Carrier-Mediated Transport of Nicotine Across the Inner Blood–Retinal Barrier: Involvement of a Novel Organic Cation Transporter Driven by an Outward H+ Gradient
Abstract
The present study was carried out to investigate the blood-to-retina transport of nicotine across the inner blood–retinal barrier (BRB). Using the in vivo vascular injection method, the blood-to-retina influx clearance of nicotine across the BRB was determined as 131 μL/(min?g retina), which is much higher than that of a nonpermeable paracellular marker, and blood-to-retina transport of nicotine was inhibited by organic cations such as pyrilamine and verapamil. The nicotine uptake by a conditionally immortalized rat retinal capillary endothelial cell line (TR-iBRB2 cells), an in vitro model of the inner BRB, exhibited time, temperature, and concentration dependence with a Km of 492 μM. These results suggest the involvement of a carrier-mediated transport process in nicotine transport in the inner BRB. The nicotine uptake by TR-iBRB2 cells was stimulated by an outwardly directed H+ gradient, and the uptake was significantly inhibited by bulky and hydrophobic cationic drugs, whereas inhibitors of organic cation transporters did not show inhibitory effect. These results suggest that the novel organic cation transport system driven by an outwardly directed H+ gradient is involved in the blood-to-retina transport of nicotine across the inner BRB. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3069–3075, 2015
Abbreviations used
-
- BRB
-
- blood–retinal barrier
-
- MPP+
-
- 1-methyl-4-phenylpyridinium
-
- nAChR
-
- nicotinic acetylcholine receptor
-
- PAH
-
- p-aminohippurate
-
- RUI
-
- retinal uptake index
-
- TEA
-
- tetraethylammonium
-
- TR-iBRB2 cells
-
- a conditionally immortalized rat retinal capillary endothelial cell line
INTRODUCTION
Nicotine is a typical nicotinic acetylcholine receptor (nAChR) agonist which affects a variety of behaviors including nociception, cognition, and memory.1 Besides the pharmacological evidence about the effects of nicotine, there is increasing evidence for carrier-mediated nicotine transport. The previous studies have shown the carrier-mediated transport of nicotine in mammalian tissues and cell lines, such as rat kidney, rabbit choroid plexus, LLC-PK1 cells, JAR cells, and Caco-2 cells,2-6 and nicotine is reported as a substrate of multidrug and toxin extrusion protein 1 (MATE1/SLC47A1) and plasma membrane monoamine transporter (PMAT/SLC29A4), and an inhibitor of organic cation transporter family (OCT1-3/SLC22A1-3) and organic cation/carnitine transporter family (OCTN1-2/SLC22A4-5).7-12 Recently, our studies have demonstrated that the nicotine influx transport in the rat blood–brain barrier (BBB) and the liver was carried out by the carrier-mediated process involving a novel organic cation transporter.13, 14 Although the physiological role is unknown, the nicotine transport system is assumed to play an important role in modulating the concentration of nicotine in various tissues including the central nervous system (CNS).
The optic nerve system is thought to be a part of the CNS, and the retina is the specific tissue where light is directly focused on the cells. For a healthy vision, it is essential to maintain retinal homeostasis, and the paracellular transport of compounds between the circulating blood and retina is regulated by the inner and outer blood–retinal barriers (BRB), that are formed by tight junctions of the retinal capillary endothelial cells and retinal pigment epithelial cells, respectively. In addition, it has been found that various transporter molecules are expressed at the BRB, and carry out endobiotic and xenobiotic transport between the retina and the blood.15 To achieve efficient and safe drug treatment of retinal diseases, it is important to increase our understanding of the blood-to-retina transport systems via the BRB as topical drug administration is known to be inefficient.16
Interestingly, previous studies have provided the pharmacological evidence that nicotine binds to retinal nAChRs to alter responses in the retina as the expression of nAChRs has been reported in retinal cells, such as amacrine and ganglion cells, of various species including rat, mouse, chick, rabbit, and pig.17-21 In humans, it has been suggested that the nAChRs are predominantly expressed in the inner plexiform layer of the retina,22 and the electroretinogram was affected by administration of nicotine gum.23 In addition, previous in vitro studies have demonstrated that nicotine exerts a protective effect against glutamate-induced excitotoxicity via the nAChR in retinal ganglion cells,24, 25 and a recent study using rat glaucoma model revealed that an α7 nAChR agonist has a neuroprotective effect against loss of retinal ganglion cells,26 showing that the nAChR agonist is a strong candidate for the drug therapy of glaucoma. These lines of evidence indicate the usefulness of nicotine or nAChR agonists for retinal diseases accompanied by neurodegeneration, and an improvement of our understanding of the nicotine transport mechanism at the BRB is important for achieving efficient drug delivery from the blood to the retina.
In the present study, the properties of blood-to-retina transport of nicotine across the inner BRB were investigated by an in vivo vascular injection technique and a cellular uptake study using a conditionally immortalized rat retinal capillary endothelial cell line (TR-iBRB2 cells), an in vitro model of the inner BRB, to clarify the nicotine transport system at the inner BRB.
MATERIALS AND METHODS
Animals
Wistar rats (6-week-old, male, 150–200 g) were purchased from Japan SLC (Hamamatsu, Japan) and kept in a controlled environment. All experiments were approved by the Animal Care Committee, University of Toyama, and conformed to the provisions of the Association for Research in Vision and Ophthalmology Statement.
Reagents
L-(-)-[N-methyl-3H]Nicotine ([3H]nicotine, 83.5 Ci/mmol) was purchased from PerkinElmer (Boston, Massachusetts). n-[1-14C]Butanol, ([14C]n-butanol, 2 mCi/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, Missouri). All other chemicals were commercially available and analytical grade.
Integration Plot Analysis
 (1)
(1)Retinal Uptake Index Method
 (2)
(2)Uptake Study in TR-iBRB2 Cells
As described previously,31 TR-iBRB2 cells, a conditionally immortalized rat retinal capillary endothelial cell line,32 was cultured at 33°C to allow temperature-sensitive large T-antigen expression. Cells were washed three times with extracellular fluid (ECF) buffer [122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 0.4 mM K2HPO4, 1.4 mM CaCl2, 1.2 mM MgSO4, 10 mM d-glucose, and 10 mM HEPES (pH 7.4)], and the uptake was initiated by applying [3H]nicotine (0.5 μCi/mL, 6 nM) dissolved in ECF buffer at 37°C in the absence or presence of inhibitors. When examining the influence of extracellular pH on nicotine uptake, media with different pH values (pH 6.4, 7.4, and 8.4) were used. NH4Cl with a concentration of 30 mM was used to increase the intracellular pH of TR-iBRB2 cells.33, 34 To examine the influence of intracellular acidification, extracellular NH4Cl was removed after preincubation with 30 mM NH4Cl for 20 min, because intracellular NH3 rapidly diffuses out of cells, resulting in the accumulation of H+ released from NH4+ in a process to produce NH3.35 Cells were rinsed three times with ice-cold buffer to terminate the uptake, and were solubilized with 1N NaOH. Subsequently, the solubilized solution was neutralized with 1N HCl. The radioactivity associated with cells was measured by liquid scintillation counting (LSC-7400; Hitachi Aloka Medical), and the cellular protein content was measured by a detergent-compatible protein assay (a DC protein assay kit; Bio-Rad, Hercules, California) with bovine serum albumin as a standard.
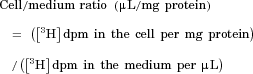 (3)
(3) (4)
(4) (5)
(5)Statistical Analysis
The parameters (Kin, retina, Km, Vmax, and Ki) were determined by least-squares regression analysis and presented as the means ± SD. Other data represent the means ± SEM. Student's t-test was used in order to determine the significance of differences between two unpaired group means. One-way ANOVA followed by Dunnett's test was carried out in order to assess the statistical significance of differences among means of more than two groups.
RESULTS
Blood-to-Retina Transport of [3H]Nicotine Across the BRB
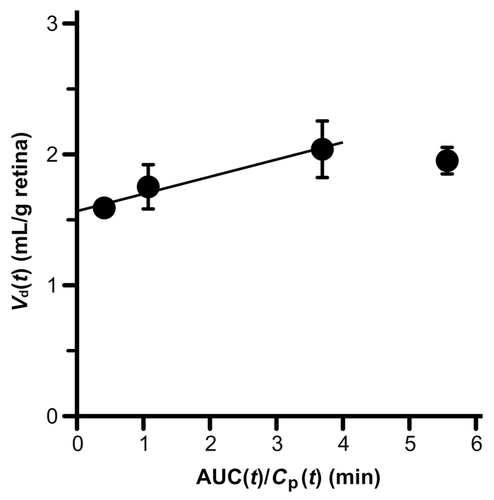
The in vivo blood-to-retina transport of nicotine across the BRB was investigated using integration plot analysis after intravenous administration of [3H]nicotine to rats. According to Eq. 1, the apparent blood-to-retina influx clearance (Kin, retina) of [3H]nicotine was estimated as 131 ± 25 μL/(min·g retina) (Fig. 1).
To investigate the mechanism of in vivo blood-to-retina transport of nicotine in rats, an inhibition study was performed using the RUI method. The RUI value of [3H]nicotine was found to be 312%, and this value was significantly reduced by 30% and 49% in the presence of 30 and 100 mM unlabeled nicotine, respectively (Table 1). In addition, 40 mM pyrilamine and 3 mM verapamil reduced the RUI value of [3H]nicotine by 45% and 30%, respectively (Table 1). These results suggest that a carrier-mediated transport process with cationic drug sensitivity is involved in nicotine transport to the retina from the blood.
| Compounds | RUI (%) | Percentage of Control |
|---|---|---|
| Control | 312 ± 21 | 100 ± 7 |
| Nicotine (30 mM) | 218 ± 20** | 70.1 ± 6.5** |
| Nicotine (100 mM) | 159 ± 15** | 50.9 ± 4.7** |
| Pyrilamine (40 mM) | 173 ± 5** | 55.5 ± 1.7** |
| Verapamil (3 mM) | 218 ± 19** | 69.9 ± 6.1** |
- [3H]Nicotine (3.4 μCi/rat) and [14C]n-butanol (0.4 μCi/rat) dissolved in 200 μL Ringer-HEPES buffer (pH 7.4) were injected into the common carotid artery in the absence (control) or presence of inhibitors. Each value represents the mean ± SEM (n = 3–6). **p < 0.01, significantly different from control.
[3H]Nicotine Uptake by TR-iBRB2 Cells
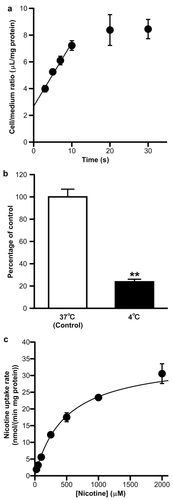
To characterize the nicotine transport across the inner BRB, a [3H]nicotine uptake study was performed using TR-iBRB2 cells. [3H]Nicotine uptake increased lineally for at least 10 s (Fig. 2a), suggesting that nicotine uptake during 0–10 s mostly shows the influx into cells, and the uptake at 10 s was significantly reduced by 76% at 4°C (Fig. 2b). The nicotine uptake exhibited a concentration dependence with a Km of 492 ± 41 μM and a Vmax of 35.3 ± 2.0 nmol/(min?mg protein) (Fig. 2c). These results suggest that a carrier-mediated transport process is involved in nicotine uptake by TR-iBRB2 cells.
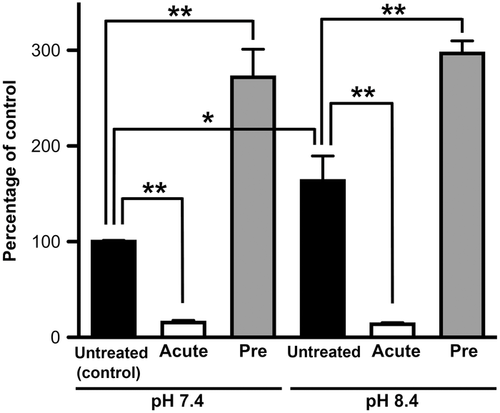
In the study of pH dependence, [3H]nicotine uptake was significantly increased to 163% at an extracellular pH of 8.4 (Fig. 3). Acute treatment with NH4Cl (intracellular alkalization) reduced [3H]nicotine uptake at pH 7.4 and 8.4 by 85% and 92%, respectively. In addition, pretreatment with NH4Cl (intracellular acidification) increased [3H]nicotine uptake at pH 7.4 and 8.4 to 272% and 182%, respectively (Fig. 3). These results suggest the presence of a pH-dependent transport system for nicotine in TR-iBRB2 cells.
Inhibition Study of [3H]Nicotine Uptake by TR-iBRB2 Cells
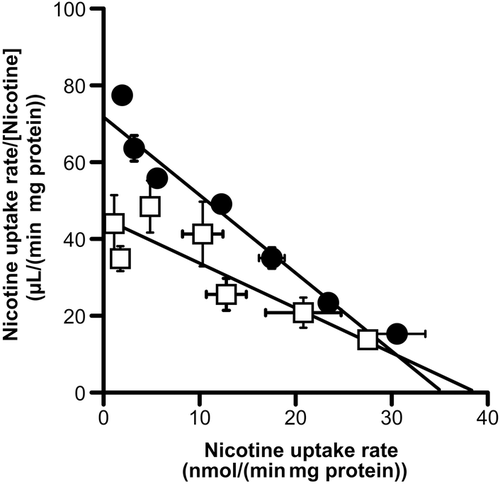
Table 2 shows the inhibitory effect of cationic compounds on [3H]nicotine uptake by TR-iBRB2 cells. Cationic drugs, such as nicotine, pyrilamine, verapamil, propranolol, clonidine, desipramine, and timolol, significantly inhibited [3H]nicotine uptake by more than 81%. On the contrary, no inhibitory effect on [3H]nicotine uptake was shown by tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP+), choline, l-carnitine, serotonin, thiamine, and p-aminohippurate (PAH), which are substrates and/or inhibitors of typical organic cation transporters and organic anion transporters. In the Eadie–Scatchard plot analysis, a Vmax and Km value of nicotine uptake in the presence of pyrilamine were estimated as 38.7 ± 5.1 nmol/(min·mg protein) and 848 ± 257 μM, respectively (Fig. 4). The Km value in the presence of pyrilamine was significantly increased compared with that in the absence of pyrilamine (Km = 492 μM) (p = 0.000059), and the Vmax value in the presence of pyrilamine showed no significant difference compared with that in the absence of pyrilamine [Vmax = 35.3 nmol/(min·mg protein)] (p = 0.13), indicating the competitive inhibition of [3H]nicotine uptake by pyrilamine (Ki = 67.7 ± 45.7 μM).
| Compounds | Percentage of Control |
|---|---|
| Control | 100 ± 5 |
| Nicotine | 26.4 ± 1.3** |
| Propranolol | 8.19 ± 0.73** |
| Pyrilamine | 11.6 ± 1.1** |
| Verapamil | 13.2 ± 1.1** |
| Desipramine | 14.4 ± 3.4** |
| Clonidine | 15.2 ± 1.6** |
| Timolol | 19.2 ± 2.1** |
| Thiamine | 77.7 ± 15.1 |
| Serotonin | 102 ± 2 |
| l-Carnitine | 113 ± 12 |
| Choline | 116 ± 13 |
| 1-Methyl-4-phenylpyridinium | 130 ± 18 |
| Tetraethylammonium | 167 ± 21** |
| p-Aminohippurate | 138 ± 19* |
- [3H]Nicotine uptake (0.5 μCi/mL, 6 nM) was measured at 37°C for 10 s in the absence (control) or presence of 1 mM compounds. Each value represents the mean ± SEM (n = 3–9).
- *p < 0.05, **p < 0.01, significantly different from control.
DISCUSSION
Our previous in vivo and in vitro studies revealed the carrier-mediated transport of cationic drugs, such as verapamil and pyrilamine, and their transport properties apparently differ from well-characterized organic cation transport systems, such as the OCT1-3, OCTN1-2, MATE1, and PMAT.38 These transports are inhibited by several neuroprotective drugs, such as timolol and desipramine,39, 40 showing the possible advantage of these transport systems at the inner BRB for the delivery of neuroprotective drugs to the retina. In addition, the transport of propranolol, a β-adrenergic antagonist, and clonidine, an α2 agonist, in the inner BRB was investigated because of their neuroprotective effect, and suggested the involvement of novel organic cation transporters in such transport.33, 34 Interestingly, propranolol and clonidine transport in the inner BRB was inhibited by nicotine. Moreover, it has been suggested that a similar transport system, as yet unidentified, is involved in pyrilamine transport in the inner BRB and BBB.38 These lines of evidence suggest the possible presence of an influx transport system that is involved in nicotine transport at the BRB, and the clarification of the transport properties of nicotine at the BRB will help increase our understanding of the mechanisms of cationic drug transport at the BRB. The present study provides in vivo and in vitro evidence that nicotine is transported from the circulating blood to the retina across the inner BRB via a carrier-mediated process involving a novel H+/organic cation antiporter.
In the integration plot analysis, [3H]nicotine was transported from the circulating blood to the retina across the BRB with a Kin, retina of 131 μL/(min·g retina) that is much higher than that of d-mannitol and sucrose, nonpermeable paracellular markers (Fig. 1),41 indicating the influx transport system of nicotine at the BRB. In addition, the involvement of a carrier-mediated process in blood-to-retina transport of nicotine across the BRB was also implied as an influx clearance for the blood-to-retina transport of nicotine is similar to that of the blood-to-brain transport of nicotine [272 μL/(min·g brain)].13 The blood flow rate of retina has been reported to be 700 μL/(min·g retina),42 and it is 5.3-fold greater than the Kin, retina value of [3H]nicotine, suggesting that the membrane transport at the BRB is a rate-limiting step in nicotine uptake into retina. In a previous study, the correlation between the lipophilicity of compounds and the RUI values was reported,29 and the RUI value of nicotine is roughly estimated as 60% from the lipophilicity of nicotine.43 However, the actual RUI value of nicotine is 5.2-fold greater than the estimated value, and this value was significantly inhibited by unlabeled nicotine, pyrilamine, and verapamil. In the inhibition study of RUI method, inhibitors at high concentration have been often applied because of mixing effect, that dilutes inhibitors in the circulating blood, and it is unlikely that the inhibitors exert the unexpected effects, such as toxicity, during a very short uptake time after injection (15 s), supporting that the reduction of RUI was caused by the inhibition of nicotine transport, not by their toxicity. Taking these into consideration, it is suggested that the carrier-mediated process, which can be interacted with pyrilamine and verapamil, plays a role in the influx transport of nicotine at the BRB.
The [3H]nicotine uptake by TR-iBRB2 cells exhibited time, temperature, and concentration dependence with a Km of 492 μM, which is comparable to that of [3H]nicotine transport in TR-BBB13 cells, an in vitro rat BBB model, isolated rat hepatocytes, and LLC-PK1 cells (92–360 μM).5, 13, 14 The cell/medium ratio in TR-iBRB2 cells is smaller than that observed in TR-BBB13 cells (∼58 μL/mg protein) and rat hepatocytes (∼62 μL/mg protein),13, 14 and their differences in nicotine accumulation implied that the lower expression and/or function of nicotine transport system in TR-iBRB2 cells than those in TR-BBB13 cells and rat hepatocytes.
In addition, the Km value of nicotine uptake by TR-iBRB2 cells (492 μM) is much greater than the clinical concentration of nicotine (179–370 nM),44 supporting that nicotine transport at the inner BRB is hardly saturated. This also suggests the proportional increase of the nicotine concentration in the retina with that in the circulating blood, and the study of a carrier-mediated nicotine transport process at the inner BRB would be helpful to predict and to modulate pharmacological effects, such as neuroprotection, of nicotine in the retina.
The pH dependence of [3H]nicotine uptake by TR-iBRB2 cells was investigated because our previous studies showed the pH-dependent transport of organic cation drugs such as pyrilamine, propranolol, and clonidine in TR-iBRB2 cells.33, 34, 38 The [3H]nicotine uptake was increased at an extracellular pH of 8.4. Intracellular acidification and intracellular alkalization produced a significant increase and decrease of [3H]nicotine uptake, respectively, suggesting that nicotine transport in TR-iBRB2 cells is driven by an outwardly directed H+ gradient.
Furthermore, the inhibition study suggested that an organic cation-sensitive transport system is involved in the blood-to-retina transport of nicotine as in vitro [3H]nicotine uptake by TR-iBRB2 cells was significantly inhibited by bulky and hydrophobic cationic drugs such as pyrilamine, verapamil, propranolol, clonidine, desipramine, and timolol (Table 2). Interestingly, the inhibitory potential of unlabeled nicotine seemed to be different between in vivo and in vitro assays. Differing from TR-iBRB2 cells, an in vitro model of the inner BRB, the RUI method represents the combined transport at the inner and outer BRB. In light of this point, the difference of apparent nicotine sensitivity to the [3H]nicotine transport between in vivo and in vitro studies could be caused by the nicotine transport across the outer BRB within the in vivo uptake into the retina. In contrast to the cationic drug described above, TEA, l-carnitine, MPP+, choline, serotonin, thiamine, and PAH, which are reported to be substrates and/or inhibitors of OCTs, OCTNs, MATE1, PMAT, and OATs,9-12, 35, 45-47 did not have any inhibitory effect on [3H]nicotine uptake, suggesting the involvement of the novel organic cation transporter in the blood-to-retina transport of nicotine.
In regard to the novel organic cation transporter, the functional expression of the transport systems for verapamil, pyrilamine, propranolol, and clonidine has been demonstrated at the inner BRB.33, 34, 38 It is suggested that the transport system of nicotine in the inner BRB is not identical with that of verapamil because nicotine transport is l-carnitine-insensitive and pH-sensitive, whereas verapamil transport has been reported to be l-carnitine-sensitive and pH-insensitive.38 Although propranolol transport at the inner BRB is reported to be pH-sensitive, the inhibitory effect of nicotine on propranolol uptake by TR-iBRB2 cells was much lower than that predicted from the affinity of nicotine transport (Km = 492 μM).33 In addition, nicotine uptake by TR-iBRB2 cells is serotonin-insensitive, whereas the propranolol uptake is sensitive. These results suggest that the molecules responsible for nicotine and propranolol transport are different. A similar affinity for pyrilamine has been shown between the high-affinity pyrilamine transport system in the inner BRB (Km = 20 μM) and the putative pyrilamine transport system at the BBB (Km = 28 μM),38, 48 and the nicotine affinity at the inner BRB (Km = 492 μM) also resembles that at the BBB (Km = 92 μM).13 In addition, the nicotine uptake by TR-iBRB2 cells was inhibited by pyrilamine in a competitive manner, and the Ki value was very similar to the Km value of the high-affinity process of pyrilamine uptake by TR-iBRB2 cells (Km = 20 μM).38 These data indicate the potential identity of the molecules involving the nicotine and pyrilamine transport at the inner BRB, and this transport system may play a significant role in regulation of the nicotine concentration with modulating nicotine-induced neural responses in the retina. On the contrary, it would be difficult to define the physiological meaning of nicotine uptake into the retina as nicotine is thought to be originally inessential to the normal retinal function, and it is a possible story that nicotine transport system is principally involved in the transport of unknown endobiotics across the inner BRB.
From a pharmacological view point, evidence that nicotine exerts a neuroprotective effect has been presented in a number of previous reports, and recent studies suggest the usefulness of the nAChR agonist in drug therapy for glaucoma.26 On the contrary, nicotine is considered to be a risk factor for age-related macular degeneration (AMD) as it has been shown that nicotine increases the size and severity of experimental choroidal neovascularization.49 In addition, it has been reported that nicotine exhibits a proangiogenic effect in human retinal endothelial cells,50 suggesting that nicotine exposure may affect the pathogenesis of AMD. Taking these pieces of evidence into consideration, it seems that the nAChR agonist is able to prevent neurodegeneration by intraocular pressure elevation, although the treatment using nAChR agonist is difficult in retinal diseases accompanied by neovascularization, such as AMD and retinal neuropathy. The findings in the present study provide helpful information about how to deliver nicotine and/or nAChR agonist to the retina for the efficient treatment of glaucoma.
CONCLUSIONS
In the present study, our findings from in vivo and in vitro analyses suggest that a carrier-mediated transport process is involved in nicotine transport from the circulating blood to the retina across the inner BRB, and show that the nicotine transport is outward H+ gradient dependent. The inhibition study suggests the minor contribution of typical organic cation transporters to the blood-to-retina transport of nicotine across the inner BRB, and the novel transporter, which has a high sensitivity to cationic drugs, play a major role in nicotine transport at the inner BRB. The potential identity of molecules involving nicotine and pyrilamine transport at the inner BRB was indicated because nicotine transport was competitively inhibited by pyrilamine. These findings help increase our understanding of drug transport at the BRB, allowing the efficient delivery of cationic neuroprotectants to the retina via the BRB.
ACKNOWLEDGMENTS
This work was partly supported by a Grant-in-Aid for Scientific Research (B) (25293036) and Scientific Research (C) (26460193) from the Japan Society for the Promotion of Science (JSPS).
The authors declare that there is no conflict of interest.




