.1 M AAPH at 40°C for 24 h resulted in 84% of tryptophan oxidation and 58% of methionine oxidation.22 After reacting human growth hormone with AAPH, Steinmann et al.30 reported a multitude of oxidized residues, including 20% tryptophan oxidation and significant methionine oxidation (100% M14; 80% M125; 70% M170).
Indeed, the use of established conditions for forced oxidation available in literature did not allow us to selectively oxidize neither methionine nor tryptophan amino acids in a mAb model protein as depicted in Figures 2–4, and Supplementary Tables 1 and 2. Instead, extensive co-oxidation of both methionine and tryptophan was present under these conditions.
To identify optimal conditions leading to selective oxidation of methionine or tryptophan in a mAb, a large set of parameters were evaluated based on published studies and previous in-house experiments (data not shown).12, 14, 22 A model IgG1 was incubated with different oxidants while the reaction times and incubation temperatures were varied as described in Table 2. To curtail oxidation of the amino acids methionine or tryptophan, the corresponding free amino (L-methionine or L-tryptophan) acids were added to the reaction mixtures. After completion of all reactions, samples were screened for total oxidation levels using protein A analytical chromatography and RP-HPLC. Selected samples were further analyzed using LC–MS and LC–MS/MS in order to identify and semiquantify the methionine and tryptophan oxidation sites in the amino-acid sequence.
| Oxidation with/Without Tryptophan Protection | |||||
|---|---|---|---|---|---|
| Modified | 1% H2O2 | 1% H2O2 | 2% t-BHP | 2% t-BHP 25°C | |
| Amino Acids | Control | 5°C 24 h | 5°C 24 h with Tryptophan | 25°C 120 h | 120 h with Tryptophan |
| LC M4ox | 0.3 | 4.4 | 0.9 | 4.1 | 3.8 |
| HC M34ox | 0.7 | 7.4 | 2.5 | 6.9 | 7.4 |
| HC M83ox | 0.6 | 4.1 | 0.7 | 4.2 | 4.0 |
| HC M258ox | 4.4 | 99.6 | 99.6 | 99.7 | 99.7 |
| HC M364ox | 0.4 | 90.8 | 92.9 | 91.0 | 91.6 |
| HC M434ox | 1.2 | 96.1 | 96.1 | 96.1 | 96.7 |
| LC W35ox+4 | 0.2 | 0.6 | 0.5 | 0.6 | 0.5 |
| LC W35ox+16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| LC W96ox+4 | 0.1 | 0.3 | 0.2 | 0.4 | 0.2 |
| LC W96ox+16 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| HC W50ox+4 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
| HC W50ox+16 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 |
| HC W50ox+32 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| HC W108ox+4 | 0.0 | 0.0 | 0.0 | 1.5 | 0.3 |
| HC W108ox+16 | 0.2 | 0.3 | 0.2 | 2.6 | 0.6 |
| HC W108ox+32 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| HC W283ox+4 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| HC W283ox+16 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
| HC W319ox+4 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| HC W319ox+16 | 0.4 | 0.8 | 0.6 | 0.8 | 0.7 |
| HC W319ox+32 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
- The mAb was denatured and the disulfide bridges were reduced using DTT. Alkylation of free cysteines was performed using iodoacetic acid. After a buffer exchange, the mAb was finally digested with trypsin. LC stands for light chain, HC stands for heavy chain. Methionine (M) and tryptophan (W) oxidation levels were quantified.
In the model IgG1 used in this study, several methionine residues were identified as susceptible to oxidation (M4, M34, M83, M258, M364, and M434). Basal levels of oxidation of these susceptible residues were identified in the starting material (Table 1). From the oxidized tryptophan identified by LC–MS/MS (W35, W50, W96, W108, W283, and W319), only surface-exposed W50 and W108 showed significant changes between unstressed and stressed samples.
Selective Methionine Oxidation
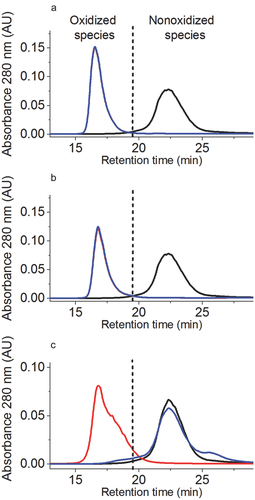
Oxidation levels of Fc methionine were first quantified by protein A chromatography. The nonoxidized antibody species eluted at 23 min (Fig. 2). The oxidized species formed upon incubation with oxidant eluted earlier (before 19.8 min) because of the lower affinity of oxidized Fc methionine to protein A.7, 12 As depicted in Figures 2a and 2b, the incubation of the mAb with 1% H2O2 for 24 h at 5°C as well as the incubation with 2% t-BHP for 120 h at 25°C allowed for the nearly complete oxidation of the methionine residues from the Fc region.
The extent of methionine oxidation was also determined by selected ion current chromatogram analysis of the oxidized peptides (Table 1). We found more than 91% oxidation for M364, 96% oxidation for M434, and 99% oxidation for M258 according to LC–MS results.
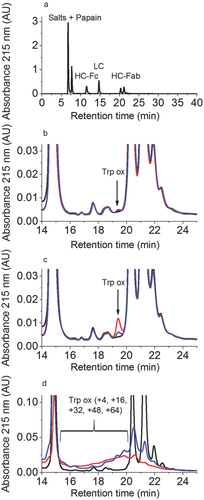
In order to assess the total tryptophan oxidation of the HC-Fab, the oxidized samples were first digested with papain and then the disulfide bridges were reduced with DTT. The generated LC, HC-Fc, and HC-Fab were then separated by RP-HPLC (for details see Materials and Methods) as presented in Figure 3a, and the tryptophan oxidation of the HC-Fab was quantified. The oxidized species because of tryptophan oxidation elute before the HC-Fab peaks. The peak with a retention time of 19.5 min represents in that case mainly oxidized tryptophan W108 of the HC-Fab (Figs. 3b and 3c; signal labeled as “Trp-ox”).
As previously reported, the oxidation of mAb with H2O2 or t-BHP induced relatively low tryptophan oxidation levels (see Figs. 3b and 3c).22 The well-resolved signal with a retention time of 19.5 min (corresponding to tryptophan oxidation products) increased not only with increasing H2O2 or t-BHP concentrations, but also when increasing the reaction time and the temperature of incubation (data not shown). In order to exclude the possibility of metal ion contamination resulting in Fenton-like reactions, protein oxidation reactions were carried out in the presence and absence of EDTA. As no differences in the oxidation levels of Trp were detected (data not shown), the mechanism of Trp oxidation in these reactions remains unclear. To avoid this unwanted tryptophan oxidation, reaction conditions were set as H2O2 oxidation at 5°C for 24 h and t-BHP oxidation at 25°C for 120 h. Moreover, the tryptophan oxidation could be inhibited using addition of free tryptophan at concentrations of 19.6 mM during the incubations as depicted in Figures 3b and 3c (for detailed LC–MS, results see Table 2).
LC–MS analyses confirmed the low abundance of tryptophan oxidation (Table 1), and as a consequence the protective effect of free tryptophan against tryptophan oxidation. According to LC–MS results, the total oxidation of W50 and W108 was lower than 1% when adding free tryptophan before oxidation with t-BHP (2%, 25°C, 120 h). In the case of H2O2 oxidation (1%, 5°C, 24 h), where tryptophan oxidation products were present at very low level (<1%), the addition of free tryptophan could not protect against the formation of low levels of residual tryptophan oxidation products (see Table 1).
In addition to free tryptophan, which has been shown to protect tryptophan residues in proteins from oxidation, other antioxidants could potentially be used to avoid tryptophan oxidation.20, 21 However, the addition of antioxidant should be investigated in detail before use to avoid unexpected effects.31
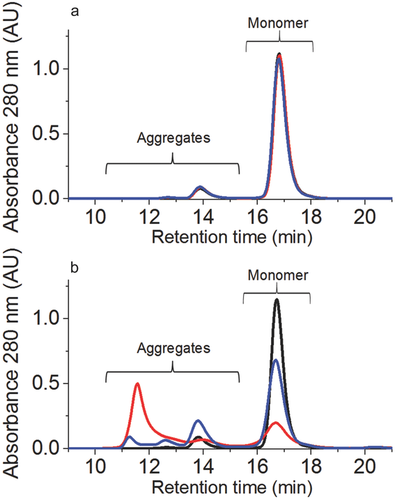
Size-Exclusion HPLC was used to assess the sample integrity after oxidation. Both H2O2 (1%, 5°C, 24 h with free tryptophan) or t-BHP (2%, 25°C, 120 h with free tryptophan) oxidation induced only minimal changes in aggregate and fragment formation. In comparison to the reference material, the incubation with 1% H2O2 induced 0.8% soluble aggregates, whereas the oxidation with 2% t-BHP induced 1.4% soluble aggregates (Fig. 5a). Thus, the application of the developed model stress system does not significantly impact molecular integrity and the biological consequences of selective methionine oxidation could be further analyzed by functional testing.
Selective Tryptophan Oxidation
2,2-Azobis(2-amidinopropane) dihydrochloride is a reagent that has been used for forced tryptophan oxidation studies.22 In our hands, the simultaneous oxidation of several tryptophan residues upon stress (mass additions of +4, +16, +32, +48, +64) resulted in a complex mixture of reaction products that were difficult to separate well by RP-HPLC (Fig. 3d). The prepeaks were broad and poorly resolved. Nevertheless, it was possible to detect tryptophan-oxidized species in the oxidized sample as early-eluting peaks (retention times from 16 to 19.6 min in Fig. 3d) and to identify the different tryptophan oxidation sites by subsequent LC–MS analyses. Upon incubation at 40°C for 120 h with AAPH (0%–5%), a considerable tryptophan oxidation (between 12% and 95%) was observed, as seen in Supplementary Tables 2 and 3.
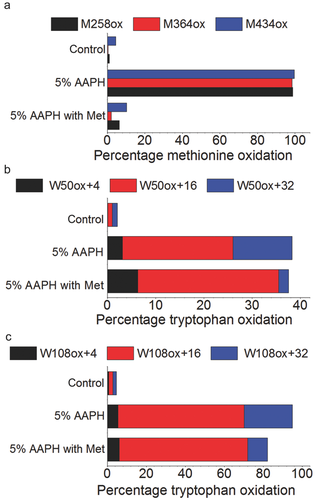
As reported in previous studies, the reaction of proteins with alkyperoxyl radicals (generated by AAPH that reacts with oxygen) also induced methionine oxidation.22, 32 As seen in Figures 2c and 4a, oxidation with 5% AAPH lead to almost complete oxidation of Fc methionine measured by protein A chromatography and LC–MS peptide mapping.
The addition of a large excess of free methionine into solution before oxidation at 40°C for 120 h allowed protection of Fc methionine as seen in the protein A chromatogram (Fig. 2c). As depicted in Supplementary Figures 1 and 2, the low levels or the absence of peptide signal in the TIC plots and extracted ion chromatograms for M258ox (RT = 12.65 min), M364ox (RT = 1.78 min), and M434ox (RT = 18.45 min) when adding free methionine into the mAb solution before oxidation with 5% AAPH (at 40°C for 120 h) is a clear confirmation of the protective effect of free methionine against methionine oxidation.
The results from LC–MS analyses of AAPH-oxidized samples are presented in Tables 1 and 2 of the Supplementary Material. These results clearly demonstrate that methionine residues otherwise susceptible to oxidation by AAPH can be selectively protected by addition of free methionine, even in the harshest reaction conditions used in this study [longest incubation time (120 h), highest oxidant concentration (5%), and highest temperature of incubation (40°C)]. This specific sample showed 10.3% oxidation for M258, whereas the same sample without methionine addition showed oxidation of 99.7%. Similarly, 6.3% versus 98.8% M434ox was verified by LC–MS. The oxidation of M364 was lower than 1% in the protected sample (99% oxidation in the sample without addition of free methionine). It has to be mentioned that 4.5% M258ox, 0.4% M364ox, and 1.1% M434ox were found in the nonstressed reference material (Fig. 4a).
Figures 4a–4c show a comparison of the methionine (M258, M364, and M434) and tryptophan (W50 and W108) distribution between AAPH-stressed samples with and without addition of free methionine. Over and above the decrease of methionine oxidation, the presence of free methionine in the mAb solution for AAPH incubation slightly changed the oxidation profile of tryptophan residues as seen in Figures 4b and 4c. As already observed in the RP-HPLC data, the total amount of tryptophan oxidation (as quantified by LC–MS) slightly decreased but remained considerable when adding free methionine to the solution (Figs. 4 and 5). On the one hand, a higher percentage of hydroxytryptophan (+16) for W50 and W108 was measured in the samples containing free methionine; on the other hand, a lower percentage of N-formylkynurenine (+32) was found for W50 and W108 when adding free methionine (Supplementary Figs. 3 and 4). Presumably, this effect is related to shift in the equilibrium between reaction intermediates caused by the addition of the antioxidant. Further elucidation of this mechanism may be of interest as a topic of future studies.
Significant formation of soluble aggregates was detected by size SEC correlating well with the increase in AAPH concentration. The addition of free methionine, however, had a protective effect against aggregation as seen in Figure 5b. This effect correlates well with overall reduction on oxidation. The monomer content represented 30% of the total area in the AAPH sample without methionine addition, whereas 65% of monomers were still present in the sample containing free methionine during incubation with 5% AAPH for 120 h at 40°C. The content of soluble fragments remained below 0.3% for the control and the stressed samples in all studies. The correlation between Met oxidation and protein aggregation observed in this study was not unexpected, as such correlation has been reported previously by others and studied in some detail.11, 33 These studies have demonstrated that Met oxidation in mAbs may result in changes of the secondary and tertiary structure and in turn significantly affect the physical stability of the protein resulting in increased aggregation propensity. Thus, the decrease of aggregation upon the addition of free Met can be attributed to the oxidation protective effect of Met.
The experimental approach of using combinations of oxidative reagents and antioxidants presented here provided an efficient strategy for selective oxidation of Met and Trp residues in therapeutic IgG molecules. Although residual low levels of undesirable oxidation were detected in some cases (which presumably would need to be accounted for in follow-up biological studies), we believe that this approach will find broad application in studying the biological effects of protein oxidation.
CONCLUSIONS
Free methionine and/or tryptophan are used as pharmaceutical antioxidants because of their protective action against oxidation of amino acids in protein therapeutics. In the present report, we explore the potential of these antioxidants to selectively generate methionine- or tryptophan-oxidized residues. A combination of oxidative (H2O2, t-BHP, and AAPH) and protective (free tryptophan or methionine) components will serve as a strategy to address questions related to the impact of oxidation-induced changes on the biological properties of protein therapeutics (e.g., bioactivity, PK/PD, immunogenicity, etc.). Such mechanistic studies will be instrumental to better understand and control oxidative degradation of antibodies.
ACKNOWLEDGMENT
We thank Y. John Wang, Ulla Grauschopf, and Kishore Ravuri for insightful discussions and comments on the manuscript.




