Kinetics of the Absorption, Distribution, Metabolism, and Excretion of Lobeglitazone, a Novel Activator of Peroxisome Proliferator-Activated Receptor Gamma in Rats
Abstract
This study was performed to determine biopharmaceutical properties of lobeglitazone (LB), a novel thiazolidinedione-based activator of peroxisome proliferator-activated receptor gamma, in rats. In parallel artificial membrane permeability assay and Madin–Darby canine kidney (MDCK) cell permeability assays of LB, the activator was found to interact with multidrug-resistance protein 1 (MDR1) and OATP1B1. The concentration resulting in 50% inhibition value for LB in MDR1 expressing MDCK cells was approximately 12.5 ± 3.61 μM. LB had adequate stability (i.e., 56% remaining at 0.5 h) in rat liver microsomes. A cytochrome P450 (CYP) inhibitory potency study indicated that LB is primarily interacted with CYP1A2, 2C9, and 2C19. In rats, LB appeared to be readily absorbed after an oral administration (an absolute bioavailability of ∼95%). Following intravenous administration, LB exhibited linear pharmacokinetics in the dose range of 0.5–2 mg/kg. The primary distribution site was the liver but it was also distributed to heart, lungs, and fat tissue. The excretion of LB to the urine, bile, feces, and intestine was insignificant (i.e., <10% of the dose) in rats. These observations suggest that, despite the fact that it interacts with some drug transporters and metabolizing enzymes, the pharmacokinetics of LB were linear with a high oral bioavailability. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3049–3059, 2015
Abbreviations used
-
- LB
-
- lobeglitazone
-
- PAMPA
-
- parallel artificial membrane permeability assay
-
- MDCK
-
- Madin–Darby canine kidney
-
- MDR1
-
- multidrug-resistance protein 1
-
- OATP
-
- organic anion transporting polypeptide
-
- OAT
-
- organic anion transporter
-
- OCT
-
- organic cation transporter
-
- BCRP
-
- breast cancer-resistance protein
-
- IC50
-
- concentration resulting in 50% inhibition
-
- CYP
-
- cytochrome P450
-
- TZD-PPAR
-
- thiazolidinedione-peroxisome proliferator-activated receptor
-
- log P
-
- partition coefficient
-
- HPLC
-
- high-performance liquid chromatography
-
- DMSO
-
- dimethyl sulfoxide
-
- TEER
-
- transepithelial electrical resistance
-
- HEPES
-
- N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid
-
- LC–MS/MS
-
- liquid chromatography–tandem mass spectrometry
-
- CO2
-
- carbon dioxide
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate oxidase
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- RED
-
- rapid equilibrium dialysis
INTRODUCTION
Lobeglitazone (LB), an activator of thiazolidinedione-peroxisome proliferator-activated receptor gamma (TZD-PPAR-γ), is a new drug that is under development for use in the treatment of diabetes in Korea. The compound is reported to have at least a 1.11- and 16.6-fold higher affinity for PPAR-γ in comparison to pioglitazone and rosiglitazone (i.e., clinically used TZD-PPAR activators), respectively. As a result, it is expected that LB would have a lower effective dose and reduce cardiovascular side effects (i.e., common for TZD-PPAR activators), provided that the kinetic properties of LB are comparable to the other PPARs activators. Unfortunately, however, a comprehensive pharmacokinetic study of TZD-PPAR activators, including LB, has not been reported in the literature.
In the case of the biopharmaceutical properties of TZD-PPAR activators, the metabolic characteristics are relatively well understood. For example, although pioglitazone and rosiglitazone are primarily eliminated via hepatic metabolism, the major cytochrome P450 (CYP) isozyme for their elimination is different (i.e., CYP2C8/3A4 for pioglitazone; CYP2C8/2C9 for rosiglitazone). As the activators, including pioglitazone and rosiglitazone, contain a common TZD moiety but with different side chains, this structural difference appears to be the main determinant of the heterogeneity in metabolic outcome. TZD-PPAR activators are relatively hydrophobic [i.e., partition coefficient (log P) of 2.95, 3.17, and 4.16 for rosiglitazone, pioglitazone, and troglitazone, respectively; the values were determined using the ALOGPS software] and their extent of absorption is relatively high (95%, 83%, and 50% for rosiglitazone, pioglitazone, and troglitazone, respectively).1-3 The involvement of drug transporters in their pharmacokinetics has not been systemically studied in the case of TZD-PPAR activators.
The objective of this study was to characterize the biopharmaceutical properties of LB. Although such properties have been shown to be important in understanding the pharmacokinetics, the information may also be closely linked to clinically relevant issues (e.g., drug–drug interaction) in the later stages of the development of a new drug. We were particularly interested in the in vitro properties of the metabolism and transport of LB, and their relationship to pharmacokinetics, as such information may have a direct consequence in the elucidation/prediction of drug interactions. Our findings indicate that, despite its interaction with transporters and metabolizing enzymes, LB exhibited linear kinetics with a nearly complete bioavailability.
MATERIALS AND METHODS
Chemicals and Reagents
Lobeglitazone (98.5% purity), LB sulfate (99.1% purity), and rosiglitazone [99.0% purity, an internal standard (IS) of LB assay] were provided by Chong Kun Dang Pharmaceuticals (Seoul, Korea). Acetonitrile [high-performance liquid chromatography (HPLC) grade] and formic acid were obtained from J. T. Baker (Phillipsburg, New Jersey) and from Fluka (Cambridge, Massachusetts), respectively. Other chemicals, including testosterone and 7-hydroxy coumarin, were purchased from Sigma–Aldrich (St. Louis, Missouri). Pooled rat liver microsomes and uridine diphosphate (UDP) reaction mix solution were purchased from Corning Gentest (Woburn, Massachusetts). Solvents were of HPLC grade (Fisher Scientific, Pittsburgh, Pennsylvania), and other chemicals were of the highest grade available.
In Vitro Absorption, Distribution, and Elimination Studies
Parallel Artificial Membrane Permeability Assay
To estimate the intestinal permeability of LB via diffusional transport, parallel artificial membrane permeability assay (PAMPA) was carried out following the standard procedure.4, 5 Briefly, a dodecane solution containing phosphatidyl choline (20 mg/mL) was added to the membrane (multiscreen PAMPA assay plate, #MAIP-N4550; millipore, Bedford, Massachusetts) of the insert. Phosphate-buffered saline (PBS) containing LB (200 μg/mL) in 15% dimethyl sulfoxide (DMSO) and 15% polyethylene glycol (PEG) 400, or LB sulfate (100 μg/mL) in 10% DMSO, was added to the well before artificial membrane material had evaporated (<10 min). The reaction was initiated by inserting the donor plate into the acceptor plate, and allowing the process to proceed at room temperature. When necessary, the permeability of verapamil (i.e., the high-permeability marker for this assay) was measured in parallel. The acceptor buffer was collected at 16 h after the initiation and the concentration was measured using an HPLC–UV spectrophotometer (Waters e2695, 2489, Waters, Milford, Massachusetts). The mobile phase involved isocratic condition of 0.1% formic acid–acetonitrile (70:30, v/v) at a flow rate of 1 mL/min at 25°C. After 50 μL of samples were injected onto a reverse-phase HPLC column (Kinetex XB-C18, 150 mm × 4.6 mm, 5 μm; Phenomenex, Torrance, California), the eluent from the column was monitored at a wavelength of 290 nm. The apparent permeability of each compound was calculated using the standard equation.4
Permeability Study in Madin–Darby Canine Kidney II Cells
Madin–Darby canine kidney II-wild type (MDCKII-WT) and MDCKII–multidrug-resistance protein 1 (MDR1) cell lines were generously provided by Dr. Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Welgene Inc., Daegu, Korea) containing 10% fetal bovine serum (FBS; Welgene Inc.), 1% nonessential amino acid solution, 100 units/mL penicillin, and 0.1 mg/mL streptomycin under a humidified atmosphere of air containing 5% carbon dioxide (CO2) at 37°C. Collagen-coated 12 mm Transwell (Costar, Corning, New York) was incubated with medium at 37°C for 1 h to improve cell attachment. Cells were seeded at a density of 2.5 × 105 cells/well and the medium was replaced at 2-day intervals. Bidirectional transport experiments were performed on 5 days after seeding. The confluence of the cell monolayer and the integrity of tight junction were confirmed by microscope and measurement of transepithelial electrical resistance (TEER; 130–180 Ω), respectively. Apical or basolateral chambers were washed twice and preincubated with the transport buffer [25 mM N-2-hydroxyethylpiperazine-N'-2-ethane sulfonic acid (HEPES), 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose (pH7.4)] at 37°C for 30 min.
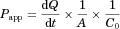
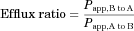
Interaction of LB with Carrier-Mediated Transports in MDCK Cells Expressing Organic Anion Transporting Polypeptide 1B1, Organic Anion Transporting Polypeptide 1B3, Organic Anion Transporter 1, Organic Anion Transporter 3, Organic Cation Transporter 2, and Breast Cancer-Resistance Protein
To determine the interaction of LB with major transporters, organic anion transporting polypeptide 1B1 (OATP1B1), OATP1B3, organic anion transporter 1 (OAT1), OAT3, and organic cation transporter 2 (OCT2) were cloned and functionally expressed in MDCK cells containing Flip-In system (Invitrogen, Carlsbad, California) (Supplementary Information). In addition, MDCKII–breast cancer-resistance protein (BCRP) cells, generously provided by Dr. Borst in the Netherland Cancer Institute, were also used. Cells were typically cultured in DMEM (Hyclone, Thermo Scientific, Rockford, Illinois) containing 10% FBS, 1% nonessential amino acids, 100 units/mL penicillin, 0.1 mg/mL streptomycin, 40 μg/mL gentamicin, and 10 mM HEPES under an atmosphere of 5% CO2 and 90% humidity.
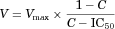
For the case of BCRP-mediated transport, MDCKII–BCRP were seeded on collagen-coated Transwell (Corning, ct#3493) inserts at a density of 2.5 × 105 cells/insert and were grown for 5 days.8 The integrity of the cell monolayer was evaluated prior to the transport experiments by measuring TEER value. Cell monolayers were considered intact and suitable for use in transport experiments when the value was more than 150–180 Ω cm.2, 9 Prior to the transport experiments, cell monolayers were washed twice and preincubated with a buffer comprising a Hank's balanced salts solution, 25 mM HEPES, and 25 mM glucose (pH 7.4). After washing, the plates were incubated in the buffer medium for 30 min at 37°C, and then TEER values were measured.8 For the transport experiment, 1.5 mL of buffer medium containing [3H]-methotrexate in the presence and absence of 100 μM of LB was added to the basolateral side, and 0.5 mL of buffer medium was added to the apical side. A 0.3-mL aliquot of buffer medium was taken from the apical side and replaced with fresh medium every 30 min for 2 h. The amount of radiolabeled methotrexate transport through the cell monolayer was determined by the liquid scintillation counter (Tri-Carb 3110 TR; PerkinElmer Life Science).
Estimation of IC50 of LB on Digoxin Efflux in MDCKII Cells Expressing MDR1
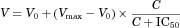
Determination of Plasma Protein Binding and the Blood–Plasma Partitioning of LB
The extent of LB binding to rat plasma proteins was estimated at 37°C using an ultrafiltration method. Rat plasma samples were spiked with LB to have the final concentrations, of 0.1, 0.5, and 2.5 μg/mL, respectively (in triplicate). After preincubation at 37°C for 30 min, an aliquot (0.4 mL) was transferred to an Amicon Microcon Centrifugal Filter Devices (30,000 Da cutoff YM-30, Millipore). The device was centrifuged at 2300 g for 20 min at 37°C to obtain the ultrafiltrates. The concentration in the filtrate was then determined by LC–MS/MS. The unbound fraction of LB in the plasma was determined from the ratio of ultrafiltrate concentration to plasma concentration.
To determine nonspecific binding to the ultrafiltration device, LB was added to water at concentrations of 0.1, 0.5, and 2.5 μg/mL, and an aliquot (0.4 mL) was transferred/centrifuged at 2300 g for 20 min. Nonspecific binding was determined by comparing the chromatographic peak area ratios for LB in water before and after the ultrafiltration procedure.
When necessary, the extent of protein binding was also determined by a rapid equilibrium dialysis (RED) method. According to the manufacturer's protocol (Thermo Scientific, Waltham, Massachusetts), LB was added to an aliquot of rat plasma at a final concentration of 2.5 μg/mL and the base plate of the RED device was then rinsed with 20% ethanol for 10 min. An aliquot of the plasma (100 μL) and PBS (300 μL) were placed into the sample chamber and the buffer chamber, respectively, and the RED device was covered/incubated on a shaker at 37°C for 4 h. After the incubation, a 50-μL aliquot was collected from each side of the chamber. A 50-μL portion of blank plasma was then added to buffer sample, and an equal volume of PBS was also added to the plasma samples to adjust for matrix effects. A 200-μL aliquot of acetonitrile containing the IS was added to each sample, and supernatants were obtained by centrifugation at 4°C for 20 min at 3200 g for use in the LC–MS/MS analysis. The fraction of unbound LB in the plasma was determined by dividing the LB concentration in the buffer compartment by that in the plasma compartment.
The blood–plasma concentration ratio of LB was also determined in fresh rat blood. LB in DMSO was added to blank blood and was diluted with the blood to obtain a final LB concentration of 0.1 and 2.5 μg/mL. Blood was then incubated at 37°C in a shaking water bath for 30 min. After incubation, the plasma was separated from whole blood by centrifugation. Control samples containing LB in the rat plasma was prepared to a final concentration of 0.1 and 2.5 μg/mL. The blood–plasma concentration ratio was calculated from the concentration difference between the control plasma and the plasma isolated from whole blood.
Liver Microsomal Stability
The time-dependent metabolic stability of LB in rat liver microsomes was determined. The reaction mixture (total volume of 0.47 mL) consisted of rat liver microsomes (Corning Gentest) in 100 mM potassium phosphate buffer (pH 7.4) and LB (10 μM, final concentration). After preincubation at 37°C for 5 min, the reaction was initiated by the addition of a nicotinamide adenine dinucleotide phosphate oxidase (NADPH)-regenerating solution (Corning Gentest) containing 1.3 mM NADP+, 3.3 mM glucose-6-phosphate, 0.4 U/mL glucose-6-phosphate dehydrogenase, and 3.3 mM MgCl2. Samples (50 μL) were collected at 0, 1, 3, 5, 15, and 30 min. The reaction was terminated by adding 150 μL of ice-cold acetonitrile-containing rosiglitazone (100 ng/mL, IS). After mixing by vortexing and subsequent centrifugation at 4°C for 5 min at 15700 g, the clear supernatant was collected and analyzed by LC–MS/MS (see below) for the quantification of LB.
When necessary, the rate of glucuronidation was determined for LB in the presence of UDP–glucuronic acid under similar reaction conditions and procedures, except that UDP–glucuronosyltransferase was added to the reaction mixture (Corning Gentest) including 50 mM Tris–HCl (pH 7.5), 8 mM MgCl2, 25 μg/mL alamethicin, and 2 mM uridine 5′-diphosphoglucuronic acid (UDPGA), instead of the NADPH-regenerating solution. Samples (50 μL) were collected at 0, 2, 5, 10, 15, and 30 min of the reaction. Testosterone (final concentration 20 μM) and 7-hydroxycoumarine (final concentration 1 μM) were used as positive controls for the oxidation and glucuronidation reactions, respectively. In vitro metabolic half-life was calculated using the slope (k), obtained from linear regression analysis, of the remaining concentration of LB versus time.10
CYP Inhibition Study
The potential of LB to inhibit major human CYP enzymes was evaluated using human liver microsomes (XenoTech, LLC, Lenexa, Kansas). The reaction mixture composed of 1 mg/mL human liver microsomes, 10 mM MgCl2, 10 μM of LB, and control in pH 7.4 potassium phosphate buffer was prepared (total volume 360 μL) and equilibrated at 37.5°C for 5 min. The reaction was initiated by the addition of 1 mM NADPH and the substrate [i.e., phenacetin (CYP1A2), tolbutamide (CYP2C9), S-mephenytoin (CYP2C19), dextromethorphan (CYP2D6), nifedipine (CYP3A4), and testosterone (CYP3A4)] of CYPs (final concentration of 100, 10, 100, 10, 5, and 50 μM for 1A2, 2C9, 2C19, 2D6, or 3A4, respectively); the mixtures were then incubated in a shaking water bath at 37.5°C for 30 min. Aliquots (100 μL) were withdrawn in triplicate at 30 min and an 100 μL of ice-cold acetonitrile–water (50:50, v/v) was added to terminate the reaction. The samples were vortexed briefly and centrifuged at 9300 g for 10 min. The LB concentration of the supernatant from each sample was then measured using LC–MS/MS.
All analyses were performed with an Applied Biosystems/MDS Sciex API3200 QTrap LC–MS/MS system interfaced with Agilent 1100 HPLC in electrospray (Turbo Ion Spray) ion source (Applied Biosystems, Foster City, California). Chromatographic separation was performed with a SB-Aq column (2.1 mm × 50 mm, 3 μm, Zorbax, Agilent, Santa Clara, California). The mobile phase consisted of solvent A, 0.1% formic acid in water, and solvent B, acetonitrile, with an A/B gradient for metabolite [i.e., acetaminophen (CYP1A2), 4-OH tolbutamide (CYP2C9), 4-OH mephenytoin (CYP2C19), dextrophan (CYP2D6), 4-OH nifedipine (CYP3A4), and 6-OH testosterone (CYP3A4)] of substrate with a flow rate of 0.3 mL/min.

In Vivo Pharmacokinetic Studies
Animals
Male Sprague–Dawley (SD) rats, 6–7 weeks old, were purchased from Orient Bio Inc. (Gyeonggi-do, Korea) and were used in all in vivo studies and in the collection of blank blood and tissue samples. Experimental protocols involving the animals used in this study were reviewed by the Seoul National University Institutional Animal Care and Use Committee according to the National Institutes of Health Publication Number 85-23 Principles of Laboratory Animal Care revised in 1985.
Administration to Rats
Male SD rats, weighing approximately 250–300 g, were anesthetized by an intramuscular administration of 50 mg/kg tiletamine HCl/zolazepam HCl (Zoletil 50, Virbac Laboratories, Carros, France) and 10 mg/kg xylazine HCl (Rompun, Byer Korea, Seoul, Korea). After confirming the induction of anesthesia, the femoral artery (for collecting blood samples) and vein (for supplementing body fluids or administration) were catheterized with polyethylene tubing (PE 50, Clay Adams, Parsippany, New Jersey), filled with heparinized saline (25 U/mL) and saline, respectively. The LB administration study was carried out after the confirmation of recovery from the anesthesia. For the intravenous administration study, solutions were injected via the intravenous catheter at the dose of 0.5, 1, or 2 mg/kg. For oral administration, the rats received LB at a dose of 0.5 or 2 mg/kg via oral gavage. The vehicle for LB administration (both routes) was DMSO–PEG400–distilled water (0.5:4:5.5) (typical dosing volume of 2 mL/kg) throughout the pharmacokinetic study.
Blood samples (150 μL for each sample) were collected from the arterial catheter at predose, 1, 5, 10, and 30 min and 1, 2, 4, and 8 h postdosing (intravenous administration) or at predose, 5, 10, and 30 min and 1, 2, 4, 8, and 12 h postdosing (oral administration). Plasma was separated from the blood samples by centrifugation (16100 g, 5 min, 10°C) and stored at −70°C. Preliminary study indicated that our blood sampling did not cause any effect on the hematocrit (i.e., hematocrit from the predose blood sample = 46.8 ± 2.2%; the hematocrit from after the 11th sampling = 42.6 ± 1.6%; not significant).
When it was necessary to determine the biliary recovery of LB, the bile duct of the rat was catheterized. Male SD rats were anesthetized and catheterized following a procedure similar to that described above, except that the bile duct was catheterized with polyethylene tubing (PE 10, Clay Adams). After recovering from the anesthesia, the rats were intravenously administered with LB at an intravenous dose of 0.5 or 1 mg/kg, and the bile was collected at designated time intervals (0–2, 2–4, 4–6, 6–8, and 8–24 h). The bile samples were weighed and stored at −80°C until used for analysis.
Pooled urine and gastrointestinal (GI) tract samples were also collected. Male SD rats were injected with LB via the tail vein at a dose of 0.5, 1, or 2 mg/kg and placed in metabolism cages with free access to water. Urine samples were collected in tube on dry ice at designated time intervals (0–8 and 8–24 h) after intravenous administration. The metabolic cage was rinsed with 10 mL of distilled water at 8 and 24 h. After measuring the exact volume of the combined urine sample, the samples were stored in a −80°C freezer until used in the analysis. To determine the fraction LB remaining in the intestine, entire GI tract, including its contents and feces, was removed at 24 h after the oral and intravenous administration, transferred into a beaker containing 100 mL of methanol to facilitate extraction of LB, cut into small pieces using scissors, and then homogenized by PowerGen 1000 Homogenizer (Fisher Scientific Inc., Waltham, Massachusetts) and stored at −70°C until the analysis.
Tissue Distribution Studies
Lobeglitazone at a dose of 2 mg/kg was administered intravenously over 1 min to SD male rats via the tail vein. The rats were sacrificed with CO2 at 1 h after the administration and blood sample was collected. In addition, approximately 1 g of tissue from the brain, liver, lung, heart, small intestine, large intestine, kidney, spleen, fat, testis, or muscle was excised immediately. The tissues were added to six volumes of mixture (acetonitrile–water = 1:1, v/v) per tissue weight, homogenized by PowerGen 1000 Homogenizer (Fisher Scientific Inc.), and stored at −70°C until used in an analysis.
Data Analysis
Standard moment analysis was carried out to calculate the pharmacokinetic parameters,11 including the area under the plasma concentration–time curve (AUC), first moment of plasma concentration–time curve (AUMC), time-averaged total body (CL), renal (CLr)/nonrenal (CLnr) clearances, terminal half-life (t1/2), mean residence time (MRT), apparent volume of distribution at steady state (Vss), and extent of absolute oral bioavailability (F). In this analysis, the area up to the last sampling time was calculated by the linear trapezoidal method added to the remaining area to the infinite time calculated by standard methods. The peak plasma concentration (Cmax) and time to reach a Cmax (Tmax) after oral administration were directly read from the concentration–time profile.
When it was necessary to compare the means between the treatments, the Student's t-test or one-way ANOVA, followed by Duncan's test, was typically used. Data are expressed as the mean ± SD.
RESULTS
Parallel Artificial Membrane Permeability Assay
The estimated Papp value for verapamil was 8.66 ± 0.0978 × 10−6 cm/s, similar to other literature reports (∼7.4 × 10−6 cm/s),4, 5 suggesting that the current assay is adequate for estimating the permeability. The estimated value for Papp of LB was approximately 1.19 ± 0.142 × 10−6 cm/s, the value corresponding to the predicted human bioavailability of 72.1%. In addition, LB sulfate, a soluble salt form of the activator, has an estimated Papp of 7.39 ± 0.0174 × 10−6 cm/s. These observations indicate that permeability of LB to the artificial membrane is high. The recoveries for LB, LB sulfate and verapamil were nearly complete (>92%).
Permeability Study with MDCKII Cells
In this study, intestinal permeability was estimated using MDCKII cell monolayers. In previous reports, the permeability of compounds across an epithelial cell monolayer was found to have a significant correlation with human bioavailability.12 The transport of LB at concentrations of 5 μM was determined at 2 h in both the apical to basolateral (A to B) and basolateral to apical (B to A) directions (Table 1) in wild-type MDCKII cell monolayers. The A to B transport was estimated to be 0.533 ± 0.0914 × 10−6 cm/s, whereas the B to A transport was estimated to be 5.02 ± 0.510 × 10−6 cm/s. The Papp for the A to B direction, the value relevant for the intestinal absorption, indicated that human bioavailability may be approximately 43.2%.13 To determine whether MDR1 is involved in the limited transport of LB in the absorptive direction, a similar bidirectional transport study was carried out for LB in MDCKII–MDR1 cell monolayers (Table 1). Although A to B permeability was relatively unchanged (i.e., from 0.533 to 0.433 × 10−6 cm/s; Table 1), the B to A transport value was significantly increased in MDR1 cells (i.e., from 5.02 to 8.28 × 10−6 cm/s, p < 0.05).
| Peapp (× 10−6 cm/s) | |||
|---|---|---|---|
| LB (5 μM) | A to B | B to A | Efflux Ratio |
| Wild type | 0.533 ± 0.0914 | 5.02 ± 0.510 | 9.42 |
| MDR I | 0.433 ± 0.123 | 8.28 ± 1.76 | 19.1 |
| Wild type + verapamil | 4.14 ± 0.691 | 5.52 ± 0.769 | 1.33 |
| MDR I + verapamil | 4.06 ± 0.618 | 6.98 ± 0.418 | 1.72 |
- Data are expressed as the mean ± SD of quadruplicate runs.
To further examine the involvement of MDR1-mediated LB efflux in MDCKII cells, a bidirectional transport study was carried out for 5 μM LB in the presence of 500 μM verapamil (i.e., an inhibitor for MDR1) in wild-type MDCKII cells as well as MDCKII–MDR1 cells (Table 1). The addition of verapamil was associated with a significant increase in A to B transport (i.e., from 0.533 to 4.14 × 10−6 cm/s for wild-type cells, p < 0.001; from 0.433 to 4.06 × 10−6 cm/s for MDR1 cells, p < 0.001), whereas the extent of change for B to A transport was much less (Table 1). As a result, the efflux ratio was reduced (i.e., from 9.42 to 1.33 for wild-type cells; from 19.1 to 1.72 for MDR1 cells) as the result of the addition of verapamil. These observations indicate that LB is transported via the MDR1 efflux transporter.
Interaction of LB with SLC, MDR1, and BCRP Transporters
In this study, we examined the possibility of LB interacting with six major transporters. These six transporters were selected based on the regulatory recommendation from the United States Food and Drug Administration.14 The cellular accumulation of the representative substrate was studied in the presence of varying concentrations of LB and the percent transport activity was plotted against the substrate concentration (Fig. 1) and the IC50 value was then estimated. In general, the inhibitory potential of LB was very weak for SLC transporters such as OATP1B3, OAT1, and OCT2, whereas the interaction was readily measured for OATP1B1 and OAT3 (Fig. 1). The estimated IC50 values were (in μM) 2.44 ± 1.23, 216 ± 187, 151 ± 36.9, and 34.3 ± 33.9 for OATP1B1, OATP1B3, OAT1, and OAT3, respectively. The plasma concentration found in our pharmacokinetic studies was consistently less than 20 μM (i.e., initial plasma concentration after 2 mg/kg intravenous injection, see below), suggesting that the inhibition of SLC transporters, except for OATP1B1, is less likely.
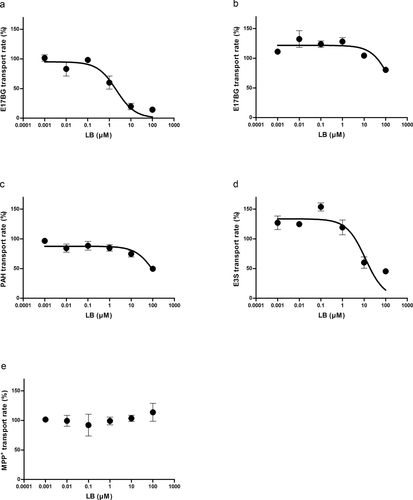
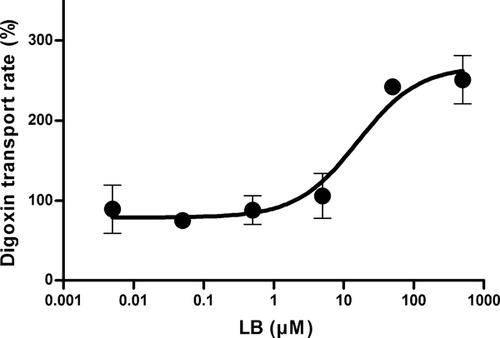
For MDR1, IC50 was estimated based on the inhibition of digoxin efflux by LB (Fig. 2). In this study, a higher cellular accumulation would be regarded as the inhibition of digoxin efflux. LB concentration dependency was readily evident with the estimated IC50 of 12.5 ± 3.61 μM. For the case of BCRP, a bidirectional transport study was carried out for methotrexate (i.e., standard substrate for the transporter) in the absence and presence of LB (i.e., 200 μM, final concentration) in MDCKII–BCRP cell monolayers. The addition of LB affected the transport to a certain extent (i.e., for A to B transport, 0.508 ± 0.0490 × 10−6 cm/s “without LB,” 1.27 ± 0.205 × 10−6 cm/s “with LB”; for B to A transport, 1.93 ± 0.0803 × 10−6 cm/s “without LB,” 3.18 ± 0.121 × 10−6 cm/s “with LB”). As a result, the efflux ratio did not appear to change (i.e., from 3.79 for “without LB” to 2.49 for “with LB”). These observations indicate that the involvement of BCRP in the transport of LB is weak.
Plasma Protein Binding and Blood–Plasma Partitioning of LB
In this study, the extent of LB binding to rat plasma proteins was examined using an ultrafiltration method. In rat plasma, the unbound fractions of LB at concentrations of 0.1, 0.5, and 2.5 μg/mL were 0.352 ± 0.194, 0.201 ± 0.0111, and 0.147 ± 0.0426%, respectively, suggesting that LB binds extensively to rat plasma proteins. The percent of unbound LB was also determined by RED. The findings indicated that plasma protein binding, as measured by the dialysis method, was also extensive (0.549 ± 0.0759%, at a final LB concentration of 2.5 μg/mL) and was not affected by the LB concentration (unpublished observation). The blood–plasma concentration ratio of LB at 0.1 and 2.5 μg/mL was 139 ± 13.0% and 126 ± 13.8%, respectively, suggesting that there is no appreciable distribution of LB to blood cells and that the concentration of LB in the plasma and the blood is almost identical.
Liver Microsomal Metabolic Stability
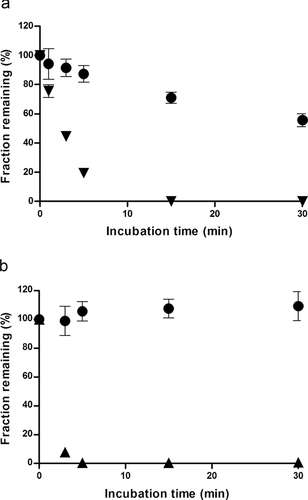
The metabolic stability of LB involved incubation for 0.5 h with rat liver microsomes in the presence of NADPH and UDPGA, respectively. As shown in Figure 3a, the LB in rat liver microsomes significantly decreased to approximately 56% (i.e., half-life of 38 min) of the initial concentration after 0.5 h of incubation in the presence of NADPH. Under similar conditions, testosterone, a positive control, decreased with a half-life of 2.1 ± 0.1 min in rat liver microsomes. In contrast, the concentration of LB in rat liver microsomes remained relatively unchanged (Fig. 3b) at 30 min of incubation in the presence of UDP–glucuronic acid. However, 7-hydroxycoumarin (7-OH), a positive control for glucuronidation, disappeared completely within 5 min of the start of the reaction, suggesting that the microsomal fraction contained adequate glucuronidation activity.
CYP Inhibition
To determine the inhibition of CYP enzyme activity for LB in humans, typical substrates [i.e., 1A2 (phenacetin), 2C9 (tolbutamide), 2C19 (S-mephenytoin), 2D6 (dextromethorphan), or 3A4 (nifedipine and testosterone)] for CYP isozymes was incubated with LB in the presence of NADPH. The metabolism of the substrate was inhibited by up to 41.0 ± 6.56%, 50.1 ± 5.71%, and 43.2 ± 3.71% for CYP1A2, 2C9, and 2C19, respectively, when LB was present (Table 2). For the case of the incubation containing substrates of other pathways, the extent of reduction was less than 20% (i.e., 4.90 ± 3.60% for 2D6, 16.1 ± 5.48% using nifedipine for 3A4 and 31.0 ± 13.1% using testosterone for 3A4) (Table 2), suggesting that these pathways play a minor role in inhibiting the CYP enzyme activity of LB.
| CYP Isozymes | Change in Activity (%) |
|---|---|
| CYP1A2 | 41.0 ± 6.56 |
| CYP2C9 | 50.1 ± 5.71 |
| CYP2C19 | 43.2 ± 3.71 |
| CYP2D6 | 4.90 ± 3.60 |
| CYP3A4 (nifedipine) | 16.1 ± 5.48 |
| CYP3A4 (testosterone) | 31.0 ± 13.1 |
- Data are expressed as the mean ± SD of triplicate runs.
In Vivo Pharmacokinetic Study
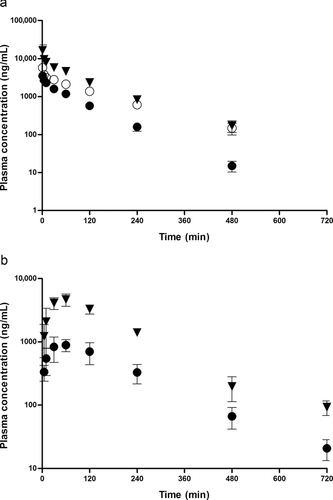
The mean plasma concentration–time curves in rats following an intravenous administration of LB at doses of 0.5, 1, and 2 mg/kg are shown in Figure 4a. The pharmacokinetic parameters, as estimated by a standard moment analysis, are listed in Table 3. The dose-normalized AUC values were determined to be 459, 514, and 481 μg min/mL for 0.5, 1, and 2 mg/kg, respectively. Consequently, the systemic clearance showed similar values, between 1.95 and 2.19 mL/min/kg, and did not change significantly with respect to the dosage, indicating that the elimination was linear for LB in rats in the dose range studied. In addition, the Vss of LB was 189–276 mL/kg and not statistically different with the dose, suggesting that the distribution kinetics of LB follows linear kinetics. The mean plasma concentration–time curves in rats following the oral administration of LB at 0.5 and 2 mg/kg are illustrated in Figure 4b, and the pharmacokinetic parameters listed in Table 3. The Tmax at 0.5 and 2 mg/kg was 67.5 and 48.8 min, respectively, indicating that the absorption of LB from the GI tract occurred very rapidly after administration. The dose-normalized AUC values were found to be 421 and 476 μg min/mL for doses of 0.5 and 2 mg/kg, respectively. The absolute bioavailability after the oral administration of LB was nearly complete and apparently not affected by the dosage [i.e., 92.1% (0.5 mg/kg) and 99.0% (2 mg/kg)]. The extent of LB remaining in the GI tract at 24 h (GI24 h) was negligible, with values less than 0.2% of the oral dose, suggesting that the intestinal absorption is complete in rats at the dose range studied. This observation is consistent with nearly complete bioavailability for LB.
| Intravenous Administration | |||
|---|---|---|---|
| Parameter | 0.5 mg/kg | 1 mg/kg | 2 mg/kg |
| AUCinf (μg min/mL) | 229 ± 18.6 | 514 ± 35.1 | 963 ± 29.6 |
| AUCinf (μg min/mL)/dose | 459 ± 37.1 | 514 ± 35.1 | 481 ± 14.8 |
| t1/2 (min) | 68.5 ± 6.0 | 110 ± 13.2 | 93.2 ± 13.2 |
| MRTinf (min) | 85.8 ± 10.1 | 142 ± 16.9 | 108 ± 19.0 |
| CL (mL/min/kg) | 2.19 ± 0.177 | 1.95 ± 0.128 | 2.08 ± 0.0650 |
| CLr (mL/min/kg) | 0.00160 ± 0.000496 | 0.00216 ± 0.000207 | 0.00187 ± 0.00123 |
| CLnr (mL/min/kg) | 2.19 ± 0.177 | 1.95 ± 0.128 | 2.08 ± 0.0638 |
| Vss (mL/kg) | 189 ± 31.8 | 276 ± 14.0 | 225 ± 39.5 |
| Ae, urine0–24 h (% of dose) | 0.0643 ± 0.0163 | 0.0749 ± 0.0117 | 0.0629 ± 0.0419 |
| Ae, feces0–24 h (% of dose) | 1.97 ± 0.610 | 2.61 ± 0.417 | 2.19 ± 0.666 |
| Ae, bile0–24 h (% of dose) | 0.367 ± 0.122 | 0.314 ± 0.133 | ND |
| GI24 h (%) | 0.105 ± 0.123 | 0.880 ± 0.0597 | 0.0417 ± 0.0305 |
| Oral Administration | |||
| Parameter | 0.5 mg/kg | 2 mg/kg | |
| AUCinf (μg min/mL) | 211 ± 40.6 | 953 ± 121 | |
| AUCinf (μg min/mL)/dose | 421 ± 81.1 | 476 ± 60.3 | |
| Cmax (μg/mL) | 0.962 ± 0.285 | 4.94 ± 0.600 | |
| Tmax (min) | 67.5 ± 37.7 | 48.8 ± 22.5 | |
| Ae, urine0–24 h (% of dose) | 0.0293 ± 0.00709 | 0.0424 ± 0.00402 | |
| Ae, feces0–24 h (% of dose) | 9.72 ± 3.99 | 9.05 ± 1.80 | |
| GI24 h (%) | 0.155 ± 0.104 | 0.0908 ± 0.0521 | |
| F (%) | 92.1 | 99.0 | |
- Data are expressed as the mean ± SD of quadruplicate runs.
- ND, not determined.
The cumulative amounts of LB excreted in the urine (doses of 0.5, 1, and 2 mg/kg) and bile (doses of 0.5 and 1 mg/kg) were determined after intravenous administration. In this study, the total recoveries of unchanged LB were 0.31%–0.37% and 0.06%–0.07% of the total dose for the biliary and urinary routes, respectively, indicating that those excretory routes represent minor elimination pathways.
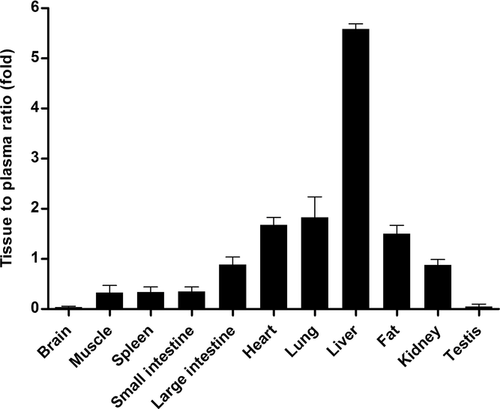
Tissue Distribution
The tissue-to-plasma (T/P) ratios of LB at 1 h after the intravenous administration of 2 mg/kg in rats are listed (Fig. 5). In general, LB was present in various tissues at concentrations above that expected from the blood remaining in the tissue. The primary distribution site was the liver (i.e., T/P ratio of 5.59 ± 0.100) for LB but it was also distributed to a less extent to the heart (i.e., T/P ratio of 1.68 ± 0.146), lung (i.e., T/P ratio of 1.83 ± 0.411), and fat (i.e., T/P ratio of 1.50 ± 0.172). In our preliminary study, the steady-state tissue to plasma ratio (Kp) value for LB was also determined in rats; the Kp values ranged from approximately 0.25–4.0 for major tissues and were comparable to the those obtained in this study.
DISCUSSION
The current study was primarily focused on the determination of the in vitro and in vivo properties for the absorption, distribution, metabolism, and excretion of LB, a new TZD-PPARs activator. In this study, the extent of absorption was first estimated based on a permeability assay with artificial membranes (i.e., PAMPA). We estimated that the LB permeability value corresponded to approximately 72% of its absolute oral bioavailability in humans, provided that the presystemic metabolism is not extensive. In this study, however, PEG400 (i.e., 15% by volume), a solvent that was reported to affect permeability measurement,15 needed to be included in order to achieve a soluble system. In literature report,15 the authors found that when 15% PEG400 is added to the donor solution the permeability decreased by approximately 50% of the Papp without the solvent. The reduction appeared similar for all test compounds, indicating that the reduction is primarily because of the chemicals in the system (i.e., by affecting the partition of test compounds from the media to the lipid layer). Therefore, it appeared likely that solubilized LB itself would be more permeable to the lipid membrane and that the bioavailability estimate might be higher than predicted. To demonstrate this possibility, the sulfate salt of LB, a water soluble form, was found to have PAMPA Papp of 7.39 ± 0.0174 × 10−6 cm/s, indicating that solubilized LB indeed would be expected to be completely bioavailable. Consistent with this, the bioavailability of LB was also predicted to be over 94% based on the correlation between the log P (i.e., LB log P of 4.20) value and human bioavailability.16
From the apical to basolateral permeability value for LB (i.e., 0.533 ± 0.0914 × 10−6 cm/s) obtained in MDCKII cell monolayer studies, it was predicted that the intestinal bioavailability would be incomplete in humans, a result that is contrary to the results of the PAMPA study. In a subsequent study, we found that the low permeability value can be attributed to MDR1-mediated efflux (Table 1). The discrepancy between the permeability estimates from the PAMPA study and the cell permeability study may be related to the active efflux of LB in the apical membrane of MDCKII–MDR1 cells. In the cellular accumulation study with MDR1-expressing MDCK cells, we found that the IC50 value was approximately 12.5 μM for LB in the efflux of digoxin. Although the Km value was not reported for digoxin, MDR1 substrates typically have Km values within the 10–100 μM range.17 Considering the digoxin concentration used in the inhibition study (i.e., 1 μM), the IC50 would be close to the Ki value for LB. Assuming the mechanism of interaction between LB and digoxin is competitive, the Ki would be regarded as reflecting the affinity of the LB for the transporter (i.e., MDR1). The intestinal fluid in 250 g rat is approximately 2.5 mL,18 and the concentration of LB in the intestine would be expected to be approximately 104 and 416 μM for 0.5 and 2 mg/kg oral administration, respectively [i.e., molecular weight (LB) of 480.2 g/mol]. The expected concentration would be significantly greater than the estimated affinity of LB (i.e., 12.5 μM) so that MDR1-mediated transport is saturated in our oral administration study. Therefore, despite the fact that the drug is subjected to MDR1-mediated efflux and has an extensive efflux ratio, an oral bioavailability of over 90% might have been possible for LB. In the literature, a number of drugs with high-efflux ratios have been reported to have a relatively high oral bioavailability (e.g., dicloxacillin19 and prazocin20, 21).
The extent of excretion to the urine, bile, feces, and intestine was minor for LB (i.e., the combined excretion was less than 10% of the dose; Table 3). Therefore, the systemic clearance may be effectively regarded as the hepatic clearance (i.e., ∼0.5 mL/min/250 g rat). As hepatic blood flow was reported to be 14.5 mL/min for a 250-g rat,22 the hepatic extraction ratio for LB would be expected to be 0.0344 (i.e., a low-extraction drug). This estimate is also consistent with the nearly by complete oral bioavailability for LB, as presystemic elimination is not likely to occur for LB. In addition, LB appeared to interact with a number of CYP isozymes (e.g., 1A2, 2C9, and 2C19; Table 2) to form at least five metabolites (unpublished observation). This aspect of LB metabolism is currently under investigation in this laboratory. In an in vitro metabolism study with rat liver microsomes, the reaction is primarily mediated by a phase I reaction, rather than a phase II reaction, suggesting that conjugation is not a major factor in the metabolic elimination of LB. The relatively good metabolic stability may also be related to the adequate biological half-life of LB (i.e., ∼110 min; Table 3) found in our in vivo study.
It is generally known that the binding of drugs to plasma proteins plays an important role in the disposition of drugs. In this study, LB was found to bind extensively to plasma proteins (i.e., up to 99.9%) with no appreciable concentration dependency on the unbound fraction. In particular, the extensive protein binding may also be involved in the absorption of LB. In our MDCKII cell monolayer study, we found that the efflux ratio was approximately 9.42 in wild-type MDCKII cells. However, this in vitro estimate may be markedly different from the in vivo situation, as LB exists primarily as the bound form in the systemic circulation. As a result, the efflux rate for LB would be substantially lowered in the case of an in vivo situation. This statement is consistent with the almost complete oral bioavailability of LB in rats (Table 3).
In addition to MDR1, interactions with other major transporters were also noted for LB. Among the SLC transporters studied, the interaction of LB with OATP1B3 and OAT1 resulted in IC50 values of over 100 μM (i.e., the highest concentration). For OAT3, the estimated IC50 was approximately 34.3 μM, suggesting that the inhibitory potential is slightly higher than those of the other two transporters. However, the plasma concentration found in our pharmacokinetic studies was consistently less than 20 μM (i.e., initial plasma concentration after 2 mg/kg intravenous injection; Fig. 4a), and, considering the IC50 of over 30 μM, the inhibition of SLC transporters, except for OAT3, is less likely in the concentration ranged studied. For OCT2, LB did not appear to affect the functional activity of the transporter at concentrations up to 100 μM. For BCRP, the efflux ratio of methotrexate was only slightly lowered by the presence of LB (i.e., 100 μM, from 3.79 to 2.49) in MDCKII–BCRP cells. In contrast, LB efflux ratio was decreased by 10-fold in the presence of verapamil (Table 1). Therefore, the interaction of LB with BCRP appeared to be relatively weak. For OATP1B1, IC50 values, that is, the apparent affinity of LB, was found to be approximately 2.44 μM. Taken together with the range of plasma concentration observed in this study, it is possible that this transporter may play a kinetic role. In our preliminary study, we functionally expressed a rat Oatp1b2 (i.e., the rat analog23, 24 of hOATP1B1) variant in MDCK cells. Interestingly, the presence of LB at concentrations up to 100 μM had no effect on the functional activity of the transporter, suggesting that the rat analog may be biochemically/functionally different from the human OATP1B1. Furthermore, considering the fact that there are three variants of the rat Oatp1b2,25 the relationship between the anion transporter and LB pharmacokinetics may be complex; this aspect of species differences for LB transport clearly warrants additional study.
In summary, LB, a new drug candidate for the use as an activator of TZD-PPARs, showed linear pharmacokinetics in terms of the absorption, tissue distribution, and elimination in rats. The activator had a nearly complete bioavailability in rats, despite the fact that the drug may be subjected to MDR1-mediated efflux in vitro. The discrepancy in LB kinetics may be attributed to a number of reasons including the high affinity of the drug for MDR1, the extensive protein binding of LB in the plasma, and/or high lipophilicity. Among the other six major SLC transporters, OATP1B1 is likely to have kinetic relevance considering the in vivo concentration levels found in this study. LB appeared to have a reasonably long metabolic half-life, primarily via CYP-dependent processes (i.e., CYP1A2, 2C9, and 2C19).
ACKNOWLEDGMENT
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A100096).




