Head-To-Head Comparison of Different Solubility-Enabling Formulations of Etoposide and Their Consequent Solubility–Permeability Interplay
Abstract
The purpose of this study was to conduct a head-to-head comparison of different solubility-enabling formulations, and their consequent solubility–permeability interplay. The low-solubility anticancer drug etoposide was formulated in several strengths of four solubility-enabling formulations: hydroxypropyl-β-cyclodextrin, the cosolvent polyethylene glycol 400 (PEG-400), the surfactant sodium lauryl sulfate, and an amorphous solid dispersion formulation. The ability of these formulations to increase the solubility of etoposide was investigated, followed by permeability studies using the parallel artificial membrane permeability assay (PAMPA) and examination of the consequent solubility–permeability interplay. All formulations significantly increased etoposide's apparent solubility. The cyclodextrin-, surfactant-, and cosolvent-based formulations resulted in a concomitant decreased permeability that could be modeled directly from the proportional increase in the apparent solubility. On the contrary, etoposide permeability remained constant when using the ASD formulation, irrespective of the increased apparent solubility provided by the formulation. In conclusion, supersaturation resulting from the amorphous form overcomes the solubility–permeability tradeoff associated with other formulation techniques. Accounting for the solubility–permeability interplay may allow to develop better solubility-enabling formulations, thereby maximizing the overall absorption of lipophilic orally administered drugs. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2941–2947, 2015
INTRODUCTION
Poor aqueous solubility is a major challenge in today's biopharmaceutics. Not only that ∼50% of marketed drugs are classified as low-solubility compounds,1-3 recent drug discovery statistics indicate that ∼70% of new drug candidates are categorized as having poor water solubility.4-7
Many formulation techniques trying to tackle low aqueous solubility are in common use. These include surface-active agents,8 lipid-based formulations,9, 10 cosolvents,11 cocrystals,12 nanonization techniques,13 cyclodextrins,14 amorphous solid dispersions (ASDs),15 and more. Although significant solubility enhancement may be achieved with these formulations, we have recently discovered that in many cases the increased apparent solubility has its price, which is a parallel decrease in the apparent permeability of the drug.16, 17 Because the intestinal permeability of the drug and the solubility/dissolution of the drug dose in the gastrointestinal milieu are the two key parameters dictating the overall drug absorption following oral administration,18-20 this tradeoff may explain why many times solubility-enabling formulations fail to improve the overall absorption. This solubility–permeability tradeoff was shown in several settings, including cases in which the increased solubility is accompanied by decreased free fraction that can explain the decreased permeability,16, 21-23 but also in cases that do not involve decreased free fraction of the drug.16, 24 It was recently discovered that this tradeoff can be overcome by using the amorphous form of the drug for solubility enhancement via supersaturation.25-28
Existing reports in the literature on the effect of formulation on the drugs’ permeability focus on a single-formulation technique, and examine its effect on the apparent solubility/permeability. Although this information is accumulating in the literature, there is no head-to-head comparison of the impact of different solubility-enabling formulations on the solubility, the permeability, and the resulted solubility–permeability interplay. The purpose of this research is to provide this assessment.
We have chosen the low-solubility anticancer agent etoposide as a model drug, and formulated it in four different solubility-enabling formulations: surfactant, cyclodextrin, cosolvent, and ASD. For each formulation, we tested several strengths, to allow drawing the relationship between the excipient level and the enhancement of etoposide's solubility. We then investigated the in-vitro permeability of etoposide from each formulation, again using different excipient concentrations for each formulation. This enabled us to model the solubility–permeability interplay from the different vehicles, and to study it in one system that allows a head-to-head comparison. Overall, this research illustrates the underlying mechanisms dictating the solubility–permeability interplay, and allows a more intelligent and efficient use of solubility-enabling formulation strategies for enhanced oral drug absorption.
MATERIALS AND METHODS
Materials
Etoposide, 2-hydroxypropyl-β-cyclodextrin (HPβCD), PEG-400, sodium lauryl sulfate (SLS), MES (4-morpholineethanesulfonic acid) buffer, trifluoroacetic acid (TFA), KCl, and NaCl were purchased from Sigma Chemicals Company (St. Louis, Missouri). Acetonitrile, water, and methanol (Merck KGaA, Darmstadt, Germany) were of ultra-performance liquid chromatography (UPLC) grade. All other chemicals were of analytical reagent grade.
Formulations Preparation
Four different etoposide solubility-enabling formulations were designed: surfactant, cyclodextrin, cosolvent, and ASD.
The preparation of the surfactant-, cyclodextrin-, and cosolvent-based formulations involved making MES buffer (10 mM; pH 6.5) solutions of the different excipients in the different strengths: (1) SLS solutions in three different levels, 2.5, 5, and 10 mM; (2) HPβCD solutions in three different levels, 5, 15, and 30 mM; and (3) PEG-400 solutions in three different levels, 10%, 20%, and 30% (v/v). After preparing the different solutions, the equilibrium solubility of etoposide in these solutions was studied, and etoposide powder was introduced to the formulations; for the permeability experiments, etoposide final concentration was made up at 60% the maximum solubility in each solution to keep thermodynamic activity constant across all formulations.
The ASD powder of 20% etoposide in copovidon was prepared by rotovap. Briefly, 20% (w/w) API and 80% (w/w) copovidon were loaded into a 500 mL round-bottom flask. Two hundred milliliters of a 50:50% (v/v) methylene chloride–methanol mixture was added to the flask. The flask was then mounted on a Buchi R215 rotovaper (Flawil, Switzerland) and rotated at 100 rpm at 40°C to dissolve the API and polymer. Once dissolved, the rotavaper was placed under vacuum to remove the solvent and dried for 30 min. The round-bottom flask was removed from the rotavaper and placed in a vacuum oven overnight at ambient temperature. The flask was removed, and the material was scraped and grinded with a mortar and pestle. Solid-state properties of the ASD versus crystalline etoposide were characterized using polarized light microscopy, X-ray diffractometer, differential scanning calorimetry, and thermal gravimetric analysis.
Solubility Experiments
Solubility studies were carried out in each formulation with different excipient strengths, to sketch the relationship between the excipient level and the enhancement of etoposide's solubility.
The equilibrium solubility of etoposide in the surfactant, cyclodextrin, and cosolvent formulations was determined at 25°C, in comparison with the drugs’ solubility in MES buffer (10 mM, pH 6.5), using the traditional shake-flask method, as we have previously reported.29 The solubility was measured at pH 6.5 to match the conditions of the permeability studies, and to mimic the small intestinal environment. Briefly, excess quantities of etoposide were added to glass vials containing the different formulations. The vials were tightly closed and placed in a shaking water bath (100 rpm) at 25°C. Equilibrium was confirmed by the comparison of 48 and 72 h samples. The vials were centrifuged (10,000 g, 15 min) and the supernatant was carefully withdrawn, filtered, and immediately assayed by UPLC.
For the surfactant-based formulations, MES buffer with different SLS levels (2.5, 5, and 10 mM) solutions were studied. For the cyclodextrin-based formulations, MES buffer with different HPβCD levels (5, 15, and 30 mM) solutions were studied. For the cosolvent-based formulations, MES buffer with different PEG-400 levels (10%, 20%, and 30% w/w) solutions were studied.
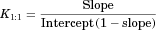
For the ASD formulation, a stability study of supersaturated solutions prepared from the etoposide ASD was carried out, as we have previously reported.32 Briefly, supersaturated etoposide solutions were prepared by dissolving appropriate amounts of the 20% etoposide ASD powder in MES buffer, to obtain supersaturated solution of 2× (392 μg/mL), 4.5× (882 μg/mL), and 6.5× (1274 μg/mL) the equilibrium solubility of crystalline etoposide (196 μg/mL). The supersaturated solutions were allowed to stand at 25°C with no agitation, and were periodically sampled and assayed for etoposide concentration (UPLC). The solution stability was studied for 5 h, which would be sufficient to run the PAMPA experiments. To demonstrate true supersaturation, the equilibrium solubility of crystalline etoposide was measured in the presence of the ASD components, eudragit L-100, and copovidon and found to be unchanged by these polymers.
PAMPA Studies
Permeability studies through artificial membrane were carried out in the precoated PAMPA assay (BD Gentest™, San Jose, CA). The PAMPA plates were handled according to the manufacturer instructions, and the permeability of etoposide from the different formulations was calculated from the drug amount transported versus time, as we have previously reported.33 Briefly, the donor wells were filled with 200 μL of the different etoposide formulations, the receiver wells were filled with 300 μL of blank buffers, and the PAMPA sandwich was incubated at 25°C. The receiver wells were collected every hour for 5 h.
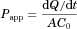
UPLC Analyses
Ultra-performance liquid chromatography analyses were carried out on a Waters Acquity UPLC H-Class system (Milford, MA) equipped with a photodiode array (PDA) detector and Empower software (Empower Software Solutions, Inc., Milford, Massachusetts). The quantification of etoposide was achieved using a Waters Acquity UPLC BEH C18 1.7-μm 2.1 × 100 mm2 column, with a gradient mobile phase, starting at 85:15 going to 15:85 (v/v) water–acetonitrile (0.1% TFA) over 7 min, at a flow rate of 0.5 mL/min. Injection volumes ranged from 5 to 50 μL, the detection wavelength was 240 nm, and the retention time of etoposide was 4.4 min.
Statistical Analysis
Solubility determinations were n = 6, and permeability experiments were replicated with n = 5. Values are expressed as mean ± SD. To determine statistically significant differences among the experimental groups, the nonparametric Kruskal–Wallis test was used for multiple comparisons and the two-tailed nonparametric Mann–Whitney U-test for two-group comparison where appropriate. A p value of less than 0.05 was termed significant.
RESULTS
Solubility
Etoposide solubility enhancement provided by the different solubility-enabling formulations is presented in Figure 1. It can be seen that the cyclodextrin-, surfactant-, and cosolvent-based formulations were able to significantly enhance the solubility of etoposide, directly proportional to the excipient level in the formulation.
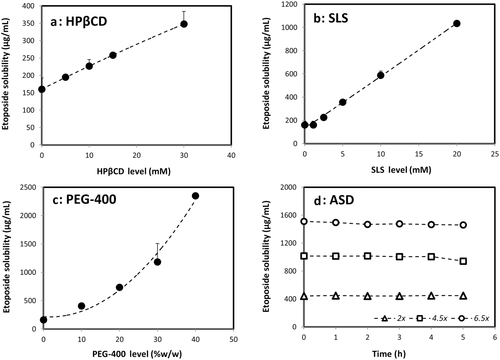
The cyclodextrin-based formulation (panel a) data were found to be of Higuchi AL-type, that is, a linear increased solubility versus HPβCD concentration, with unchanged stoichiometry, suggestive of a 1:1 complexation of etoposide with HPβCD within the concentrations range studied.30, 31 A binding constant, K1:1, of 39.4 M−1 was estimated from this phase-solubility diagram.
The surfactant-based formulation also allowed a significant solubility enhancement of etoposide (panel b). A linear relationship between etoposide solubility and SLS concentration was obtained, yet the solubility enhancement did not start right away, representing surfactant levels below the critical micelle concentration (CMC). An apparent CMC value of 1.1 mM SLS could be readily estimated from the solubility data.
Likewise, the cosolvent-based formulation also enabled a significant solubility enhancement of etoposide (panel c). Unlike the cyclodextrin- and the surfactant-based formulations, the relationship between etoposide solubility and PEG-400 levels was not linear, and could be best fitted with an exponential trendline.
As for the ASD formulation, the stability of etoposide supersaturated solutions originated by the ASD powder at 2.5× (400 μg/mL) and 5.5× (880 μg/mL) the equilibrium solubility of crystalline etoposide (160 μg/mL) is presented in Figure 1 (panel d). It can be seen that the ASD formulation allowed to achieve high levels of etoposide in solution and maintain the supersaturation for a time frame of 5 h, which enables permeability studies to be carried out with these supersaturated solutions.
Permeability
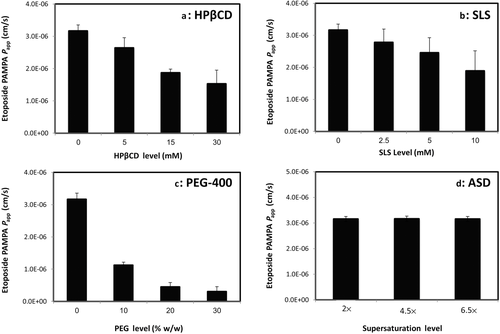
The permeability of etoposide in the parallel artificial membrane permeability assay from the different solubility-enabling formulations is presented in Figure 2. It can be seen that the cyclodextrin-, surfactant-, and cosolvent-based formulations resulted in a significantly decreased permeability, inversely proportional to the excipient level in the formulation. It was noted that the decreased permeability was proportional to the increased solubility offered by the formulation. On the contrary, the ASD formulation did not cause any change in the permeability of etoposide, in all of the strengths, 2×, 4.5×, and the 6.5× the equilibrium solubility of crystalline etoposide (panel d).
The Solubility–Permeability Interplay
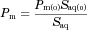
Figure 3 illustrates the concomitant effect of the excipient level on the solubility and permeability of etoposide for the cyclodextrin-, surfactant-, and cosolvent-based formulations. The solubility increase represents the best fit of the experimental values, and the permeability decrease is the theoretical model, as explained above. The solubility and permeability experimental values are also presented in Figure 3. It can be seen that excellent agreement was obtained between the theoretical permeability model and the experimental values, for all three formulations. For the ASD formulation, permeability remained constant regardless of the apparent solubility increase.
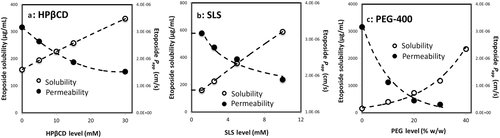
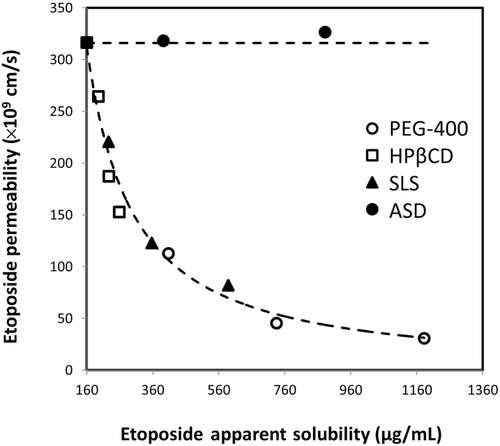
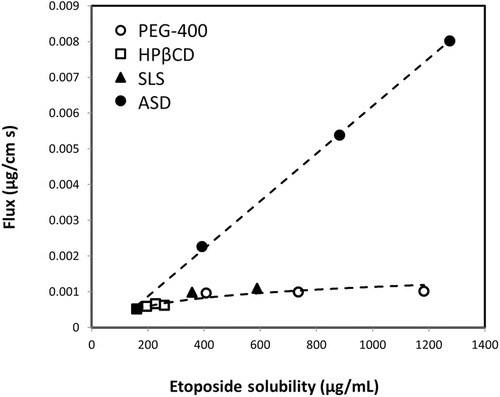
Figures 4 and 5 compare the theoretical apparent permeability and flux of etoposide as a function of apparent solubility. All of the experimental permeability values obtained for the different formulations are also presented in Figures 4 and 5. It can be seen that an excellent agreement was obtained between the theoretical prediction and the experimental permeability values for all of the formulations. As illustrated in Figure 4, enhanced solubility achieved via cyclodextrin-, surfactant-, or cosolvent-based formulations resulted in decreased apparent permeability, whereas supersaturation achieved via ASD formulation resulted in no decrease in apparent permeability with increasing apparent solubility. Consequently, etoposide flux across the membrane increased significantly with increasing apparent solubility via ASD in comparison with all other solubility-enabling formulations (Fig. 5).
DISCUSSION
In this article, we performed a head-to-head comparison of four different solubility-enabling formulations and their effects on the apparent solubility, the apparent permeability, and the solubility–permeability interplay of the lipophilic anticancer agent etoposide. All of the tested formulations were able to significantly increase the drugs’ apparent solubility; however, though the cyclodextrin-, surfactant-, and cosolvent-based formulations resulted in a concomitant decreased apparent permeability, the permeability of etoposide remained constant from the ASD formulation in spite of the significant increase in the apparent solubility. Frank et al.27 has recently reported that prolonged supersaturation is maintained by the dissolution of amorphous microparticles that present in the aqueous dispersions, which promotes the increased transmembranal flux from ASD formulation reported in this study (Fig. 5). This represents a significant advantage for using the amorphous form for oral drug delivery over other formulation techniques; whereas with other formulations, the higher solubility comes with the price of lower permeability and hence the balance between the gain and the loss must be assessed, ASD formulation provides higher apparent solubility without any payment, allowing higher flux of drug molecules across the intestinal membrane, and hence is superior to other formulation techniques from this point of view. Yet, it should be noted that further studies in the whole animal level are needed to confirm the biorelevance of the data.
The mechanism behind this fundamental difference between the ASD and the other formulations can be related to the very own nature of permeability across membranes; intestinal permeability reflects the penetration rate of a drug into the intestinal tissue, and mathematically, the permeability is equal to the diffusion coefficient of the drug through the membrane times the membrane/aqueous partition coefficient of the drug, divided by the membrane thickness. Because the membrane/aqueous partition coefficient is controlled by the drugs’ equilibrium aqueous solubility, formulations that increase the equilibrium solubility will decrease, by definition, the membrane/aqueous partition coefficient, and thereby a decreased driving force for absorption and lower intestinal permeability will be obtained. This explains the solubility–permeability tradeoff that was shown for the cyclodextrin-, surfactant-, and cosolvent-based formulations. It should be noted that this phenomenon occurs when the increased solubility involves binding/complexation that decreases the free fraction of the drug, for example, surfactant or cyclodextrins,34, 35 but also when free fraction issues are not relevant, for example, cosolvents.24 On the contrary, supersaturation involves a kinetic/nonequilibrium increase of the apparent solubility, and hence, the membrane/aqueous partition coefficient is not affected by supersaturation. For this reason, the permeability remains constant when using ASD formulations, irrespective of the increased apparent solubility provided by the formulation.
CONCLUSIONS
A head-to-head comparison of different etoposide's solubility-enabling formulations revealed that supersaturation using ASDs overcomes the solubility–permeability tradeoff that is associated with other formulation techniques. Accounting for the solubility–permeability interplay may enable to develop better solubility-enabling formulations, thereby maximizing the overall absorption of lipophilic orally administered drugs.
Acknowledgments
This work was supported by a research grant from AbbVie Laboratories. This work is a part of Avital Beig's Ph.D. dissertation.




