Zanamivir Amidoxime- and N-Hydroxyguanidine-Based Prodrug Approaches to Tackle Poor Oral Bioavailability
Abstract
The neuraminidase (NA) inhibitor zanamivir (1) is potently active against a broad panel of influenza A and B strains, including mutant viruses, but suffers from pharmacokinetic (PK) shortcomings. Here, distinct prodrug approaches are described that aimed at overcoming zanamivir's lack of oral bioavailability. Lowering the high basicity of the 4-guanidino group in zanamivir and of a bioisosteric 4-acetamidine analog (5) by N-hydroxylation was deemed to be a plausible tactic. The carboxylic acid and glycerol side chain were also masked with different ester groups. The bioisosteric amidine 5 turned out to be potently active against a panel of H1N1 (IC50 = 2–10 nM) and H3N2 (IC50 = 5–10 nM) influenza A viruses (NA inhibition assay). In vitro PK studies showed that all prodrugs were highly soluble, exhibited low protein binding, and were bioactivated by N-reduction to the respective guanidines and amidines. The most promising prodrug candidates, amidoxime ester 7 and N-hydroxyguanidine ester 8, were subjected to in vivo bioavailability studies. Unfortunately, both prodrugs were not orally bioavailable to a convincing degree (F ≤ 3.7%, rats). This finding questions the general feasibility of improving the oral bioavailability of 1 by lipophilicity-increasing prodrug strategies, and suggests that intrinsic structural features represent key hurdles. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3208–3219, 2015
INTRODUCTION
Seasonal influenza infections account for diseases of 3–5 million people and 250,000 to 500,000 deaths worldwide caused either directly by the virus or as a consequence of secondary infections.1 The last—fortunately rather harmless—pandemic occurred in 2009 (H1N1, “swine flu”), but in recent years, the highly pathogenic avian influenza viruses of subtype H5N1 (i.e., “bird flu”, 2005) and H7N9 have been alarming as they can be transmitted from animals to humans.2-5 Another major concern is the high mutagenic rate of the viruses, which contributes to the rapid development of resistances. For example, flu season 2007/2008 generated an oseltamivir-resistant H1N1 virus that spread globally and carried a H274Y mutation.6 The sporadic emergence of virus strains with resistance-conferring mutations highlights the urgent need for new therapeutic agents.7
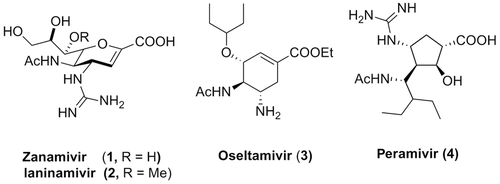
To date, approved drugs for the treatment or prophylaxis of influenza virus infections belong to the classes of adamantane-based M2 ion channel protein inhibitors and inhibitors of the viral surface protein neuraminidase (NA), which is a key enzyme in the life cycle of the virus and allows for its replication and spread.8, 9 As adamantane type of compounds are only effective against influenza A, suffer from several side effects and drug resistance,8, 9 current clinical practice mainly makes use of NA inhibitors (NAIs). Zanamivir (1, (Fig.1) represents the first marketed NAI (in 1999) and is approved for inhalative and intravenous administration.10 Laninamivir (2) was introduced later as a follow-up that only differs from zanamivir with a methoxy group in the glycerol side chain, and has been approved in Japan.11 In the form of its octanoate prodrug (CS-8958), laninamivir represents a long-acting NAI that requires only a single daily inhaled dose. This strategy emphasizes the consciousness of and necessity for the development of patient-compliant formulations of anti-flu agents.12 In fact, oseltamivir (3) is the first and only orally available NAI and because of this represents the first choice NAI, stockpiled by most countries in case of a new pandemic.13, 14 Finally, peramivir (4) was available from 2009 to 2010 for in-patient emergency treatment of severe forms of influenza infections, despite not being approved at that time.15-17 In 2014, it was approved as the third NAI by the United States Food and Drug Administration (US FDA) for the treatment of influenza infections (US FDA press announcement Dec/2014).
Notably, all clinically used NAIs suffer from distinct drawbacks. As indicated above, therapeutic treatment with zanamivir, laninamivir, and peramivir is restricted to inhalative or intravenous application, respectively.18, 19 Only oseltamivir is orally bioavailable but for this NAI many resistances have emerged. We recently reported on an attempt to overcome both issues at the same time, that is, breaking oseltamivir resistance while keeping oral bioavailability.20 Because 5-guanidino substituted oseltamivir derivatives were significantly more potent and also active against oseltamivir-resistant virus strains,10, 13 we reasoned that bioisosteric replacement of this group by a 5-amidino functionality provides access to similarly active analogs that are amenable to specific prodrug concepts. Indeed, the oseltamivir amidine turned out potently active against a large panel of H1N1 and H3N2 influenza A virus strains and effective against an oseltamivir-resistant A/H1N1 mutant. Strikingly, a simple amidoxime prodrug strategy for this compound enabled an oral bioavailability (31%, rats) that was comparable to oseltamivir (36%, rats).20
Moreover, several distinct prodrug approaches have been attempted to overcome pharmacokinetic (PK) issues of zanamivir and the more potent oseltamivir guanidine, including intra- and intermolecular ion pairing strategies or targeting of active transporters with specific amino acid conjugates.21, 22 In particular, the latter concept—that has been proposed by the Amidon group—showed some success as the oral bioavailability of oseltamivir guanidine could be increased to 23% (mice, fed state) and 48% (mice, fasted state) with a carrier-mediated l-valinate prodrug.23 The same concept was realized for zanamivir. Amidon group could demonstrate at least in vitro a fourfold higher uptake of a zanamivir l-valine prodrug in transfected hPept1/HeLa cells and an improved permeability in Caco-2 cells (ninefold) compared with zanamivir.24 Moreover, in an in situ rat perfusion model, the valine prodrug exhibited an effective permeability (Peff) that was comparable to the well-absorbed metoprolol, whereas zanamivir was not permeable at all. Therefore, it will be interesting to see how these l-valine prodrug conjugates perform in vivo.
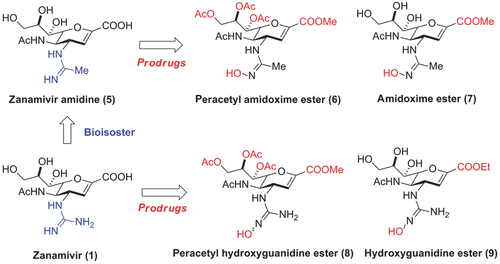
Here, we describe a prodrug approach for zanamivir that builds on our recent success with the orally bioavailable, highly potent oseltamivir amidoxime prodrug outlined above. The guanidine group in zanamivir was bioisosterically replaced by an acetamidine (zanamivir amidine, 5) (Fig. 2). For both NAI drugs, prodrug strategies were realized that make use of an N-hydroxylation of the amidine (i.e., amidoxime) and the guanidine (i.e., N-hydroxyguanidine), a concept that overcomes the high basicity and thus (almost) permanent positive charge of the compounds. The resulting less basic N-hydroxylated derivatives would later be bioactivated in vivo by N-reduction. The underlying metabolism as well as scope and limitations of this prodrug concept have been previously demonstrated.25-28 However, as the carboxylic acid and glycerol side chain in zanamivir significantly contribute to a high polarity of the parent drug and impair cellular uptake by passive diffusion, we also masked these groups by simple ester prodrugs. Therefore, for both the amidine (5) and guanidine (1) drug of zanamivir, two prodrugs were prepared that contain either a preacetylated glycerol side chain (6, 8) or an unsubstituted glycerol moiety (7, 9) along with a carboxyl ester and the respective N-hydroxylated amidine or guanidine. All prodrug candidates were extensively profiled for their in vitro and in vivo PK properties.
EXPERIMENTAL
Chemistry
General
1H (300 MHz) and 13C (75 MHz) NMR spectra were obtained on a Bruker ARX 300 spectrometer (Bruker, Bremen, Germany) at 300 K. Low-resolution mass spectra were recorded on a Bruker-Esquire-LC with electrospray ionization (ESI). Purity of synthetic products was ≥98% as determined by LC–MS. Recordings of high-mass resolution spectra (HRMS) were conducted on a Bruker 7.4 Tesla FTICR mass spectrometer BioApex II (Bruker, Bremen, Germany) equipped with an ESI-ion source (Agilent, Waldbronn, Germany); purification of synthesized compounds was performed by column chromatography using silica gel (particle size 40–63 μm). Flash chromatography on a RP18 column (43 g, RediSep®) was performed with the CombiFlashRETRIEVE® system. Starting materials were commercially available and used without further purification. All solvents were distilled and dried according to standard procedures. Zanamivir amine (10) and zanamivir hydroxyguanidine ethylester (9) were purchased from InnoChemie GmbH (Würzburg, Germany).
5-Acetylamino-7,8,9-Tri-O-Acetyl-4-(N-Acetimidamido)-2,6-Anhydro-3,4,5-Trideoxy-d-Glycero-d-Galacto-non-2-Enonic Acid Methyl Ester Hydrobromide (11)
Protected zanamivir amine precursor 10 (860 mg, 2 mmol) was dissolved in 6 mL abs. EtOH and cooled to 0°C. S-naphthylmethyl thioacetimidate hydrobromide (1.18 g, 4 mmol) was added portion wise over 60 min, and the reaction was stirred for 2 h at room temperature. The mixture was concentrated in a vacuum, taken up with approximately 30 mL water and washed thrice with approximately 20 mL Et2O. The water phase was concentrated under reduced pressure and the crude product purified twice by column chromatography on SiO2 (CH2Cl2/MeOH, 9:1, Rf = 0.42) to obtain a fine, white solid. Yield: 663 mg (60%). 1H NMR (DMSO-d6, 300 MHz): Amidine 11 appeared as rotamers/isomers predominantly observable at H-3, the amidine moiety and the NHAc group (87:13 ratio referring to the H-3 signal). Chemical shifts of the major rotamer/isomer are listed only. δ/ppm = 1.77 (s, 3H), 2.00, 2.01(3 × s, 9H), 2.10(s, 3H), 3.74 (s, 3H), 4.11 (mc, 2H), 4.35 (dd, 3J = 10.0 Hz, 3J = 2.3 Hz, 1H), 4.44 (dd, 2J = 12.3 Hz, 3J = 3.9 Hz, 1H), 4.73 (mc, 1H), 5.26 (td, 3J = 6.6 Hz, 3J = 3.0 Hz, 1H), 5.38 (dd, 3J = 6.3 Hz, 3J = 3.0 Hz, 1H), 5.84 (d, 3J = 2.4 Hz, 1H), 8.17 (d, 3J = 9.1 Hz, 1H), 8.81 (s, 1H), 9.34 (s, 1H), 9.57 (d, 3J = 8.3 Hz, 1H); 13C NMR (DMSO-d6, 75 MHz): δ/ppm = 19.0, 20.5, 22.5, 45.7, 50.9, 52.3, 61.7, 67.1, 69.3, 76.1, 108.4, 144.3, 161.1, 165.2, 169.1, 169.2, 169.7, and 170.0; MS (ESI): m/z = 472.1 [M+H]+.
5-Acetylamino-4-(N-Acetimidamido)-2,6-Anhydro-3,4,5-Trideoxy-d-Glycero-d-Galacto-Non-2-Enonic Acid (5)
Protected amidine 11 (1 mmol) was dissolved in H2O/MeOH (8:2). To this solution, 450 mg of a strongly basic anion exchanger resin (= Ionenaustauscher III; Merck) were added and the mixture very gently stirred over night at room temperature. The resin is removed by filtration and thoroughly washed with MeOH and small amounts of H2O. After concentrating this solution in vaccuo, the crude product was purified by flash chromatography on a RP-18 column. The desired title compound elutes early after the void volume. Yield: 166 mg (50%) of a colorless, crystalline solid. 1H NMR (D2O, 300 MHz): Amidine 5 appeared as rotamers/isomers (ratio: 7:3 referring to the H-11 signal) and the chemical shifts of the major rotamer/isomer are listed only. δ/ppm = 2.02 (s, 3H), 2.22 (s, 3H), 3.59−3.72 (m, 2H), 3.83−3.98 (m, 2H), 4.15−4.42 (m, 2H), 4.61 (dd, 1H, 3J = 9.0 Hz, 3J = 2.4 Hz), 5.58 (d, 1H, 3J = 2.3 Hz); 13CNMR (D2O, TPS, 75 MHz): δ/ppm = 19.5, 22.5, 47.6, 52.3, 63.7, 68.6, 70.4, 75.9, 102.5, 150.5, 166.4, 169.5, and 175.0; HRMS (ESI): m/z calculated for C13H21N3O7 [M+H]+: 332.14523, found: 332.14528; combustion analysis, calculated for C13H21N3O7 × 0.4 H2O (338.53): C 46.12, H6.49, N 12.41; found: C 46.15, H 6.88, N 12.40.
5-Acetylamino-7,8,9-Tri-O-Acetyl-2,6-Anhydro-4-[N-(N′-Hydroxy)Acetimidamido]-3,4,5-Trideoxy-d-Glycero-d-Galacto-Non-2-Enonic Acid Methyl Ester (6)
Protected zanamivir amine 10 (12 mmol) and 3.26 mL DIPEA (24 mmol) were dissolved in 60 mL of dry CH2Cl2 and stirred at 0°C. A solution of freshly prepared 2.24 g N-hydroxyacetimido chloride (24 mmol)29 in approximately 10 mL of dry CH2Cl2 was slowly added drop wise. Subsequently, the mixture was stirred at room temperature for 4 h. Fifteen milliliters of water was added and the reaction mixture vigorously stirred for 1 h, before the organic phase was separated and washed with 15 mL of brine. To improve yields, the water phase was extracted 4× with CH2Cl2 and another 4× with EtOAc. The organic phases were combined, dried with Na2SO4 and concentrated in vaccuo. The crude product (ca. 6.5 g) was purified twice by column chromatography on SiO2 (CH2Cl2/MeOH, 9:1, Rf = 0.35). Yield: 3.57 g (61%) of a fine, white solid. 1H NMR (DMSO-d6, 300 MHz): amidoxime 6 appeared as isomers (ratio 7:3) and the chemical shifts of the major isomer are listed only. δ/ppm = 1.73, 1.74 (2 × s, 6H),2.01 (3 × s, 9H), 3.70 (s, 3H), 3.87−3.99 (m, 1H), 4.06 (dd, 3J = 12.2 Hz, 3J = 2.7 Hz, 1H), 4.18 (mc, 1H), 4.31 (dd, 3J = 10.3 Hz, 3J = 1.5 Hz, 1H), 4.44 (dd, 3J = 12.3 Hz, 3J = 2.9 Hz, 1H), 5.22 (td, 3J = 6.5 Hz, 3J = 2.8 Hz, 1H), 5.30 (dd, 3J = 6.3 Hz, 3J = 2.7 Hz, 1H), 5.41 (d, 3J = 10.1 Hz, 1H), 5.75 (d, 3J = 2.3 Hz, 1H), 7.92 (d, 3J = 9.4 Hz, 1H), 8.91 (s, 1H); 13C NMR (DMSO-d6, 75 MHz): δ/ppm = 15.0, 20.4, 20.5, 20.6, 22.6, 47.1, 50.9, 52.1, 61.8, 67.3, 69.7, 76.8, 113.6, 142.8, 153.8, 161.6, 169.2, 169.3, 169.4, and 169.9; MS (ESI): m/z = 510 [M+Na]+, 488 [M+H]+, 414 [M−NHC = NOH-CH3]+; combustion analysis, calculated for C20H29N3O11 (487.46): C 49.28, H 6.00, N 8.62; found: C 49.36, H 6.45, N 8.66.
5-Acetylamino-2,6-Anhydro-4-[N-(N′-Hydroxy)Acetimidamido]-3,4,5-Trideoxy-d-Glycero-d-Galacto-Non-2-Enonic Acid Methyl Ester (7)
Peracetylated zanamivir amidoxime (6) (1 mmol) was dissolved in 5 mL of abs. MeOH under an argon atmosphere at 0°C. To this solution, sodium methoxide (0.15 mmol) was added. The mixture was stirred for 1 h at 0°C, another 3 h at room temperature and then neutralized with Amberlyst 15. After filtration and extensive washing of the resin, the solution was concentrated in a vacuum. The crude product was purified by column chromatography on SiO2 (CH2Cl2/MeOH, 7:3, Rf = 0.36). Yield: 235 mg (65%) of a fine, white solid. 1H NMR (DMSO-d6, 300 MHz): amidoxime 7 appeared as isomers (ratio 7:3) and the chemical shifts of the major isomer are listed only. δ/ppm = 1.68, 1.74 (2 × s, 6H), 3.29 (s, 3H), 4.59 (t, 3J = 3.6, 2H), 5.55 (d, 3J = 10.4 Hz, 1H), 5.69 (d, 3J = 2.4 Hz, 1H), 7.18 (d, 3J = 4.4 Hz, 1H), 8.11 (t, 3J = 8.8 Hz, 1H), 8.24 (d, 3J = 7.4 Hz, 1H), 8.92 (s, 1H); 13C NMR (DMSO-d6, 75 MHz): 15.0, 22.6, 47.1, 49.8, 52.0, 63.5, 64.9, 68.7, 69.3, 77.4, 112.0, 149.9, 162.4, and 171.3; MS (ESI): m/z = 745 [2M+Na]+, 384 [M+Na]+, 362 [M+H]+, 288 [M−NHC=NOH-CH3]+; HRMS (ESI): m/z calculated for C14H23N3O8 [M+H]+: 362.15579, found: 362.15609; m/z calculated for C14H23N3NaO8 [M+Na]+: 384.13774, found: 384.13775; the purity of this compound was further assessed by analytical HPLC and found to be ≥98.9%.
5-Acetylamino-7,8,9-Tri-O-Acetyl-2,6-Anhydro-4-[N-(N'-Benzyloxycarbonyl)-Thioureido]-3,4,5-Trideoxy-d-Glycero-d-Galacto-Non-2-Enonic Acid Methyl Ester (13)
Protected zanamivir amine 10 (3 mmol) was dissolved and stirred in 100 mL of dry CH2Cl2 and cooled to 0°C. A solution of benzyloxycarbonylisothiocyanate in CH2Cl2 (3 mmol) was added drop wise over 30 min. The reaction mixture was stirred for 2 h while warming to room temperature until TLC (thin layer chromatography) indicated complete consumption of starting material. The organic phase was washed once with 1% aqueous HCl, water and brine (20 mL each), dried with Na2SO4 and concentrated in a vacuum. The crude mixture was further purified by column chromatography (CH2Cl2/MeOH, 95:5, Rf = 0.5). Yield: 1.68 g (90%) of an off-white (slightly yellow) amorphous solid. 1H NMR (DMSO-d6, 300 MHz): δ/ppm = 1.73 (s, 3H), 1.99, 2.00 (2 × s, 9H), 3.71 (s, 3H), 4.08 (dd, 3J = 12.3 Hz, 3J = 6.6 Hz, 1H), 4.16 (mc, 1H), 4.42 (mc, 2H), 5.17 (d, 4J = 4.4 Hz, 2H), 5.18−5.28 (m, 2H), 5.36 (dd, 3J = 6.6 Hz, 3J = 1.8 Hz, 1H), 5.97 (d, 3J = 2.3 Hz, 1H), 7.40 (mc, 5H), 8.06 (d, 3J = 9.5 Hz, 1H), 9.79 (d, 3J = 7.2 Hz, 1H), 11.25 (s, 1H); 13C NMR (DMSO-d6, 75 MHz): δ/ppm = 20.5, 22.5, 45.1, 52.2, 53.9, 61.7, 66.9, 67.1, 69.3, 76.3, 109.7, 127.9, 128.2, 128.4, 135.4, 144.4, 153.1, 161.3, 169.1, 169.3, 169.5, 170.0, and 180.4; MS (ESI): m/z = 646 [M+Na]+, 624 [M+H]+, 414.
5-Acetylamino-7,8,9-Tri-O-Acetyl-2,6-Anhydro-4-{N-[N′-Benzyloxy-Carbonyl-N″-(Tetrahydro-2H-Pyran-1-yl)Oxy]guanidino}-3,4,5-Trideoxy-d-Glycero-d-Galacto-Non-2-Enonic Acid Methyl Ester (14)
Thiourea [624 mg (1 mmol)] 13, 288 mg EDCI (1.5 mmol), and 194 mg (267 μL) DIPEA (1.5 mmol) were dissolved in 60 mL of CH2Cl2 and cooled to 0°C. O-(tetrahydro-2H-pyran-2-yl)hydroxylamine (NH2OTHP) [175 mg (1.5 mmol)], dissolved in 10 mL CH2Cl2, were added dropwise over 2 h and the reaction mixture was stirred for 2 days while warming to room temperature until TLC indicated complete consumption of starting material. The CH2Cl2 phase was washed with 25 mL of 1% aqueous HCl, 25 mL of water, and 25 mL of brine. The organic phase was dried (Na2SO4) and the solvent removed in vacuo to yield a yellow oil. The crude product was purified by column chromatography on silica gel using CH2Cl2/MeOH (95:5, Rf = 0.36) as the eluant. It is to be noted that the column was preconditioned with CH2Cl2/MeOH (99:1). Yield: 671 mg (95%) of white crystals. 1H NMR (DMSO-d6, 300 MHz): The title compound appears as isomers (ratio 6:4 based on the NHAc and methyl ester signals, and the chemical shifts of the major isomer are listed only. δ/ppm = 1.44 (mc, 3H), 1.60 (mc, 3H), 1.73 (mc, 3H), 2.00(3 × s, 9H), 3.69 (mc, 3H), 4.05 (mc, 2H), 4.18 (mc, 1H), 4.29 (mc, 1H), 4.43 (dd, 2J = 12.2 Hz, 3J = 3.1 Hz, 1H), 4.88 (mc, 1H), 5.08 (d, 4J = 3.8 Hz, 2H), 5.14 (mc, 2H), 5.24 (mc, 1H), 5.30 (mc, 1H), 6.04 (d, 1H), 6.37 (mc, 1H), 7.38 (mc, 5H), 7.80 (mc, 1H), 7.98 (mc, 1H); 13C NMR (DMSO-d6, 75 MHz): δ/ppm = 19.4, 20.4, 20.5, 20.6, 22.6, 24.9, 28.7, 39.5, 45.3, 52.0, 61.4, 66.1, 66.9, 67.1, 69.3, 76.2, 100.0, 113.6, 127.6, 128.1, 128.2, 128.3, 128.4, 135.4, 142.8, 146.7, 152.8, 161.7, 169.1, 169.3, 169.5, and 169.9; MS (ESI): m/z = 729 [M+Na]+, 707 [M+H]+, 623, 414.
5-Acetylamino-7,8,9-Tri-O-Acetyl-2,6-Anhydro-4-(N-(N′-Hydroxy)-Guanidino)-3,4,5-Trideoxy-d-Glycero-d-Galacto-Non-2-Enonic Acid Methyl Ester (8)
The fully protected precursor 7 (1.32 mmol) was dissolved in 26 mL TFA (trifluoroacetic acid) and 7.8 mL thioanisole and stirred at room temperature for 45 min. The mixture was subsequently concentrated in a vacuum and taken up with 20 mL water. This solution was washed with Et2O (15 mL) and the organic phase extracted twice with water (5 mL each). The water phase was concentrated on a rotary evaporator and the crude mixture purified by column chromatography on SiO2 (CH2Cl2/MeOH, 9:1, Rf = 0.16). Yield: 387–451 mg (60%–70%) of a white amorphous solid. 1H NMR (DMSO-d6, 300 MHz): δ/ppm = 1.75 (s, 3H), 1.99, 2.00 (3 × s, 9H), 3.72 (s, 3H), 4.09 (mc, 2H), 4.28 (dd, 3J = 10.4 Hz, 3J = 1.8Hz, 1H), 4.44 (mc, 2H), 5.25 (td, 3J = 6.6 Hz, 3J = 2.9 Hz, 1H), 5.34 (dd, 3J = 6.3 Hz, 3J = 1.9 Hz, 1H), 5.77 (d, 3J = 2.3 Hz, 1H), 7.78 (s, 2H), 8.00 (d, 3J = 9.4 Hz, 1H), 9.89 (br s, 1H), 10.58 (s, 1H); 13C NMR (DMSO-d6, 75 MHz): δ/ppm = 20.5, 22.6, 46.0, 50.5, 52.2, 61.7, 67.1, 69.5, 76.5, 110.4, 143.8, 158.3, 161.3, 169.1, 169.3, 169.5, and 169.9; HRMS (ESI): m/z calculated for C19H29N4O11 [M+H]+: 489.18273, found: 489.18298; combustion analysis calculated for C19H28N4O11 × 1.5 CF3COOH (659.49): C 40.07, H 4.51, N 8.50; found: C 40.00, H 4.44, N8.77.
Biology
Control Compounds
Zanamivir (GG167) was kindly provided by GlaxoSmithKline (Uxbridge, UK). For antiviral assays, zanamivir amidine (5) stocks were prepared in H2O and stored at 4°C (storage stability was confirmed as described in detail in Supporting Information).
Cells and Viruses
MDCK cells (Friedrich-Loeffler Institute, Riems, Germany) were grown in Eagle's minimum essential medium (EMEM) supplemented with 1% non-essential amino acids (NEAA), 1 mM sodium pyruvate. EMEM medium for virus propagation, titration, antiviral tests of viruses contained 2 μg/mL trypsin, and 0.1% sodium bicarbonate. The seasonal H1N1 A/Berlin/55/08 (swab sample was published as A/342/2009),30 the H3N2 A/Sachsen/6/02, A/Berlin/10/04 and A/Rheinland-Pfalz/3911/03 (all kindly provided by the National Reference Centre of influenza viruses at the Robert Koch Institute, Berlin, Germany), pandemic H1N1 influenza virus A/Jena/5258/09 (as published),30 A/Hamburg/1580/09 (kindly provided by Ralf Dürrwald, IDT Biologica GmbH, Dessau, Germany) and the H3N2 A/HongKong/68 (strain collection of the Department of Virology and Antiviral Therapy, Jena University Hospital, Germany) were used in the present study. Furthermore, the susceptibility of H1N1 A/WSN/33 viruses with either M2-N31S mutation alone or additional NA-H275Y mutation was studied. They were derived from an eight plasmid transfection system 31 after introducing point mutational changes on the pHW-187 plasmid, encoding the A/WSN/33 matrix proteins, and pHW-186 plasmid, encoding the viral NA gene with a commercial available kit (GeneArt® Site-Directed Mutagenesis System; Invitrogen, Carlsbad, California).
NA Inhibition Assay
Inhibition of viral NA was examined using a chemiluminescence-based assay (NA-star® kit; Tropix, Applied Biosystems, Darmstadt, Germany) as described in the literature with some modifications.32
Cytopahtic Effect Inhibition Assay
Two-days-old MDCK cell monolayer without or with test compounds were infected with influenza virus in a concentration causing complete cytopathic effect (CPE) within 48 h. After incubation for 48 h at 37°C, cells were fixed and stained with crystal violet as described previously. Inhibition of virus-induced CPE was determined photometrically on a Dynatech plate reader. Antiviral efficacy was calculated by comparison of optical density of drug-treated and untreated virus-infected cells. On basis of the dose–response curve of at least three independent experiments, linear interpolation was used to calculate IC50 values. Note: None of the test compounds were toxic to MDCK cells treated for 72 h as described previously.33
Protein Binding
Protein binding was determined in a 4% albumin solution (or in human plasma) at three different concentrations (10, 25, and 50 μM) of the test compound. After shaking for 30 min at 37°C, samples were centrifuged at 10,000 rpm (10 min) in Vivaspin 500 (10 kDa cut-off) ultrafiltration units. Control samples without protein confirmed that the test compounds were not retained by the ultrafiltration unit membrane. Filtrates were analyzed by HPLC and the following analytics were used: Zanamivir amidine (5) was analyzed on a Waters Alliance system (Waters, Eschborn, Germany), Waters e2695 XC Separations Module, Waters 2998 PDA detector; LiCrospher 100 Diol (200 × 4 mm2; Merck), guard column (4 × 4 mm2); isocratic elution (2 mL/min); eluent 80% MeCN, 20% 0.1% TFA (pH 3.0); detection 210–400 nm (235 nm); retention time: 6.8 ± 0.15 min. Peracetylated amidoxime ester (6) and amidoxime ester (7) were analyzed on a Waters system consisting of a Waters 717 plus autosampler, Waters 600 Controller and Waters 2487 Dual λ Absorbance Detector (set at 230 nm); column: LiChrospher 100 RP8 (125 × 4 mm2, 5 μm), guard column (4 × 4 mm2); isocratic elution (1 mL/min); eluent: solvent A (0.1% TFA, 10 mM phosphate buffer, pH 7.0) and solvent B (MeOH) at 90/10 ratio; retention times: 3.0 ± 0.1 min (7), 4.7 ± 0.2 min (6). Zanamivir hydroxyguanidines 8 and 9 were analyzed on the same HPLC system and conditions as described for amidoximes 6 and 7, but with the following differences: LiChrospher 60 column (125 × 4 mm2, 5 μM; VDS Optilab); 3.9 ± 0.1 min (9); for 9 eluent consisted of: solvent A (0.1% TFA, 10 mM phosphate buffer, pH 7.0) and solvent B (MeOH) at 65/35 ratio 4.4 ± 0.2 min (8). Zanamivir guanidine (1) was quantified using an analytic described below in the in vitro bioactivation section.
Chemical and Plasma Stability
Chemical stability was assessed by incubating prodrugs 6–9 in 50 mM phosphate buffers (pH 2.9, 7.4, and 9.0) at room temperature and 400 μM final concentrations. Samples were taken at indicated time points and analyzed by HPLC. Incubations with human and murine plasma were performed at 37°C and also at 400 μM final prodrug concentrations. Samples (100 μL) were taken at indicated time points and 100 μL MeOH added, 10 min shaken, 10 min centrifuged at 13,000 rpm and the supernatant analyzed by HPLC. HPLC analytics were employed as described for all prodrugs above in the protein binding section.
In Vitro Bioactivation
Prodrugs were incubated with subcellular enzyme preparations from human and porcine liver and kidney (i.e., microsomes, mitochondria, 9000g supernatants, cytosol). A typical incubation sample consisted of 500 μM prodrug, 500 μM NADPH, 1 U carboxyl esterase (pig liver), and 0.3 mg of the respective enzyme preparation in 250 μL potassium phosphate buffer (100 mM, pH 6.3). Samples were incubated at 37°C for 30 min and reactions stopped by addition of 250 μL MeCN. After centrifugation (10,000 rpm, 15 min) the supernatant was analyzed by HPLC using the following methods: Amidoxime prodrug 7 was analyzed on a Waters Alliance system (Waters, Eschborn, Germany), Waters e2695 XC Separations Module, Waters 2998 PDA detector; Waters Atlantis HILIC Silica (100 × 3 mm2, 3 μm); eluent: solvent A (10 mM NH4OAc, pH 5.2), solvent B (MeCN); gradient elution (0.45 mL/min): 0–5 min (25%–35% solvent A), 5–6 min (35% solvent A), 6–9 min (35%–25% solvent A); detection 210–400 nm (235 nm); retention times: 2.5 ± 0.2 min (7), 3.0 ± 0.2 min (amidoxime free acid metabolite), 6.1 ± 0.2 min (5), 7.4 ± 0.2 min (amidine methylester metabolite). Hydroxyguanidine prodrug 9 was analyzed on the same system as described above but with the following differences: eluent: solvent A (0.1% formic acid, pH 2.7), solvent B (MeCN); gradient elution (0.6 mL/min): 0–8 min (15%–18% solvent A), 8–10 min (18% solvent A), 10–11 min (18%–15% solvent A), 11–14 min (15% solvent A); retention times: 5.2 ± 0.3 min (guanidine ester), 9.6 ± 0.3 min (1).
In Vivo Bioavailability
Oral bioavailability of zanamivir amidoxime ester (7) and hydroxyguanidine ester (9) was examined with Wistar rats. The study was conducted according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and authorized by the local regulatory authority (=Ministerium für Energiewende, Landwirtschaft, Umwelt und ländliche Räume des Bundeslandes Schleswig-Holstein). The animals were kept at r.t. with a 12:12 h dark (8 AM–8 PM)–light (8 PM–8 AM) cycle. They received a standard diet and water ad libitum. Rats (385 ± 9 g) were habituated to research assistants and vice versa 2 weeks before drug treatment was initiated. Rats were randomized into groups receiving either zanamivir (1) i.v. (n = 15), amidine drug (5) i.v. (n = 15), amidoxime ester (7) p.o. (n = 10), and hydroxyguanidine ester (9) p.o. (n = 8).
Surgery
Two days before the PK study, chronic polyethylene catheters have been inserted during pentobarbitone anesthesia (67 mg/kg) into the right femoral vein and artery. Catheters were tunneled under the back skin, exteriorised in the region of the cervical vertebra, and fixed at the skin. Thereafter, rats were housed individually in cages (height × width × length: 20 × 22 × 25 cm3) until the end of the study.
Intravenous administration of guanidine and amidine drugs (1, 5) was performed at 10 mg kg−1. Blood samples were collected 5, 10, 15, 20, 40, 60, 90, and 120 min after i.v. injection. For oral administration, compounds were suspended in a solution of gum arabic (10%, H2O) and administered orally by gavage at 50 mg kg−1 body weight. Blood samples (ca. 300 μL) were taken with microvettes (EDTA-coated) after 15, 30, 45, 60, 90, 120, and 240 min via an arterial implanted catheter. Plasma samples were obtained by centrifugation at 14,000g for 10 min and were immediately frozen (−80°C). Animals were sacrificed by decapitation under isofurane anesthesia 360 min after application of test compounds. Plasma samples were treated with equal volumes of MeCN (0.1% TFA) for analysis of amidine-based compounds and MeCN (0.1% formic acid) for analysis of guanidine-based compounds. Samples were shaken for 15 min and frozen (−80°C). After thawing and shaking for 15 min, samples were centrifuged at 13,000g for 15 min. The supernatant was analyzed by HPLC/UV or HPLC/MS. Analysis of zanamivir amidine (5) and guanidine (1) i.v. samples was performed by HPLC as described above in the in vitro bioactivation section. Samples from amidoxime ester (7) were analyzed for 7, different metabolites and the amidine drug 5 by LC/MS: Agilent 1100 HPLC system with a 1100 binary pump, 1100 PDA detector (set at 235 nm), and a Bruker Esquire ESI-MS; column: Waters Atlantis HILIC Silica (100 × 3 mm2, 3 μm); eluent: solvent A (10 mM NH4OAc, pH 5.2), solvent B (MeCN); gradient elution (0.45 mL/min): 0–8 min (25% solvent A), 8–9 min (25%–35% solvent A), 9–11 min (35% solvent A), 11–12 min (35%–25% solvent A); retention times: 2.3 ± 0.1 min (7), 4.5 ± 0.3 min (amidoxime free acid metabolite), 6.9 ± 0.2 min (5), 9.0 ± 0.3 min (amidine ester metabolite). Samples from hydroxyguanidine ester (9) were analyzed for the guanidine drug 1 by LC/MS using the same system as described above but with the following differences: eluent: solvent A (0.1% formic acid, pH 2.5), solvent B (MeCN) at a ratio of 19:81 (A:B); flow rate: 0.6 mL/min; retention time: 6.7 ± 0.6 min (1). PK parameters were calculated using PK solutions 2.0 software (Summit Research Services, USA).
RESULTS AND DISCUSSION
Chemistry
The concept of bioisosteric replacement of the 4-guanidine group in zanamivir by a 4-amidine is theoretically possible in two ways, either by replacing a distal NH2 group with a CH3 generating a 4-acetamidine or by substituting the proximal heterocycle-bound 4-NH group with a CH2 affording a 4-(2-amino-2-iminoethyl) functionality. For the sake of synthetic feasibility, we aimed at preparing 4-acetamidine-based zanamivir derivatives which was possible from readily available zanamivir 4-amine (10). Amine 10 was thus reacted with S-(2-naphtylmethyl)-thioacetimidate34 and afforded the protected amidine 11 in 60% yield (Scheme 1). Deprotection was performed with a strongly basic anion exchanger and the desired zanamivir amidine drug (5) was obtained in moderate yield (50%) and high purity. The amidoxime prodrugs of zanamivir were accessible through reacting amine 10 with freshly prepared acetohydroximoyl chloride.29 This way, peracetylated amidoxime ester 6 was synthesized in 61% yield. The acetyl groups were then removed by treating 6 with sodium methoxide, furnishing the amidoxime ester prodrug 7 in good yields (65%). Furthermore, as an extension of the herein described prodrug principle, a pro-prodrug concept was realized by introducing an O-succinate (12) substituent to amidoxime 6 (see Supporting Information).
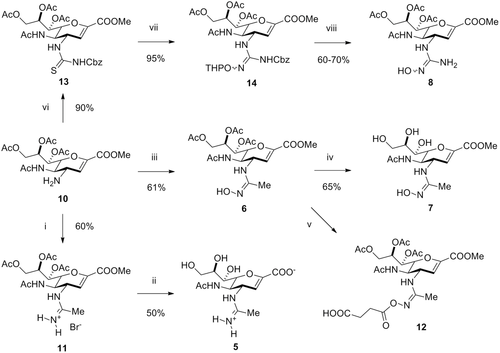
As the hydroxyguanidine ester prodrug (9) was obtained commercially, we only had to prepare peracetyl hydroxyguanidine ester (8) which was carried out according to established synthetic routes for N-hydroxyguanidines.27, 35, 36 First, amine 10 was converted to the Cbz-protected thiourea 13 using benzyloxycarbonylisothiocyanate in very good yields (90%). Subsequently, the hydroxyguanidine moiety was installed by reacting 13 with O-THP-protected hydroxylamine in the presence of a desulfurizing reagent (i.e., EDCI), furnishing 14 in excellent yields (95%). Finally, the desired peracetyl hydroxyguanidine ester prodrug 8 was obtained removing the THP protecting group with TFA/thioanisol in good yields (typically 60%–70%).
Antiviral Activity
In order to confirm that bioisosteric replacement of the 4-guanidino group by a 4-acetamidine renders this zanamivir analog active, several influenza A/H1N1 and A/H3N2 subtypes, including two oseltamivir-resistant strains harboring the H275Y mutation,30 were tested against amidine 5 using an established chemiluminescence-based NA assay (Table 1).37, 38 The original guanidine 1 served as a reference compound and potently inhibited different H3N2 viruses (0.20–0.66 nM, IC50), wild type pandemic H1N1 (0.03–0.05 nM, IC50), A/WSN/33-M2-N31S as well as two H275Y mutant H1N1 strains (0.26 and 0.29 nM, IC50). In comparison, amidine 5 was about 10-fold less potent against these influenza A viruses suggesting that the guanidino group indeed contributed to higher NA affinity than an amidine group. These data paralleled the trend observed in a complementary cell-based assay that addresses the CPE of influenza infections (Table 2).37, 38 However, zanamivir amidine 5 is still a highly potent NAI considering inhibition potencies in the single digit range.
| Influenza Virus of Subtype H1N1 | |||||
|---|---|---|---|---|---|
| IC50 Values (nM) | |||||
| Compound | A/Jena/5258/2009 | A/WSN/33-M2-N31S | A/WSN/33-M2-N31Sb (H275Y Mutant) | A/Berlin/55/08b (H275Y Mutant) | |
| Zanamivir amidine (5) | 2.20 ± 1.13 | 2.23 ± 0.40 | 4.33 ± 0.95 | 9.97 ± 1.50 | |
| Zanamivir (1) | 0.03 ± 0.01c | 0.21 ± 0.11 | 0.26 ± 0.05 | 0.29 ± 0.17c | |
| Influenza Virus of Subtype H3N2 | |||||
| IC50 Values (nM) | |||||
| Compound | A/Hong Kong/68 | A/Sachsen/6/02 | A/Berlin/ 10/04 | A/Rheinland-Pfalz/3911/03 | Overall anti H3N2 Activity |
| Zanamivir amidine (5) | 6.33 ± 2.33 | 10.47 ± 4.78 | 8.89 ± 0.82 | 4.91 ± 1.91 | 7.65 ± 2.50 |
| Zanamivir (1) | 0.66 ± 0.56c | 0.49 ± 0.16c | 0.45 ± 0.07c | 0.20 ± 0.01c | 0.45 ± 0.19c |
- a Mean IC50 ± SD of at least 2–3 independent experiments.
- b Oseltamivir-resistant influenza A, mutant H1N1 (H275Y).
- c Data as previously describe by us [20].
| IC50 Values (μM) | |||
|---|---|---|---|
| H3N2 A/Hong | H1N1 A/WSN | H1N1 A/Berlin | |
| Compound | Kong/68 | /33-M2-N31S | /55/08b |
| Zanamivir amidine (5) | 0.23 ± 0.13 | 0.58 ± 0.31 | 3.84c |
| Zanamivir (1) | 0.07 ± 0.09 | 0.013 ± 0.003 | 0.40c |
- a Mean IC50 ± SD of at least three independent experiments.
- b Oseltamivir-resistant influenza A, mutant H1N1 (H275Y).
- c Data from single experiments (three technical replicates).
In Vitro PK
The herein presented prodrug candidates of zanamivir were primarily designed to improve membrane permeability, and thereby oral bioavailability, by increasing passive diffusion processes. Consequently, polar functional groups were masked by esterification and ionizable moieties (i.e., amidine and guanidine) were converted to functionalities (i.e., amidoxime and hydroxyguanidine) that react neutral at physiological pH. Hence, more lipophilic structures were generated and their solubilities needed to be determined (Fig. S1, Supporting Information) to ensure that a low solubility does not become the limiting factor for the desired oral administration and bioavailability, respectively. However, our results revealed that amidine 5 and its two amidoxime-based prodrugs 6 and 7 were highly soluble at pH 2, 7.4 and 9.0 (i.e., >50 mg/mL) underlining their value for diverse administration routes (Fig. S1, Supporting Information). N-hydroxyguanidine ester 9 was comparably soluble at >50 mg/mL (>100 mM). The solubility of zanamivir (1) ranged between 17 and 23 mg/mL (48–66 mM) depending on the pH. However, for the peracetyl hydroxyguanidine ester (8) solubility could only be accurately determined at pH 2 (>50 mg/mL) because of its instability at higher pH values (see below). Together, all new drug and prodrug candidates exhibited sufficient solubilities for various types of administration, even beyond peroral applications.
As indicated above, stability issues became obvious with prodrug 8, and thus, chemical and plasma stabilities were investigated for all new zanamivir drugs and prodrugs (Fig. 4). Zanamivir amidine was highly stable at all tested pHs (pH 2, 7.4, and 9.0) as well as towards murine and human plasma (see Fig. S1, Supporting Information). Amidoxime ester 7 was highly stable at pH 2 and at physiological pH 7.4 but susceptible to hydrolysis of the methyl ester at pH 9. Methyl ester hydrolysis was then, of course, also observed in murine and human plasma but still left this prodrug with an acceptable plasma half-life (t1/2 ca. 2 h). In contrast, because of the many O-acetyl groups, hydrolysis was already observed at pH 7.4 for prodrug 6, and was even more pronounced at pH 9. Similarly, 6 exhibited a very short plasma half-life (t1/2 < 20 min) which is not necessarily a red flag in this context, considering that these hydrolytic reactions are eventually needed for a complete bioactivation. Moreover, as an attempt to take advantage of potential active transport mechanisms, an O-succinate (12) substituent was introduced into the amidoxime-based prodrugs. However, we found that this “pro-prodrug” conjugate was quite labile under all tested conditions, and therefore, was not further pursued (data not shown).
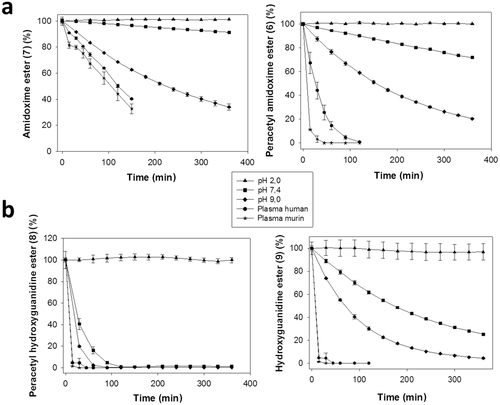
Interestingly, the hydroxyguanidine ethyl ester prodrug 9 showed a higher instability at pH 7.4 (t1/2 ca. 3.5 h) and 9.0 (t1/2 ca. 1.5 h) compared with its amidoxime methyl ester analog 7 suggesting that additional degradation reactions occurred (not further investigated). For the peracetylated prodrug 8 chemical and plasma stabilities were rather poor, questioning its utility as a prodrug candidate. In summary, these data underlined the particular stability of amidoxime ester prodrug 7 which is relevant for a reasonable survival of this compound under conditions found in the stomach and intestine after oral administration. Although less stable than 7, prodrug 9 also exhibited a sufficient degree of stability that encouraged in vivo applications.
In addition to stability studies, in vitro bioactivation of the different prodrug candidates was evaluated using distinct liver enzyme preparations to justify further whole animal studies. Notably, only amidoxime ester 7 and hydroxyguanidine ester 9 were tested for two reasons. For one, monitoring bioactivation via N-reduction of the amidoxime and N-hydroxyguanidine moieties was essential and would have been complicated from a technical point of view when using the peracetylated prodrugs which gave rise to more metabolites. Second, bioactivation by esterases is a known powerful metabolism which we have already characterized in incubations with human and rat plasma sources (Fig. 4). Therefore, amidine 5 and the amidine methyl ester metabolite were quantified by HPLC from incubations of amidoxime ester 7 with porcine and human liver (and kidney) enzyme preparations. The same was performed for prodrug 9 for which the guanidine 1 as well as the guanidine ethyl ester metabolite were quantified. As shown in Table 3, both prodrugs 7 and 9 were activated to the corresponding amidines at moderate to high turnover rates. However, amidoxime ester 7 appeared to be more efficiently bioactivated than hydroxyguanidine ester 9, and particularly by porcine enzyme sources.
| Conversion Rate (nmol/min/mg Protein) | ||||
|---|---|---|---|---|
| Amidoxime Ester (7) | Hydroxyguanidine Ester (9) | |||
| Enzyme Source | Amidine Drug (5) | Amidine Ester | Guanidine Drug (1) | Guanidine Ester |
| PL Ms | 0.06 ± 0.02 | 5.70 ± 0.33 | 0.02 ± 0.01 | 0.05 ± 0.01 |
| PL Mt | 0.07 ± 0.02 | 4.42 ± 0.24 | 0.02 ± 0.01 | 0.05 ± 0.01 |
| PL 9000g | 0.10 ± 0.04 | 2.57 ± 0.43 | 0.02 ± 0.01 | 0.04 ± 0.01 |
| PL Cyt | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.05 | 0.06 ± 0.01 |
| PK 9000g | 0.07 ± 0.01 | 1.35 ± 0.05 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| PK Ms | 0.05 ± 0.03 | 4.42 ± 0.62 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| HL Ms | 0.00 ± 0.02 | 0.14 ± 0.01 | n.d. | 0.08 ± 0.02 |
| HL Mt | 0.03 ± 0.01 | 0.80 ± 0.02 | n.d. | 0.04 ± 0.01 |
| HL 9000g | 0.01 ± 0.01 | 0.05 ± 0.01 | n.d. | 0.04 ± 0.02 |
| HL Cyt | 0.03 ± 0.01 | 0.01 ± 0.01 | n.d. | 0.04 ± 0.02 |
- a Liver enzyme sources: pig liver (PL), pig kidney (PK), human liver (HL), microsomes (Ms), mitochondria (Mt), cytosol (Cyt), 9000g supernatant (9000g); n.d. (not detectable).
Finally, as part of the in vitro PK profiling, we examined protein binding (albumin) of all new compounds which turned out to be similar or less pronounced compared to zanamivir (1, <10%)39 (Table 4). Only the peracetylated amidoxime ester 6 exhibited higher protein binding (20%).
| Protein Binding Albumin (%) | ||||
|---|---|---|---|---|
| Compound | 10 μM | 25 μM | 50 μM | Mean |
| Amidine (5) | 6.9 ± 0.9 | 16.4 ± 3.6 | 9.1 ± 4.3 | 11.8 ± 4.0 |
| Peracetyl amidoxime ester (6) | 15.2 ± 4.7 | 22.2 ± 1.1 | 22.7 ± 1.0 | 20.0 ± 4.2 |
| Amidoxime ester (7) | 4.7 ± 4.2 | 9.8 ± 1.1 | 1.1 ± 3.3 | 5.4 ± 4.4 |
| Peracetyl hydroxyguanidine ester (8)a | n.d. | n.d. | n.d. | n.d. |
| Hydroxyguanidine ester (9) | 9.0 ± 7.8 | 12.2 ± 4.3 | 7.3 ± 0.1 | 9.5 ± 2.5 |
- a n.d., not determined because of instability (hydrolysis, see Fig. 4) under incubation conditions.
Together, the herein presented data were important to nominate preferred prodrug candidates for in vivo bioavailability studies. All compounds exhibited excellent solubilities and low protein binding. However, limitations loomed ahead with regards to the N-hydroxyguanidine prodrugs 8 and 9. Although 9 was chemically labile to an acceptable degree (t1/2 ca. 3.5 h at pH 7.4), its peracetylated analog 8 was not. Moreover, both N-hydroxyguanidine-based prodrugs appeared to be bioactivated to a lesser extent compared with the amidoxime prodrugs 6 and 7.
In Vivo PK
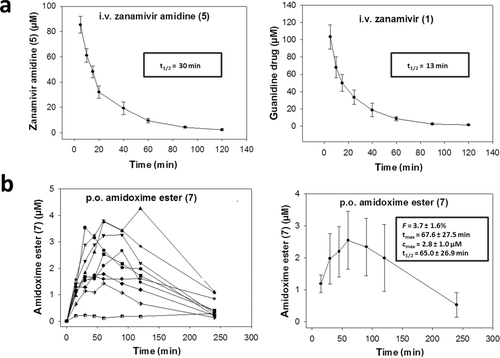
Based on the above described in vitro PK data, amidoxime ester 7 and hydroxyguanidine ester 9 were nominated for a comparative in vivo bioavailability study in rats. Amidine (5) and guanidine (1) drugs were administered intravenously (10 mg/kg for each compound) to obtain reference plasma levels for calculating bioavailability later after oral administration of the prodrug candidates. Interestingly, amidine 5 showed a markedly longer plasma half-life of t1/2 = 30 min compared with guanidine 1 (t1/2 = 13 min) (Fig. 1a). This terminal half-life is in accordance with literature data on zanamivir (1) in rats.40 It appeared, that 5 was not as quickly eliminated from circulation as 1 which may have consequences for the maintenance of therapeutically relevant plasma concentrations. Prodrugs 7 and 9 were administered perorally at 50 mg/kg, and plasma samples were analyzed for concentrations of the prodrugs and their respective drugs by LC–MS. The determined invivo PK parameters are depicted together with the corresponding plasma curves in Figure 1b. A low oral bioavailability of F = 3.7 ± 1.6% (n = 9) was detected for amidoxime ester prodrug 7. The respective drug, amidine 5, could only be detected at very low levels, close to the detection limit (=0.2 μM) which did not allow for determining an F value. On the other hand, prodrug 9 as well as its drug form zanamivir (1) could not be quantified at all. The detection limit for these compounds was 0.5 μM which meant that the oral bioavailability was <3.5%. Thus, the oral bioavailability of 9 was comparably low to that of amidoxime ester prodrug 7. The disappointing outcome of this in vivo data suggested that neither the amidoxime nor the N-hydroxyguanidine prodrug strategy could successfully overcome zanamivir's lack of oral bioavailability which has been stated to be in similar range in healthy humans (F = 2%).19 It is a matter of debate why our success with same amidoxime prodrug strategy for oseltamivir could not be transferred to zanamivir. Although the N-hydroxyguanidine prodrug principle also did not lead to an orally available oseltamivir (F = 1.3%, rats), the amidoxime indeed gave rise to a remarkable bioavailability (F = 33%, rats).20 Therefore, we can only speculate that zanamivir's intrinsic physicochemical features, likely due to its highly polar glycerol side chain, along with its pronounced and rapid elimination from the circulation (i.e., short plasma half-life) are the main reasons for a low systemic availability after oral administration. An extensive metabolism can be excluded as it has been demonstrated that zanamivir (1) is almost completely (90%) excreted in the urine as the unchanged drug.19 Together, our data raise the question whether it is feasible to overcome zanamivir's lack of bioavailability by prodrug strategies that solely mask its polar and ionic structural properties. Currently, data from the Amidon lab are most encouraging as they could at least in vitro show increased cell permeability by targeting active uptake mechanisms.24 It will be interesting to see whether rapid elimination after oral administration, as observed in studies from us (and others), also turns out to be a key bottleneck to reach a reasonable bioavailability.
CONCLUSIONS
In case of influenza pandemic, oseltamivir is currently the drug of choice and has been stockpiled by most countries, primarily because of the possibility for oral administration in the form of capsules. All remaining clinically relevant NAIs cannot be administered orally but instead by inhalation or intravenous injection, respectively (i.e., zanamivir and peramivir). Since the occurrence of oseltamivir-resistant influenza strains, there is an urgent need to identify and develop novel agents, particularly ones that are orally bioavailable. To date, no resistance phenomena have been reported for zanamivir (1) which prompted us to explore strategies to overcome its restriction to inhalative administration.
We used distinct prodrug strategies that were aimed at lowering the basicity of the guanidine (1) and its bioisosteric amidine (5) by N-hydroxylation. Of course, also the carboxylic acid and the polar glycerol side chain were masked with different ester functionalities. First, we could show that a bioisosteric 4-acetamidino zanamivir (5) was still potently active against a panel of H1N1 and H3N2 influenza A strains including an oseltamivir-resistant mutant. In vitro PK studies revealed that all prodrugs were highly soluble, exhibited low protein binding and were bioactivated (i.e., N-reduction) by different liver and kidney enzyme preparations at moderate to good levels. However, some limitations became obvious regarding the N-hydroxyguanidine prodrugs 8 and 9. Both of these prodrugs appeared to be bioactivated to a lesser extent compared with the amidoxime prodrugs 6 and 7. Moreover, the peracetylated N-hydroxyguanidine ester prodrug 9 was chemically labile to an unacceptable degree. Therefore, the best performing amidoxime ester 7 as well as N-hydroxyguanidine ester prodrug 8 were nominated for in vivo bioavailability studies (rats). Disappointingly, however, we were not able to detect a convincing oral bioavailability for both prodrugs (F ≤ 3.7%) which is comparable to the parent zanamivir (F = ca. 2%, humans).
It is tempting to speculate that intrinsic physicochemical features of zanamivir, for instance derived from its highly polar glycerol side chain together with its short plasma half-life, represent hurdles that cannot be overcome by a simple lipophilicity-increasing prodrug strategy. A complementary approach via targeting active transport mechanisms, such as the in vitro promising l-valyl-ester approach for zanamivir by the Amidon group, will have to show whether permeability denotes the actual bottleneck or zanamivir's fate after absorption.
ACKNOWLEDGMENTS
We thank Dr. Ulrich Girreser and Sven Wichmann for performing and supporting NMR- and MS-experiments and gratefully acknowledge the excellent technical assistance of Melissa Zietz. Financial support by the Dritte Patentportfolio Beteiligungsgesellschaft GmbH & Company KG (to B.C.) and the European Social Fund and the Thuringian Ministry of Economy, Labour and Technology (2011FGR0137) (to M.S.) is acknowledged.




