Quantitative Determination of Luminal and Abluminal Membrane Distributions of Transporters in Porcine Brain Capillaries by Plasma Membrane Fractionation and Quantitative Targeted Proteomics
Abstract
Abluminal or luminal localization of transporter in plasma membranes at the blood–brain barrier (BBB) is critical for their physiological and pharmacological roles. Therefore, the purpose of this study was to develop a new method to investigate membrane localization of transporters, through quantitative measurement of protein expression levels in fractionated plasma membrane prepared from porcine brain capillaries. Luminal–abluminal distribution ratios were calculated from the results of quantitative targeted absolute proteomics of fractionated membranes, after correction for cross-contamination based on measurements of luminal and abluminal membrane markers. BCRP expression was greater at the luminal membrane than at the abluminal membrane, supporting the usefulness of the distribution ratio as a quantitative indicator of localization. The distribution ratios suggested luminal-dominant localizations of GLUT1 and OATP3A1, and abluminal-dominant localizations of ABCA1 and FATP1. For OATP3A1, ABCA1 and FATP1, these results require reconsideration of their functions at the BBB. Species differences were examined using expression levels normalized to Na+/K+–ATPase. BCRP expression is dominant over multidrug resistance 1 expression in porcine BBB, as in other primates including humans. This methodology for quantitative measurement of protein localization is expected to improve our understanding of the roles of transporters at the BBB, and should be applicable to other polarized cells. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3060–3068, 2015
Abbreviations used
-
- ATA1
-
- amino acid transporter system A1
-
- ATA2
-
- amino acid transporter system A2
-
- ABC
-
- ATP-binding cassette
-
- BCRP
-
- breast cancer resistance protein
-
- FATP1
-
- fatty acid transport protein 1
-
- GLUT1
-
- glucose transporter 1
-
- MCT1
-
- monocarboxylate transporter 1
-
- MDR1
-
- multidrug resistance 1
-
- MRP5
-
- multidrug resistance-associated protein 5
-
- OAT3
-
- organic anion transporter 3
-
- OATP3A1
-
- organic anion transporting polypeptide 3A1
-
- SLC
-
- solute carrier
INTRODUCTION
The blood–brain barrier (BBB), which is formed by brain capillary endothelial cells linked via tight junctions, serves to separate neural tissue from blood. It possesses vectorial transport systems because of polarized expression of various transporter proteins, and these systems regulate the exchange of endogenous and exogenous compounds between brain and blood.1, 2 Multidrug resistance 1 (MDR1/P-gp/ABCB1) and breast cancer resistance protein (BCRP/ABCG2), which are representative ATP-binding cassette (ABC) transporters, are localized at the luminal membrane of the brain capillary endothelial cells in humans and rodents, in accordance with their function to restrict drug entry to the brain.3-6 Amino acid transporter system A2 (ATA2/SLC38A2), a solute carrier (SLC) transporter, is involved in cellular uptake of small neutral amino acids, and is the major subtype of system A at the abluminal membrane in rodents.7-10 Organic anion transporter 3 (OAT3/SLC22A8) is also localized at the abluminal membrane in rodents and mediates trans-BBB elimination of anions.11
Identification of the localization of transporters in brain capillary endothelial cells is essential to reach an understanding of their physiological and pharmacological roles at the BBB. So far, transporter localization has been analyzed by immunohistochemical methodologies, although this approach is limited by the availability of specific antibodies. In addition, there other issues, for example, there are conflicting reports about the localization of glucose transporter 1 (GLUT1/SLC2A1) in brain capillary endothelial cells: abluminal-dominant localization has been reported in rat,12 and luminal-dominant localization in human.13 Simpson et al explained this discrepancy in terms of restricted access of anti-GLUT1 antibody to the C-terminus of the transporter at the luminal membrane.14 There are also conflicting reports on multidrug resistance-associated protein 5 (MRP5/ABCC5), with luminal-dominant, and abluminal-dominant or intracellular localizations suggested in humans and rats.15, 16
The specificity and reactivity of antibodies are unavoidable issues in immunohistochemical analysis, and thus, alternative methodology is needed to validate the localization of proteins. One approach is membrane fractionation. Betz et al fractionated plasma membrane of bovine brain capillary endothelial cells, and compared the activity and cytochemical localization of enzymes, concluding that the luminal and abluminal membranes tended to be enriched in the lower- and higher-density layers, respectively.17 They examined the membrane distributions of enzymatic activities, such as phosphatase and 5′-nucleotidase, to investigate functional differences between the luminal and abluminal sides of the BBB.17 Fractionated membrane was also adopted in a functional study of amino acid transport at the abluminal membrane of the BBB.9, 10 However, membrane fractionation analysis can only reveal trends of distribution because of incomplete separation of luminal and abluminal plasma membranes.
We have established quantitative targeted absolute proteomics (QTAP) as a highly sensitive, simultaneous, antibody-free protein quantification method using liquid chromatography–mass spectrometry (LC–MS/MS).18, 19 We have employed QTAP to evaluate the absolute expression levels of ABC transporters and SLC transporters in brain capillaries of human, cynomolgus monkey, common marmoset, rat and mouse,18-22 although these studies did not deal with localization. In the present study, we combined the two methodologies, fractionation and QTAP, to overcome the limitations of each method. Thus, QTAP analysis of fractionated plasma membrane of brain capillaries provides both protein expression levels and localization of transporters. The influence of cross-contamination of luminal and abluminal membranes can be corrected based on the absolute amounts of marker proteins that are specifically localized in luminal or abluminal membranes, and then the luminal–abluminal distribution ratios of each transporter can be calculated. This methodology makes it possible to achieve a comprehensive localization analysis of membrane proteins at the BBB and other polarized cells without the use of antibodies.
During method development, large amounts of brain tissues are required to prepare membrane fractions of brain capillary endothelial cells, which represent no more than 0.1% of brain volume.23 We chose porcine brain, because the pig is a large domesticated animal with a low risk of transmissible spongiform encephalopathy24; it is widely used as an animal model for the in vivo and in vitro studies of the BBB,25-29 and large amounts of porcine brain are readily available. Here, we describe the developed methodology and its application to obtain the localization ratios of transporters between luminal and abluminal plasma membranes of porcine brain capillaries.
MATERIALS AND METHODS
Isolation of Porcine Brain Capillaries
The contents of buffer solutions are given in Supplemental Information. Brain capillaries were prepared as described elsewhere.21, 30 Briefly, porcine brains were obtained at a wholesale market authorized by the Ministry of Health, Labour and Welfare of Japan, and packed in ice for transport to the laboratory. After removal of the surface blood vessels and meninges, brains were stored at −80°C. Brain blocks (∼100 g), including gray matter and white matter, were minced in ice-cold solution A. The minced brain was homogenized with a 100-mL Teflon glass homogenizer in four volumes of solution A with 25 up-and-down hand strokes and no rotation, followed by centrifugation for 15 min at 1,000g and 4°C. The pellet was resuspended in solution A (similar volume to that used in the homogenization), and the suspension was mixed with an equal volume of 35% dextran-containing solution A, followed by centrifugation for 30 min at 4,500g and 4°C. The pellet was suspended in solution B and passed through 210- and 20-μm nylon mesh sheets. The porcine brain capillaries retained by the 20-μm nylon mesh sheet were suspended in solution B, and centrifuged for 10 min at 1,000g and 4°C. The pellet was rinsed with solution A, and stored as brain capillary-rich fraction at −80°C. Microscopic analysis was performed to confirm that the prepared samples contained predominantly brain capillaries (Supplemental Fig. 1), and the protein content was determined using a DC protein assay kit (Bio-Rad, Hercules, California).
Preparation of Fractionated Membrane of Porcine Brain Capillaries
The fractionated membrane was prepared as described elsewhere.9, 17 Briefly, brain capillary-rich fraction (∼10 mg protein) was suspended in 1 mL hypotonic buffer, and homogenized with a loose-fitting Teflon-glass homogenizer (1,800 rpm, 10 strokes), and then a tight-fitting one (1,800 rpm, 40 strokes) on ice, followed by centrifugation for 10 min at 2,000g and 4°C. The resultant pellet was resuspended in 13 mL hypotonic buffer with a tight-fitting Teflon-glass homogenizer (1,800 rpm, 20 strokes), and disrupted by means of nitrogen cavitation (800 psi, 15 min, 4°C), followed by centrifugation for 10 min at 2,000g and 4°C. The supernatant was collected in a clean tube. Resuspension of the pellet, nitrogen cavitation, centrifugation, and collection of the supernatant were repeated three times. After addition of 1 M MgSO4 (final concentration: 10 mM), the collected supernatant was centrifuged for 10 min at 3,000g and 4°C. The resultant supernatant was centrifuged for 60 min at 90,000g and 4°C, and a 25-gauge needle was used to suspend the resultant pellet in 3 mL Tris/sucrose/ethylenediaminetetraacetic acid (TSE) buffer. The suspension was layered on top of a discontinuous gradient (5, 10, 15 and 20% Ficoll in TSE buffer), and centrifuged for 2.5 h at 162,500g and 4°C. The fluffy materials at each of the interfaces between Ficoll layers were collected using a 200-μL micropipette whose top had been chopped off, without ruffling the interface, and rinsed in TSE buffer by centrifugation twice for 1 h at 90,000g and 4°C to remove Ficoll. The fractions from the interfaces between the 0% and 5%, 5% and 10%, 10% and 15%, and 15% and 20% Ficoll layers were assigned as fractions #1, #2, #3 and #4, respectively, and fractions #1 and #4 were used as the luminal- and abluminal-rich fractions, respectively, based on the previous report.17 The pellets were each suspended in 150 μL TSE buffer using a 27-gauge needle, and stored as fractionated membranes at −80°C. The protein content was determined using a DC protein assay kit (Bio-Rad).
Quantification of Membrane Proteins by LC–MS/MS
Ten transporters and Na+/K+–ATPase were quantitated using the same probe peptides for human transporters as used in our previous studies on human, monkey, marmoset, mouse, and rat (Table 1).19-22, 31 We confirmed that identical sequences were present in the porcine orthologs listed on the Pig Genomic Informatics System (http://pig.genomics.org.cn) or Sus scrofa genome of National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/genome?term=sus%20scrofa). The amino acid identity of residues on the N-terminal side of probe peptide sequences was also confirmed as sample pretreatment included digestion with trypsin that specifically cleaves the carboxylic side of l-lysine and l-arginine residues. Membrane protein quantification was performed as described previously,19 and sample pretreatments were as described in Supplemental Information. Quantification was performed by applying the supernatant derived from 1.6 or 25 μg protein of membrane fraction to a high-performance liquid chromatography system (Agilent 1100; Agilent, Santa Clara, California) connected to an electrospray ionization-triple quadrupole mass spectrometer (QTRAP5500; AB Sciex, Foster City, California). A C18 column (Waters XBridge BEH130 C18, 1.0 mm ID × 100 mm, 3.5 μm particles; Waters, Milford, Massachusetts) was used. In the elution of peptides, a linear gradient of 1%–50% acetonitrile in 0.1% formic acid was applied at a flow rate of 50 μL/min. Mass spectrometry for peptide detection was set up to run a selected/multiple reaction monitoring (SRM/MRM) experiment with 10 ms of dwell time per transition, and four transitions specific for each target peptide (Table 1). Analyst software ver. 1.5 (AB Sciex) was used for data analysis, and quantitative values were calculated from the peak area ratios of analytes and stable isotope-labeled peptides. Signal peaks with a peak area count of over 5000 detected at the same retention time as an internal standard peptide were defined as positive.32 When positive peaks were observed in three or four sets of SRM/MRM transitions, the molecules were considered as existing in the membrane fractions of brain capillaries, and their protein expression was calculated as the average of the three or four quantitative values. The transporter protein expression in membrane fraction is presented as transporter protein amount per microgram protein of the membrane fraction (fmol/μg protein). The value of the limit of quantification for each SRM/MRM transition was defined as the quantitative value (fmol/μg protein) that would give a peak area of 5000 counts in the measurement of the membrane fraction, and the minimum value among the four transitions was used as the limit of quantification for the protein amount per microgram protein (fmol/μg protein) of target protein in the membrane fraction of brain capillaries.
| SRM/MRM Transition (m/z) | |||||||
|---|---|---|---|---|---|---|---|
| Gene Name | Alias | Probe Peptide Sequence | Q1 | Q3-1 | Q3-2 | Q3-3 | Q3-4 |
| ABCA1 | ABC1 | FVSPLSWDLVGR | 688.4 | 1129.6 | 1042.6 | 832.4 | 945.5 |
| FVSPLSWDL*VGR | 691.9 | 1136.6 | 1049.6 | 839.4 | 952.5 | ||
| ABCB1 | MDR1 | NTTGALTTR | 467.8 | 618.4 | 719.4 | 490.3 | 561.3 |
| NTTGAL*TTR | 471.3 | 625.4 | 726.4 | 497.3 | 568.3 | ||
| ABCC5 | MRP5 | SLSEASVAVDR | 567.3 | 646.4 | 717.4 | 933.5 | 559.3 |
| SL*SEASVAVDR | 570.8 | 646.4 | 717.4 | 933.5 | 559.3 | ||
| ABCG2 | BCRP | SSLLDVLAAR | 522.8 | 644.4 | 757.5 | 529.3 | 430.3 |
| SSLLDVL*AAR | 526.3 | 651.4 | 764.5 | 536.3 | 437.3 | ||
| SLC2A1 | GLUT1 | TFDEIASGFR | 571.7 | 537.3 | 894.4 | 779.4 | 650.4 |
| TFDEIA*SGFR | 573.7 | 541.3 | 898.4 | 783.4 | 654.4 | ||
| SLC16A1 | MCT1 | SITVFFK | 421.2 | 641.4 | 294.2 | 441.3 | 540.3 |
| SITVFF*K | 426.2 | 651.4 | 304.2 | 451.3 | 550.3 | ||
| SLC27A1 | FATP1 | LLPQVDTTGTFK | 660.4 | 1093.6 | 227.2 | 868.4 | 769.4 |
| LLPQVDTTGTF*K | 665.4 | 1103.6 | 227.2 | 878.4 | 779.4 | ||
| SLC38A1 | ATA1 | NELPSAIK | 436.3 | 515.3 | 628.4 | 418.3 | 331.2 |
| NELPSAI*K | 439.8 | 522.3 | 635.4 | 425.3 | 338.2 | ||
| SLC38A2 | ATA2 | AFGLVGK | 346.2 | 473.3 | 303.2 | 620.4 | 416.3 |
| AFGL*VGK | 349.7 | 480.3 | 303.2 | 627.4 | 423.3 | ||
| SLCO3A1 | OATP3A1 | SGELQGDEAQR | 595.3 | 675.3 | 803.4 | 503.3 | 916.5 |
| SGELQGDEA*QR | 597.3 | 679.3 | 807.4 | 507.3 | 920.5 | ||
| Na+/K+–ATPase | AAVPDAVGK | 414.2 | 685.4 | 586.3 | 489.3 | 374.2 | |
| AAVPDAV*GK | 417.2 | 691.4 | 592.3 | 495.3 | 380.2 | ||
- The quantification was performed by means of QTRAP5500. Doubly charged precursor ions were selected (Q1). Four transitions per peptide (Q3–1, -2, -3 and -4), corresponding to high-intensity fragment ions, were selected.
- Bold letters with asterisks indicate amino acid residues labeled with stable isotope (13C and 15N).
Normalization of Protein Expression Levels with Respect to Na+/K+–ATPase
Theoretical Background of Marker Protein Correction for Polarized Transporter Protein Analysis
 (2)
(2) (3)
(3) (4)
(4) (5)
(5)The transporter protein amounts per microgram protein in the four membrane fractions were expressed as P1, P2, P3 and P4. Pu, PL and PA were defined as total transporter protein expressions, that is, the sum of the transporter protein amounts per microgram protein in the four fractions (P1+P2+P3+P4), for the molecule of interest, the luminal marker, and the abluminal marker, respectively. We assigned MDR1 and ATA2 as luminal and abluminal markers, respectively. The fraction distribution of protein was expressed as U; for example, Uu1 is obtained by dividing Pu1 by Pu. Then, we can convert Eq. 4 into Eq. 5. The transformations for fL, fA and fL+fA are shown in detail in Supplemental Information.
RESULTS
Fractionation of Plasma Membrane of Porcine Brain Capillaries
Preparation of fractionated membrane was performed eight times in total. On average, 100 g (wet weight) of porcine brain provided 18.8 mg protein of brain capillaries, and homogenization and ultracentrifugation provided 3.73 mg protein of crude membrane fraction. Density-gradient centrifugation separated the crude membrane into plasma membrane fractions #1–#4 that contained 164, 134, 166 and 129 μg protein, respectively, so that 593 μg protein of plasma membrane was recovered in total. Each preparation gave similar values, and we considered that the quality of samples was rather uniform. Quantification of transporter proteins by LC–MS/MS was performed with each preparation. In the present study, fractions #1 and #4 were assumed to be the luminal- and abluminal-rich fractions, respectively, based on the previous report that examined the fractional distribution of activities and cytochemical localization of enzymes.17 The organelle-rich fraction was observed as a pellet at the bottom of the tube after density-gradient centrifugation.
Protein Expression Levels of Transporters in the Fractions of Porcine Brain Capillary Plasma Membrane
Protein expression of transporters was determined in membrane fractions of porcine brain capillaries. The transporters to be quantified were selected based on the following criteria: (1) the amino acid sequence was identified for the porcine molecule in Pig Genomic Informatics System or Sus scrofa genome of NCBI; (2) the target peptide and adjacent amino acids for QTAP quantification of the porcine molecules were identical to those used in our previous work on the expression levels of transporters, receptors, and tight junction molecules in human brain capillaries20; and (3) the transporter is expressed on plasma membrane and its function has been reported. Nine transporters, excluding organic anion transporting polypeptide 3A1 (OATP3A1/OATP-D/SLCO3A1), as shown in Table 1, were selected according to these criteria. The OATP family includes important drug transporters, and Oatp2 and OATP1A2 (OATP-A) were reported to be present at the rodent and human BBB, respectively.34, 35 Porcine OATP3A1 was the only molecule in the porcine OATP family meeting criteria 1 and 2. Although its BBB expression was unknown, we selected it for QTAP quantification. We also quantified Na+/K+–ATPase as a plasma membrane marker.
Among 11 molecules measured, 10 were detected (Table 2), and amino acid transporter system A1 (ATA1/SLC38A1) was below the limit of quantification (Table 2). Among ABC transporters, ABCA1 (ABC1), MDR1, MRP5, and BCRP were quantified, and the protein expression of BCRP was the greatest among the examined ABC transporters in all fractions (Table 2). Among SLC transporters, GLUT1, monocarboxylate transporter 1 (MCT1/SLC16A1), fatty acid transport protein 1 (FATP1/SLC27A1), ATA2, and OATP3A1 were quantified, and GLUT1 exhibited the greatest expression among them in all fractions (Table 2).
| Protein Expression (fmol/μg protein) | |||||
|---|---|---|---|---|---|
| Alias | Fraction #1 (0/5) | Fraction #2 (5/10) | Fraction #3 (10/15) | Fraction #4 (15/20) | Limit of Quantification (fmol/μg protein) |
| ABCA1 | 1.59 ± 0.18 | 2.24 ± 0.48 | 2.09 ± 0.40 | 2.32 ± 0.17 | 0.915 |
| MDR1 | 33.4 ± 0.6 | 26.4 ± 2.6 | 17.5 ± 0.7 | 8.89 ± 1.07 | 0.313 |
| MRP5 | 0.911 ± 0.031 | 0.651 ± 0.039 | 0.676 ± 0.144 | 0.708 ± 0.227 | 0.0913 |
| BCRP | 166 ± 6 | 141 ± 3 | 111 ± 4 | 70.0 ± 2.4 | 6.27 |
| GLUT1 | 374 ± 15 | 400 ± 15 | 337 ± 8 | 197 ± 5 | 0.406 |
| MCT1 | 0.698 ± 0.183 | 0.350 ± 0.074 | 0.559 ± 0.080 | 0.807 ± 0.263 | 0.0400 |
| FATP1 | 1.81 ± 0.75 | 1.68 ± 0.47 | 2.17 ± 0.30 | 2.86 ± 1.10 | 0.171 |
| ATA1 | ULQ (<0.0313) | ULQ (<0.0313) | ULQ (<0.0313) | ULQ (<0.0313) | 0.0313 |
| ATA2 | 1.04 ± 0.23 | 0.268 ± 0.127 | 0.703 ± 0.306 | 2.15 ± 0.92 | 0.122 |
| OATP3A1 | 0.652 ± 0.118 | 0.584 ± 0.063 | 0.254 ± 0.033 | 0.340 ± 0.038 | 0.120 |
| Na+/K+–ATPase | 116 ± 2 | 116 ± 5 | 130 ± 5 | 121 ± 7 | 0.428 |
- The quantification was performed by means of QTRAP5500. Fraction #1, #2, #3 and #4 correspond to the membrane fractions collected from the interfaces between 0% and 5%, 5% and 10%, 10% and 15%, and 15% and 20% Ficoll layers in discontinuous density gradient centrifugation, respectively. Each value represents the mean ± SEM (n = 3–4 transitions).
- ULQ, under the limit of quantification; a value in brackets following “ULQ” represents the value of the quantification limit (fmol/μg protein).
Luminal and Abluminal Distribution Ratios of Transporters in Plasma Membrane of Porcine Brain Capillaries
The luminal (fL) and abluminal distribution ratios (fA) were calculated to investigate the membrane localization of transporters in porcine brain capillary endothelial cells, employing MDR1 and ATA2 as luminal and abluminal marker proteins, respectively.3, 4, 7 Among all quantified molecules, MDR1 showed the greatest fraction distribution ratio (0.388) in fraction #1, and ATA2 showed the greatest ratio (0.517) in fraction #4, without correction for cross-contamination. Although a relatively high ratio (0.250) of ATA2 was found in fraction #1, this was considered to have had only a very small effect on the calculation of distribution ratios. The ratio of cross-contamination in each fraction was assumed to be the same for all transporters, and equations were written to correct for cross-contamination of the respective membranes.
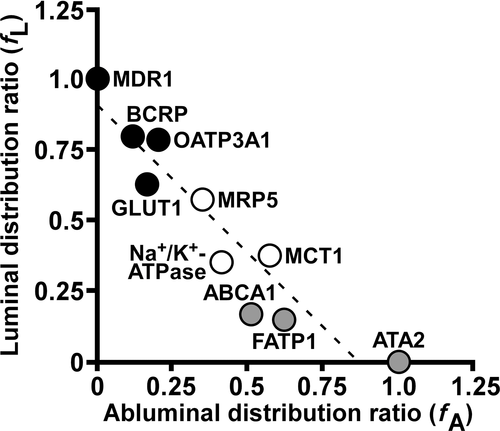
Table 3 summarizes the fL and fA values for transporters and Na+/K+–ATPase, and the results are illustrated graphically in Figure 1. The sum of fL and fA is 1 theoretically, and the plot was almost linear, as fL+fA = 1 (fL = −1.03 × fA + 0.905; r2 = 0.887) (Fig. 1), suggesting that the basis for our calculation of fL and fA from the four fractions with cross-contamination compensation is valid. If we define dominant localization in terms of at least threefold greater distribution ratio in one membrane than in the other, BCRP, GLUT1 and OATP3A1 are luminal-dominant transporters, in addition to MDR1, which is a luminal marker (Table 3). FATP1 and ABCA1 are abluminal-dominant transporters, in addition to ATA2, which is an abluminal marker (Table 3). MRP5, MCT1 and Na+/K+–ATPase did not show an obviously polarized distribution (within 1.6-fold difference) in plasma membrane of porcine brain capillaries (Table 3).
| Alias | Luminal Distribution Ratio (fL) | Abluminal Distribution Ratio (fA) | fL /fA |
|---|---|---|---|
| MDR1 | 1.00 | 0.00 | - |
| BCRP | 0.802 | 0.118 | 6.80 |
| OATP3A1 | 0.789 | 0.202 | 3.91 |
| GLUT1 | 0.631 | 0.166 | 3.80 |
| MRP5 | 0.572 | 0.351 | 1.63 |
| Na+/K+–ATPase | 0.352 | 0.414 | 0.850 |
| MCT1 | 0.377 | 0.572 | 0.659 |
| ABCA1 | 0.168 | 0.511 | 0.329 |
| FATP1 | 0.148 | 0.620 | 0.239 |
| ATA2 | 0.00 | 1.00 | 0 |
- The calculation of luminal and abluminal distribution ratios (fL and fA) were performed by using the values listed in Table 2. MDR1 and ATA2 were assigned as luminal and abluminal marker proteins, respectively.
Comparison of Protein Expression Levels of Transporters in Porcine Brain Capillary with Those in Primates and Rodents
Our previous QTAP studies clarified the species differences of transporter protein expression levels at the BBB among human, monkey and rodents.18-22 In the present study, the protein expression levels of transporters in porcine brain capillaries were compared with those in other species including human. In our previous reports, the absolute expression levels of transporters were quantified in whole lysate of brain capillaries, whereas in the present porcine study, expression levels are given as the sum of those in the four fractions prepared from plasma membrane of brain capillaries. This difference may mean that the previous and present data are not strictly comparable, but we think the potential issue is at least ameliorated by the data normalization using Eq. 1. With this proviso, Na+/K+–ATPase localization at the plasma membrane and its expression in brain capillaries are similar among the different species: 35.1, 39.4, 68.6, 35.1 and 31.5 fmol/μg protein of Na+/K+–ATPase in human, mouse, rat, monkey and marmoset.19-22
The protein expression level of MDR1 in porcine brain capillaries was 1.03 times that in human, suggesting very similar expression levels of MDR1 in porcine and human brain capillaries. The difference of expression levels of MDR1 was suggested to be within 1.53-fold in brain capillaries of porcine and primates, such as human, cynomolgus monkey and common marmoset (Table 4; Supplemental Fig. 2). The most marked difference between primates and rodents is in expression of MDR1 and BCRP: BCRP is dominant in primates and MDR1 is dominant in rodents. In porcine brain capillaries, the protein expression level of BCRP was suggested to be 4.36 times that in human brain capillaries. Thus, expression of BCRP at porcine BBB is 5.66-fold greater than that of MDR1 at primate BBB (Table 4). Porcine and human brain capillaries appear to show comparable expression levels of GLUT1, but expression of MCT1 in porcine brain capillaries would be much lower than that of human (Table 4; Supplemental Fig. 2).
| (Expression of Protein)/(Expression of Na+/K+–ATPase) | ||||||
|---|---|---|---|---|---|---|
| Alias | Human | Porcine | Cynomolgus Monkey | Common Marmoset | Rat | Mouse |
| MDR1 | 0.173a | 0.178 | 0.134b | 0.206c | 0.277c | 0.393d |
| (1.03-fold) | (0.777-fold) | (1.19-fold) | (1.60-fold) | (2.28-fold) | ||
| BCRP | 0.232a | 1.01 | 0.405b | 0.524c | 0.0605c | 0.102d |
| (4.36-fold) | (1.74-fold) | (2.26-fold) | (0.261-fold) | (0.440-fold) | ||
| GLUT1 | 3.96a | 2.71 | 3.68b | 4.60c | 1.22c | 2.28d |
| (0.684-fold) | (0.928-fold) | (1.16-fold) | (0.309-fold) | (0.577-fold) | ||
| MCT1 | 0.0647a | 0.00500 | 0.0238b | 0.0965c | 0.169c | 0.602d |
| (0.0773-fold) | (0.367-fold) | (1.49-fold) | (2.61-fold) | (9.30-fold) | ||
- The protein expressions of Na+/K+–ATPase, MDR1, BCRP, GLUT1 and MCT1 in porcine brain capillary were calculated as the sum of protein amounts in each fraction listed in Table 2, and their values were 483, 86.2, 488, 1310 and 2.41 fmol/μg protein, respectively. These values were used in the normalization of expression level for porcine brain capillary. The normalization of expression level with Na+/K+–ATPase, for human, cynomolgus monkey, common marmoset, rat, and mouse, were performed by referring to our previous reports.19-22 aUchida et al; bIto et al; cHoshi et al; dKamiie et al. Data in the previous reports and the present study were obtained in tissue lysates and membrane fraction, respectively. Although their comparison is not entirely appropriate in a precise sense of sample preparation, the differences of expression level from human are calculated for reference, and are shown in parentheses.
DISCUSSION
We have developed a new method to investigate the membrane localizations of transporter proteins at the BBB by using QTAP analysis to measure absolute amounts of multiple proteins simultaneously in the fractionated plasma membrane of porcine brain capillaries. This enables us to calculate the luminal–abluminal distribution ratios of transporters by using the absolute amounts of luminal and abluminal marker proteins to compensate for cross-contamination of luminal and abluminal membrane fractions, on the assumption that the ratio of cross-contamination in each fraction is the same for all transporters.
As marker proteins, we selected MDR1 and ATA2. Luminal localization of MDR1 has been established by immunohistochemistry.3, 4 ATA2, which functions as a system A transporter for l-alanine, l-proline, and glycine, is localized in abluminal membrane.7-10 The present results (Table 2) indicate that MDR1 was the most enriched protein in fraction #1 (luminal membrane-enriched fraction), whereas ATA2 was the most enriched protein in fraction #4 (abluminal membrane-enriched fraction), supporting the validity of using these markers to compensate for cross-contamination. It should be noted that luminal–abluminal distribution ratios of 1 and 0 do not necessarily imply exclusive localization at the respective membranes. Theoretically, the calculations could give values of more than 1 and less than 0, and it remains possible that better markers might be identified in the future, although MDR1 and ATA2 are currently believed to be the best.
Previous immunohistochemical analyses revealed luminal localization of BCRP.5, 6 Our present finding that the BCRP protein expression level in luminal membrane was 6.80-fold greater than that in abluminal membrane (Table 3) not only supports the immunohistochemical results, but also provides for the first time a quantitative measure of the polarized distribution of transporters. Eq. 5 indicates that the sum of fL and fA approaches 1 when a target protein exhibits a similar profile of fraction distribution to the marker proteins. The sum of fL and fA for ABCA1 (0.679) was relatively low compared with that for BCRP (0.920), indicating that the fraction distribution of ABCA1 is not the same as that of the marker proteins, so the above assumption may not be valid for this protein. Eq. 5 also indicates that the sum of fL and fA could exceed 1 when inappropriate markers are used in calculations. Therefore, the trend line in Figure 1, being closely similar to fL+fA = 1, supports the validity of the equations and markers used in this study.
GLUT1 is a representative nutrient transporter; immunohistochemical analysis suggested its abluminal-dominant localization at the BBB, although luminal-dominant localization was also reported.12, 13 Simpson et al employed immunoblot analysis to examine the fractionated membrane fraction of bovine brain microvessels; using three antibodies against different epitopes in GLUT1, they found that the distributions of antibody reactivity are different depending upon the epitope to which the antibodies are directed.14 Glucose transport activity and cytochalasin B-binding activity exhibited similar luminal-dominant distribution, with 2.3-fold greater activity in the luminal fraction than in the abluminal fraction.14 Our results show that the luminal distribution ratio of GLUT1 was 3.80-fold greater than the abluminal distribution ratio, which is consistent with the results of transport and binding activities. These results strongly support luminal-dominant localization of GLUT1 in brain capillaries. MCT1 is another nutrient transporter, which supplies ketone bodies and lactate from blood to brain. Our results show that MCT1 was equally localized in luminal and abluminal membranes of porcine brain capillaries (Table 3). This is consistent with the previous immunohistochemical finding of a symmetrical distribution of MCT1 at the luminal and abluminal membranes of rat brain capillary endothelial cells.36
In this study, we quantified ABCA1, MRP5, ATA2 and OATP3A1 in fractions of porcine brain capillaries, though these transporters were under the limit of quantification in our previous QTAP analysis of human BBB.20 In the previous study, we had performed quantification using whole tissue lysate of brain capillaries because of the limited sample amounts. In the present study, plasma membrane was prepared from larger amounts of porcine brain capillaries, and further fractionation was carried out, resulting in a higher concentration of membranes in the final fractions. Indeed, the protein amounts per microgram protein of membrane fraction for MDR1 and BCRP in fraction #1 were 5.52- and 20.4-fold greater than their protein amounts per microgram protein of tissue lysate in human brain capillaries (Table 2).20 Therefore, concentration of membrane proteins during the fractionation process made it possible to quantify these transporters in the present study.
Equal localization of MRP5 at luminal and abluminal membranes of porcine BBB was suggested by the distribution ratio (Table 3), in contrast to previous reports.15, 16 Immunohistochemical analysis had suggested luminal localization of MRP5 in human brain capillary endothelial cells,15 whereas abluminal-dominant or intracellular localization was suggested in rat.16 MRP5 was characterized as the efflux transporter of organic anions, including cyclic nucleotides,37 so the present finding of equal distribution at luminal and abluminal membranes implies that further functional studies are needed to clarify the role(s) of MRP5 at the BBB.
Regarding FATP1 and ABCA1, their relatively low fA values suggested abluminal-dominant localizations at the BBB (Table 3). FATP1 is a fatty acid transporter, and is one of the major FATP isoforms at the BBB.38, 39 Previous in vivo studies showed the blood-to-brain transport of fatty acid,40 and an in vitro study with human brain microvessel endothelial cells suggested that luminal-to-abluminal transport of fatty acid was greater than abluminal-to-luminal transport. FATP1 knockdown resulted in significant decreases of both vectorial transports,38 supporting the idea of dual efflux and influx functions of FATP1 at the BBB. The abluminal-dominant localization of FATP1 found here supports a contribution to the transport of long-chain fatty acids into the brain. ABCA1 is considered to be involved in efflux transport of cholesterol from brain capillary endothelial cells, and a decrease of apolipoprotein E (ApoE) in the brain was reported in ABCA1-deficient mice.41, 42 Polarized distribution of ABCA1 in brain capillary endothelial cells was previously suggested.41 Our finding of abluminal-dominant localization suggests that ABCA1 is involved in cholesterol transport at the abluminal membrane, and it may contribute to brain cholesterol homeostasis by facilitating the lipidation of ApoE in concert with glial cells.
The distribution ratios also revealed luminal localization of OATP3A1 (Table 3), which transports benzylpenicillin and prostaglandin E2,43 suggesting involvement of OATP3A1 in organic anion transport at the luminal membrane of porcine BBB. In rodents, OAT3 and organic anion transporting polypeptide 1a4 (Oatp2/Slco1a4) are expressed at the BBB and mediate transport of anion compounds, such as homovanillic acid and dehydroepiandrosterone sulfate, across the BBB.11, 34 However, our previous QTAP study revealed that the human homologues of these molecules were under the detection limit in human brain capillaries, suggesting involvement of other transporter(s) in anion transport at the human BBB. The present result raises the possibility that OATP3A1 plays some role in anion transport at the human BBB.
It should be noted that we could not completely exclude the possibility of contamination with neurons, astrocytes and pericytes, even though the preparation protocol could efficiently remove these cells to provide high-grade porcine brain capillary samples.20, 30, 31 In particular, a previous report suggested that porcine brain capillary samples contain some astrocytes,31 which have been reported to express ABCA1, MDR1, MRP5, BCRP, GLUT1, MCT1 and ATA2.15, 36, 44-48 However, the expression levels in astrocytes seem unlikely to be much higher than those in brain capillary endothelial cells, and we consider that the contamination would have had very small effect on the localization study for most transporters; the validity of our approach is supported by the fact that the reported localizations of MDR1, ATA2, BCRP, GLUT1 and MCT1 were confirmed in the present quantification (Tables 2 and 3).3-10, 13, 14, 36 Nevertheless, immunohistochemical confirmation may be desirable for transporters that are much more highly expressed in neurons, astrocytes, or pericytes than in brain capillary endothelial cells.
We have previously quantified transporter proteins at the BBB of various mammals, including human, uncovering species differences in the protein expression of efflux transporters between primates and rodents.19-22 The normalized expression values obtained in the present study suggested comparable protein expression of MDR1 at porcine BBB to that of primates as porcine, human, common marmoset and cynomolgus monkey all showed similar protein expression levels of MDR1. Comparable expression of GLUT1 was also suggested between porcine and primates (Table 4; Supplemental Fig. 2). Furthermore, rodents showed MDR1-dominant expression, that is, the expression of MDR1 was greater than that of BCRP, whereas BCRP-dominant expression was observed in primates (Table 4). In porcine brain capillaries, BCRP-dominant expression was observed, and this suggests that substrates of BCRP would have relatively lower permeability at the porcine BBB. Monocarboxylates would also show lower permeability at the porcine BBB, as the expression of MCT1 in porcine brain capillaries was much lower than that in human (Table 4; Supplemental Fig. 2).
In conclusion, we have developed a new method to investigate the membrane localization of transporters expressed at the BBB, by using QTAP to quantitate protein expression in fractionated plasma membrane. This method provides quantitative information about protein localization within plasma membranes for the first time, in terms of the distribution ratio, and it should be applicable to various kinds of polarized cells. It is noteworthy that the predominant expression of BCRP over MDR1 at the porcine BBB is consistent with the situation in other primates. We believe the methodology and results described here will contribute to a better understanding of the membrane localizations, roles, and species differences of transporters at the BBB.
ACKNOWLEDGMENTS
This study was supported in part by a Global COE Program from the Japan Society for the Promotion of Science and Grants-in-Aid for Scientific Research (A) (KAKENHI: 24249011). The authors thank Dr. Katsuaki Ito for his technical support and valuable discussions concerning isolation of porcine brain capillaries and the quantification of membrane proteins, and Dr. Ken Ohmine and Dr. Wataru Obuchi for their technical support during protein quantification with LC–MS/MS.
Tetsuya Terasaki and Sumio Ohtsuki are full professors of Tohoku University and Kumamoto University, respectively, and are also directors of Proteomedix Frontiers Company Ltd. Their positions at Proteomedix Frontiers did not affect the design of the study, collection of the data, analysis or interpretation of the data, decision to submit the manuscript for publication, or writing of the manuscript. The other authors declare no competing interest.





