Capric Acid Absorption in the Presence of Hydroxypropyl-β-Cyclodextrin in the Rat Ileum using the In Situ Single-Pass Perfusion Technique
Abstract
The purpose of the present study was to gain quantitative mechanistic insight into the role cyclodextrin carriers may play in the intestinal absorption of highly lipophilic molecules. The physical model approach was employed to investigate capric acid absorption in the rat ileum using the in situ single-pass method with 2-hydroxypropyl-β-cyclodextrin (HPB) present in the perfusate. Two physical models were examined: the flat surface model in which the intestinal wall was treated as a hollow, smooth, circular cylinder, and the villus model in which the intestinal surface allowed for the presence of villi. Capric acid absorption was found to be essentially 100% aqueous boundary layer controlled at low HPB concentrations and increasingly membrane controlled at the higher HPB concentrations. Theoretical calculations based on the experimental data and model parameters were found to be consistent with: at low HPB concentrations, capric acid was mainly absorbed at the villus tips and there was very little capric acid penetration into the intervillus space; in contrast, at 50 mM HPB, there was considerable capric acid penetration into the intervillus space, this corresponding to around a 4.5-fold increase in the accessible area for absorption when compared with 0 mM HPB. © 2014 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2832–2844, 2015
INTRODUCTION
Although there has been much progress from the practical standpoint in the area of oral drug formulation development involving cyclodextrins,1-5 there has been relatively little progress from the standpoint of obtaining a quantitative mechanistic understanding of the interplay of key variables involved in the intestinal absorption enhancement by cyclodextrins. Recently, we developed a physical model approach involving a model which we called the flat surface model (FSM) that should represent an important step toward the mechanistic understanding of the transport of highly lipophilic, ionizable drug molecules between two aqueous compartments separated by a lipophilic membrane, and where a carrier such as a cyclodextrin is present in the aqueous phase.6 Capric acid permeability coefficients predicted by the FSM equations describing the permeant transport behavior in the presence of a carrier were found to be in good agreement with the experimental results for capric acid transport between two aqueous compartments separated by a silicone polymer membrane over wide ranges of pH and the 2-hydroxypropyl-β-cyclodextrin (HPB) concentrations. The good agreement of experimental data with the FSM predictions over the range of conditions demonstrates the rigor of the approach and the appropriateness of extending the applications of the FSM approach to other transport situations and conditions. The FSM was able to quantitatively demonstrate the interplay of key variables such as solution pH, capric acid pKa, caprate species-HPB binding constants, aqueous boundary layer (ABL) thickness, diffusion coefficients, and the capric acid lipophilicity and to demonstrate the relative importance of each of these variables for a given set of transport conditions.
A main purpose of establishing the FSM approach in this previous study6 was to establish a sound physical model framework that could be used to probe the effects of cyclodextrins on the transport of lipophilic molecules across biological membranes, more specifically the intestinal membrane. Accordingly, the aim of the present study was to gain insight into the role of cyclodextrin carriers in improving the oral bioavailability of highly lipophilic molecules by applying the FSM approach to results from intestinal absorption experiments involving the rat ileum. Capric acid and HPB are used to represent a typical, highly lipophilic weak acid molecule and a carrier molecule, respectively. The interplay of key variables, their relative importance under various experimental conditions, and the mechanism by which cyclodextrins may improve oral bioavailability were to be examined. An additional aim of this study was to extend the FSM to allow for a more detailed examination of the rat ileal intestinal absorption problem. To this end, a villus model (VM) that takes into account the villus architecture of the ileum is also presented. Each of the models is examined in light of the experimental data for capric acid absorption in the rat ileum at pH 7.4 in the presence of 0–50 mM HPB.
STRATEGY
The Experimental Method and the Estimation of Key FSM Parameters
The technique of in situ single-pass perfusion of an isolated rat ileal segment and local mesenteric venous blood collection is used.7, 8 As the perfusing permeant (i.e., capric acid) solution flows down the rat ileum, taken as a cylinder with a smooth inner surface, at a constant rate, the permeant is absorbed across the epithelial cells into a blood sink. The perfusion flow rate is strictly controlled using a perfusion pump, and the exit concentration of the permeant and the osmolarity of the perfusate are assayed at regular time points. Mesenteric venous cannulation provides the means for the determination of the permeant appearance rate in the blood draining the intestinal segment. This allows for a more sensitive determination of steady-state flux as compared with methods that determine the permeant flux from the disappearance kinetics in the lumen. The gut length and the inside diameter are measured for the flat surface area determination (flat surface area refers to treating the intestinal gut segment as a hollow, smooth circular cylinder), and the mean permeant concentration in the lumen is determined from initial and exit concentrations of the permeant. With the mean luminal concentration, membrane surface area, and appearance rate of permeant in the blood, the permeant flux (Jb) and the total permeability coefficient (PT) are directly determined. Further details are explained in the methods and data treatment sections.
Key parameters of the FSM developed in the previous study6 were intended for application in the intestinal perfusion studies. With the exception of the ABL thickness (hABL) and intrinsic membrane permeability coefficient (Pi), the parameters determined in the previous study are applicable to the intestinal perfusion of capric acid in the presence of HPB and are listed, along with the appropriate hABL and Pi for the present study, in Table 1. The hABL is dependent upon the hydrodynamics and therefore must be determined experimentally under the same experimental conditions as those of the perfusion experiments in this study. The hABL was determined to be 0.117 cm by a best-fitting procedure (as described in detail in the results section) involving the experimental data over the range of HPB concentrations at pH 7.4. Taurocholate, which is actively absorbed in the rat ileum and which is completely ABL controlled,7 was used to confirm the hABL thickness. A value of 0.102 cm was obtained using tracer levels of taurocholate, which was consistent with the hABL determined previously in our laboratory (0.109 cm) for the same perfusate flow rate.7 The best-fit hABL of 0.117 cm was chosen for the purposes of this study. The estimation of Pi was initially thought to be problematic. Although capric acid alone transport across the silicone polymer membrane was shown in the previous study6 to be essentially membrane controlled at pH 7.4, preliminary intestinal perfusion experiments indicated that capric acid alone absorption was 100% ABL controlled at pH 7.4 in the rat ileum and therefore Pi was thought to be insensitive to quantification. However, as will be seen, the HPB carrier investigation itself provided a means for obtaining a reasonably good estimate of Pi because, as was shown in the previous study, with increasing HPB concentration capric acid transport becomes increasingly membrane controlled. Thus, the capric acid transport experiments in the presence of varying concentrations of HPB (0–50 mM) provided a means for determining both hABL and Pi by best fitting of both parameters to the entire range of experimental data.
| Parameter | Symbol | Value |
|---|---|---|
| Effective aqueous boundary layer thickness (cm) | hABL | 0.117(±0.02) |
| Capric acid intrinsic permeability coefficient (FSM case) (cm s−1) | Pi,FSM | 3.5(1.7–10) |
| Capric acid intrinsic permeability coefficient (VM case) (cm s−1) | Pi,VM | 0.48(0.15–2.1) |
| Capric acid (HA) or caprate ion (A−) aqueous diffusion coefficient (cm2 s−1) | Df | 6.9(±0.5) × 10−6 |
| A−·HPB or HA·HPB diffusion coefficient (cm2 s−1) | D* | 2.9(±0.2) × 10−6 |
| Taurocholate diffusion coefficient (cm2 s−1) | 5.6 × 10−6a | |
| HA·HPB binding constant (mM−1) | Ku | 7.5(±0.3) |
| A−·HPB binding constant (mM−1) | K− | 2.5(±0.2) |
| Buffer solution pH | pH | 7.40(±0.01) |
| pKa of capric acid | pKa | 4.53(±0.03) |
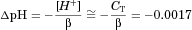 (1)
(1)Secondly, the microclimate pH about the rat ileal membrane surface, owing to the continuous flow of alkalinized secretions from intervillus secretory cells, has been found to be pH 7.3,10, 11 which is essentially the same as the pH of the phosphate buffer used in the present study with the rat ileum. In contrast, the secretory cells in the rat jejunum would maintain a more acidic microclimate pH 6.0 environment; in the latter case, there may be the potential to sustain pH gradients if the luminal solution pH was buffered at pH 7.4,12 and for this latter more complex situation, a physical model has yet to be written. On a somewhat different but related issue, the open-ended intraluminal perfusion of pH 7.4 buffered solution of capric acid and HPB mixtures from an infinite reservoir at 0.2 mL/min (or linear flow velocity of 1.6 cm/min) would also preclude the existence of any longitudinal pH gradient along the 10 cm ileal segment as well as any significant accumulation of secretions in the perfusate with time.
Flat Surface versus Villus Surfaces
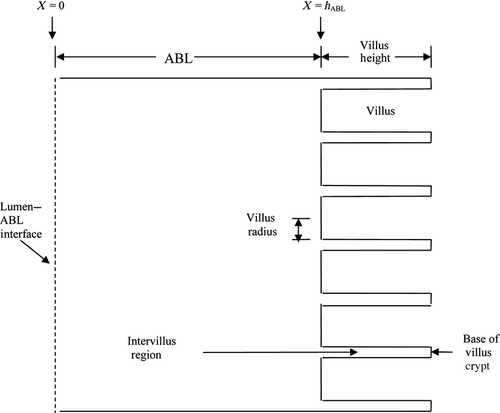
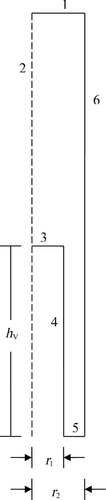
In addition to different FSM hABL and Pi parameter values when compared with the in vitro silicone polymer membrane transport system,6 the in situ single-pass perfusion system involves key fluid flow and geometry differences. The permeant solution flows into and out of the isolated ileal segment in the intestinal perfusion experiment, whereas the permeant solution is confined to a magnetically stirred donor compartment in the side-by-side diffusion cell experiment. Another key difference between the intestinal perfusion experiments and the silicone polymer membrane experiments is that the surface of the rat ileum is covered with villi which may significantly increase the effective surface area utilized for absorption compared with the smooth (flat) surface of the silicone polymer membrane. Accordingly, we also examine a modified physical model which should be more appropriate to the intestinal transport experiments and is termed the VM for intestinal absorption. This VM incorporates the height, diameter, and density of the villi as well as the mean intervillus space. Winne13, 14 and Oliver et al.15 have previously idealized the structure of the villi using a two-dimensional (2D) model with an intervillus space of given height and width that represents the villi as rectangular columns with a mean villus diameter. Using a similar approach, we idealize the villus structure using a 2D axial symmetry model (see Figs. 1 and 2, and Table 2) that represents the villi as circular-cylindrical columns of a given height and diameter with a defined intervillus space represented by an annular cylinder, coaxial with the villus, having a cross-sectional area of the average intervillus space per villus unit of  where r2 is the outer radius of this annular cylinder and r1 is the villus radius. This model allows for diffusion of capric acid, the complexes, and HPB in the aqueous phase (ABL and intervillus space), rapid equilibrium between capric acid and HPB, and absorption of neutral free capric acid across the biomembrane at the various surfaces (i.e., tip, lateral surface, and crypt) of the villi into a blood sink. The villi density is the number of villus units per cm2 of flat surface area and the cross-sectional area of the average intervillus space is the total intervillus cross-sectional area per cm2 of flat surface divided by the villus density.
where r2 is the outer radius of this annular cylinder and r1 is the villus radius. This model allows for diffusion of capric acid, the complexes, and HPB in the aqueous phase (ABL and intervillus space), rapid equilibrium between capric acid and HPB, and absorption of neutral free capric acid across the biomembrane at the various surfaces (i.e., tip, lateral surface, and crypt) of the villi into a blood sink. The villi density is the number of villus units per cm2 of flat surface area and the cross-sectional area of the average intervillus space is the total intervillus cross-sectional area per cm2 of flat surface divided by the villus density.
| Aqueous boundary layer thickness | 0.117 cma |
| Villus height (hV) | 0.0399 cmb |
| Villus radius (r1) | 0.0037 cmb |
| Radius of villus plus intervillus space radius (r2) | 0.0049 cmb |
| Villus density | 1.32 × 104 villi per cm2 of flat surfaceb |
| Cross-sectional area of average intervillus space | 3.24 × 10−5 cm2 per villus; area = π(r22 − r12) |
- a Determined in the present study.
- b Taken from Ref. 15.
Although previous researchers have used similar VMs to demonstrate the effects of the villus geometry on drug absorption,13-15 this study was to examine for the first time the concept that including a cyclodextrin in the formulations may increase the effective epithelial surface area utilized for absorption of highly lipophilic molecules by providing a means for the permeant to diffuse further into the intervillus space than without a carrier. As will be seen, the extent to which the additional surface area is effectively utilized is dependent on the various parameters (Table 1) and the experimental conditions, these including the intrinsic bio-membrane permeability coefficient (Pi,VM), the aqueous diffusion coefficients, the HPB concentration, and the binding affinity of the permeant to HPB.
 (2)
(2) (3)
(3)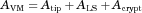 (4)
(4)MATERIALS AND METHODS
Chemicals and Reagents
For the in situ single-pass perfusion system, the phosphate buffer solution consisted of 57 mM NaH2PO4·H2O and 79 mM Na2SO4. The pH was adjusted to 7.4 with 10 M NaOH. Capric acid was obtained from Sigma–Aldrich (St. Louis, Missouri); [14C]Mannitol, [14C]capric acid, and [14C]sodium taurocholate were purchased from Moravek Biochemicals Inc. (Brea, California). HPB (Kleptose HPB) was obtained by a generous gift from Roquette America (Keokuk, Iowa). The purity of the radioisotopes was checked using high pressure liquid chromatography and found to be >97% pure. Solvable tissue solubilizer and Ultima Gold scintillation cocktail were obtained from PerkinElmer (Boston, Massachusetts). Other chemicals were of reagent grade.
In Situ Single-Pass Perfusion Experiments
The procedures used for the single-pass perfusion experiments in this study essentially followed the procedures used by Tsutsumi et al.7 Briefly, Male Sprague–Dawley rats weighing 300–400 g were obtained from Charles River (Wilmington, Massachusetts) and were used under the approval of the Institutional Animal Care and Use Committee at the University of Utah. The animals had free access to food and water prior to the experiment. The animals were under general anesthesia with halothane in oxygen and were kept warm with a 37°C circulating water heating pad. A 5 cm long silastic cannula was implanted into the jugular vein which was used for blood collection or blood infusion for the donor and experimental rats, respectively. Heparin sodium was used to prevent coagulation. For the case of perfusion experiment rats, the ileum and mesenteric vein were isolated and cannulated as described previously.7 The perfusion studies were run at 0.2 mL/min and 37°C blood was replaced into the rat jugular vein at approximately the same rate it was collected from the mesenteric vein.
For capric acid with or without HPB, the perfusion solution consisted of 0.2–0.6 μCi/mL [14C]capric acid, 0.05 mM cold capric acid, and an appropriate amount of HPB in the pH 7.4 buffer. The final osmolarity of the buffer was adjusted to 300 ± 10 mOsm/kg using reagent grade Na2SO4, and the final ionic strength was 0.37. Taurocholate experimental buffer solutions were prepared in the same manner except the buffer contained only [14C]taurocholate (no cold taurocholate or HPB). Each formulation was perfused through the rat ileum at 0.2 mL/min using a model 100 perfusion pump (KD Scientific, Holliston, Massachusetts). The animal was kept at 37 ± 1°C using a circulating water blanket, and the mean perfusate temperature was 30 ± 2°C. The perfusate was collected at 10-min intervals using preweighed 20 mL scintillation vials. Perfusate samples were weighed to determine the perfusion outflow rate, and a 100 μL sample of perfusate was assayed via scintillation counting to determine the permeant concentration in the perfusate. Water absorption/secretion was determined to be less than 5% by comparing the outflow of the perfusion solution with the inflow. The osmotic pressure change of the solution was found to be less than 5% from periodic osmolarity determinations using a micro osmometer (model 3300 Advanced Instruments Inc. Norwood, Massachusetts). Mesenteric blood flow from the isolated intestinal segment was collected at 10 min intervals in preweighed 20 mL scintillation vials. The vials were weighed and the blood was analyzed using the following method. Exactly 500 μL of whole blood was pipetted into a fresh 20 mL scintillation vial along with 100 μL of heparin (5000 units/mL) and 550 μL of tissue solubilizer and incubated for 1 h in a 50°C shaking water bath. Then 100 μL of 100 mM of EDTA at pH 7.4 and 300 μl 30% H2O2 were added to the sample and incubated at 50°C for another hour. The sample was then mixed with 10 mL scintillation cocktail and assayed by scintillation counting. For each experiment, an additional 500 μL sample of whole blood was taken from the 10 min time point and spiked with 100 μL of donor solution and then analyzed as above as an internal standard to correct for any quenching due to the blood or reagents. The disintegrations per minute (DPM) of the 10-min sample alone was subtracted from the internal standard and the remaining DPM were compared with 100 μL of donor solution in saline. The internal standard DPM was generally within 10% of the saline DPM. The blood samples were corrected by multiplying the blood DPM by the ratio of saline donor DPM to internal standard DPM. At the end of the transport experiment, the length of the intestinal segment was measured with silk thread. The outside diameter and intestinal wall thickness of the ileum were measured using a micrometer to determine the inside radius of the ileum. These measurements were used to calculate AFSM.
VM Simulations
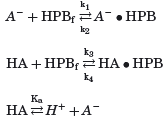
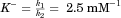 ; k3 = 7.5 × 106 mM−1 s−1, k4 = 1 × 106 s−1, and
; k3 = 7.5 × 106 mM−1 s−1, k4 = 1 × 106 s−1, and 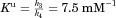 ); as the intermolecular interaction between capric acid or the caprate ion with a cyclodextrin molecule is basically a rather conventional interaction between two hydrophobic groups in aqueous media, the complexation rates between the two entities would be expected to be many orders of magnitude greater than bulk diffusion rates in the ABL or in the intervillus space; for a discussion of diffusion-controlled chemical reactions see, for example, Levine.16 At steady state, the condition expressed by Eq. 5 must apply simultaneously for all species.
); as the intermolecular interaction between capric acid or the caprate ion with a cyclodextrin molecule is basically a rather conventional interaction between two hydrophobic groups in aqueous media, the complexation rates between the two entities would be expected to be many orders of magnitude greater than bulk diffusion rates in the ABL or in the intervillus space; for a discussion of diffusion-controlled chemical reactions see, for example, Levine.16 At steady state, the condition expressed by Eq. 5 must apply simultaneously for all species.
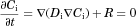 (5)
(5) (6)
(6) (7)
(7) (8)
(8)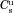 is the villus surface site free capric acid concentration. It is to be noted that, from here on, Pi,VM will represent the Pi for the VM and Pi,FSM that for the FSM.
is the villus surface site free capric acid concentration. It is to be noted that, from here on, Pi,VM will represent the Pi for the VM and Pi,FSM that for the FSM.Treatment of Experimental Data using the FSM
 , is taken as the effective concentration of capric acid in the lumen.
, is taken as the effective concentration of capric acid in the lumen.
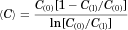 (9)
(9)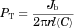 (10)
(10)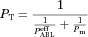 (11)
(11) is the effective permeability coefficient in the ABL. In the case of the present study, only free unionized capric acid (HA) is assumed to be absorbed; therefore, Eq. 11 becomes:
is the effective permeability coefficient in the ABL. In the case of the present study, only free unionized capric acid (HA) is assumed to be absorbed; therefore, Eq. 11 becomes:
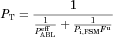 (12)
(12)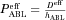 , Eq. 12 becomes
, Eq. 12 becomes
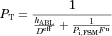 (13)
(13) (14)
(14)RESULTS AND DISCUSSION
Intestinal Absorption
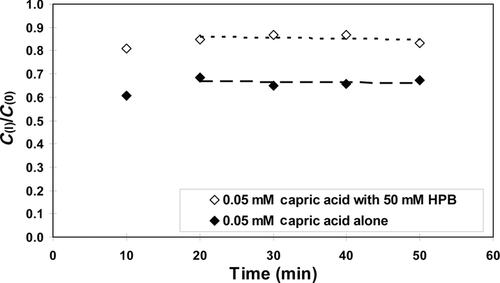
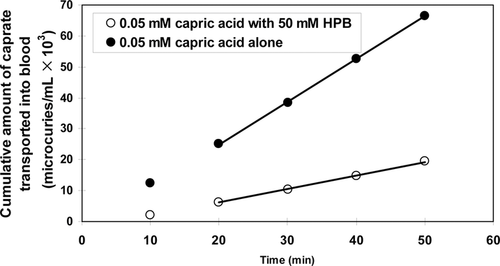
Figure 3 shows typical experimental results of the fractions of [14C]-capric acid remaining in the ileal segment of length ∼10 cm, C(l)/C(0), in the absence and presence of 50 mM HPB. Here, in 20 min C(l)/C(0) is seen to attain steady-state values of 0.7 and 0.9 in the absence and presence of 50 mM HPB, respectively. Figure 4 shows the cumulative amounts of [14C]caprate transported into the blood for examples of caprate alone and caprate plus 50 mM HPB. Consistent with the results of Figure 3, these data also show steady state of caprate absorption was generally attained in about 20 min and was essentially independent of the HPB concentration. The perfusion rate, the blood flow rate, and the osmotic pressure of the solution in the intestinal lumen were generally constant during an experiment and did not vary greatly from run to run (see Supplementary Material). Water absorption/secretion was ±5% and inconsequential. Figures 3 and 4 represent typical displays from absorption studies conducted with 0.05 mM capric acid in HPB concentrations from 0 to 50 mM (i.e., molar ratios of [HPB]/[HA] ranging from 0 to 1000:1). Similar patterns as seen in Figures 3 and 4 were also observed in the [14C]taurocholate experiments conducted with phosphate buffer at pH 7.4. Table 3 summarizes the flux of capric acid, the mean luminal concentration and the total permeability coefficient (calculated using Eq. 10).
| Species | HPB Concentration (mM) | Jb × 109 mol/(cm2 × s) | PT × 105 (cm/s) |
|---|---|---|---|
| Taurocholate | 0 | ***** | 5.50 (±0.48) |
| Caprate | 0 | 2.88 (±0.69) | 5.76 (±1.37) |
| Caprate | 0.5 | 1.85 (±0.35) | 3.69 (±0.70) |
| Caprate | 3 | 1.45 (±0.16) | 2.89 (±0.31) |
| Caprate | 10 | 1.07 (±0.24) | 2.14 (±0.49) |
| Caprate | 30 | 1.01 (±0.22) | 2.02 (±0.43) |
| Caprate | 50 | 0.78 (±0.25) | 1.56 (±0.51) |
Analysis of the Experimental PT data with the FSM
The principal outcomes of the FSM analysis of the experimental results are presented in Table 4 and Figure 5. The experimental PT values (column 3 of Table 4) were best-fitted to Eq. 12 in the following manner. As it was apparent from a preliminary analysis that, at low HPB concentrations (≤3.0 mM), capric acid absorption would be essentially 100% ABL controlled, a first approximation for the best hABL value was obtained based on this consideration. Then a first approximation of a best-fit value for Pi,FSM was found using only the PT results from the three highest HPB concentration experiments. Subsequent iterations employing all of the data did not significantly change these results. The individual hABL values at the three lowest HPB concentrations, and the individual Pi,FSM values at the three highest HPB concentrations are all presented in Table 4. The individual hABL values are in good agreement showing modest variability and good consistency with the reference value obtained with [14C]taurocholate. The individual Pi,FSM values show greater variability, as was expected. The capric acid absorption for the three highest HPB cases was still 78%–59% ABL controlled and was therefore still relatively insensitive to Pi,FSM. The large variability in the individual Pi,FSM values also obscures any indication of a trend in Pi,FSM with increasing membrane-controlled capric acid absorption (that would have been expected as discussed later in the VM analysis). Nonetheless, the experimental PT values correlate with the FSM deduced values reasonably well (see Fig. 5).
| Permeant | HPB Concentration (mM) | PT × 105 (cm s−1)a | Fub | Deff × 106 cm2 s−1b | hABL (cm) | Pi,FSM Fu × 105 (cm s−1) | Pi,FSM (cm s−1) | ABL Controlled (%)c |
|---|---|---|---|---|---|---|---|---|
| Taurocholate | 0 | 5.5 (±0.48) | 0 | 5.6 | 0.102 (±0.010) | – | – | 100.0% |
| Capric acid | 0 | 5.8 (±1.4) | 1.26×10−3 | 6.7 | 0.115 (±0.027) | – | – | 98.9% |
| 0.5 | 3.7 (±0.7) | 5.75×10−4 | 4.7 | 0.126 (±0.024) | – | – | 90.5% | |
| 3 | 2.9 (±0.3) | 1.50×10−4 | 3.4 | 0.118 (±0.013) | – | – | 96.7% | |
| 10 | 2.1 (±0.5) | 4.85×10−5 | 3.1 | 9.8 | 2.0 (±2.6) | 78.3% | ||
| 30 | 2.0 (±0.4) | 1.65×10−5 | 3.0 | 8.4 | 5.1 (±3.5) | 76.3% | ||
| 50 | 1.6 (±0.5) | 1.26×10−5 | 3.0 | 4.6 | 3.7 (±3.8) | 59.2% | ||
| Best fit | – | – | – | 0.117 | – | 3.5 (range 1.7–10) | – |
- a n = 3.
- b Fu (fraction of free unionized capric acid) and Deff (effective aqueous diffusion coefficient of capric acid–HPB systems) were calculated using experimentally determined diffusion coefficients, pKa and binding constants of capric– and caprate–HPB complex.
- c Fraction ABL controlled = PT/
 where
where  = Deff/hABL.
= Deff/hABL.
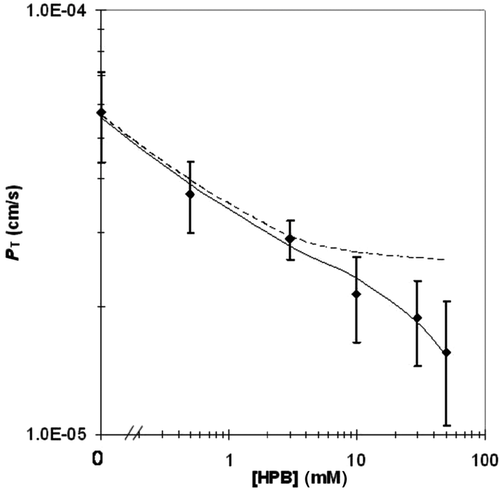
In applying the FSM, it was assumed that the ABL can in fact be treated like a stagnant layer and that molecules cross the ABL by molecular diffusion only. It is important to note that all of the hABL values listed in column 6 of Table 4 are essentially the same within the data scatter, whereas Deff varied by almost a factor of two. This evidence supports the assumption implied in Eq. 12 that the ABL can be treated as a stagnant diffusive layer for the molecules in the present study. If the ABL were to be characterized by a significant contribution from convective diffusion, the hABL determined would have been expected to have a dependence upon the diffusion coefficient to the nth power (Dn)18-20 where n may be a fraction greater than zero. Although the experimental error in the hABL values is too large to definitively state that there is no significant amount of convective diffusion in the ABL, the results suggest that transport across the ABL is predominantly diffusion controlled and that convection does not contribute significantly to overall transport across the ABL, this supporting the appropriateness of the stagnant layer assumption inherent in Eq. 12.
It is of interest to note that the Pi,FSM for capric acid has been found to be 5–6 orders of magnitude larger than the permeability coefficient of the ABL (PABL); this is why absorption of capric acid from a solution of capric acid species alone would be essentially completely insensitive to Pi,FSM. At high HPB concentrations and a high pH of 7.4, however, Fu is sufficiently small that the product of Pi,FSM and Fu is comparable to PABL; this then affords reasonable sensitivity to Pi,FSM (see Eq. 12. This example illustrates that, although lipophilic weak acid molecules can have a very high intrinsic membrane permeability coefficient compared with the ABL permeability coefficient, by reducing Fu sufficiently (i.e., high HPB and high pH) an accurate Pi,FSM determination may be possible. This also illustrates the important role played by HPB in obtaining Pi,FSM values under experimental conditions that otherwise would have been insensitive to Pi,FSM. This role of cyclodextrins and other carriers to improve sensitivity of the determination of Pi,FSM for lipophilic molecules has not, to our knowledge, been previously reported.
At this point, it should be helpful to comment on the reasonableness of the intrinsic permeability coefficient deduced from the FSM (Pi,FSM), particularly in light of the large error bars (see Fig. 5). An attempt can be made to examine the consistency of the Pi,FSM value of 3.5 in Table 4, column 8 with data from the work of Ho et al. who had obtained permeability coefficients of a series of n-alkanoic acids up to n-octanoic acid employing a modified Doluisio in situ technique using rat jejunal segments. By extrapolation of the shorter chain fatty acids, Ho has deduced a Pi,FSM value for capric acid absorption in the rat jejunum (unpublished results) that appears to be within a factor of two of the Pi,FSM value for capric acid transport in the ileum after the above 3.5 cm/s value is corrected for (a) the effect of the presence of HPB and (b) the effect of the differences between the ileum and jejunum villus architecture. Details are presented elsewhere.21 The closeness of the Pi,FSM values for the ileum and the jejunum seems reasonable in light of the known constituent compositions of the ileum and the jejunum, especially those components associated with the lipoidal structures.
A final point of interest is that the goodness of fit of the FSM to experimental data (see Fig. 5) provides an opportunity to examine the effects of HPB on the transport behavior of capric acid for equal thermodynamic activity of capric acid in the lumen (for example, as would be the case for considering saturated solutions of capric acid in the lumen; this consideration should be important in the formulation optimization of very low solubility lipophilic drugs). Figure 6 shows that the capric acid activity would drop by 98.9% across the ABL for the capric acid alone case compared with a 59.2% drop for the capric acid plus 50 mM HPB case. This would translate into a 37-fold increase in the surface thermodynamic activity (and therefore the flux) of capric acid in the presence of 50 mM HPB compared with the capric acid alone condition for equal capric acid thermodynamic activity conditions in the lumen. Thus, Figure 6 demonstrates that the HPB as a drug carrier can improve bioavailability for lipophilic permeants by increasing the surface thermodynamic activity of the permeant at the membrane surface by reducing the permeant concentration gradient across the ABL.

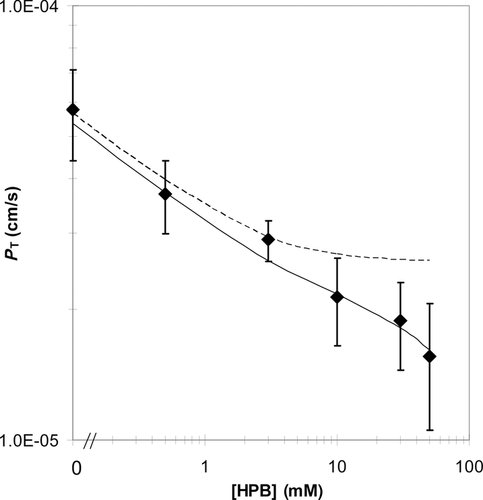
Analysis of the Experimental Results with the VM
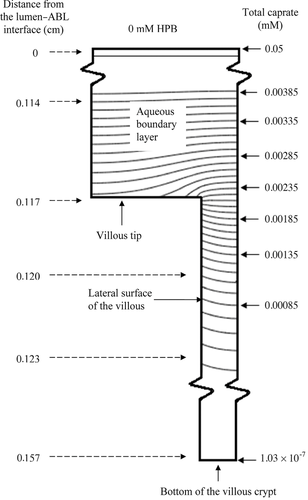
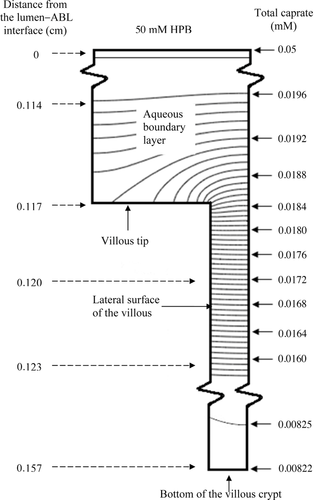
The villus Pi,VM values were deduced using the database of the fluxes of capric acid at 10, 30, and 50 mM. The estimates of Pi,VM ranged from 0.39 to 0.89 cm s−1 in the various cases (Table 5). The Pi,VM by least squares fitting was found to be 0.48 cm/s which is sevenfold smaller than the Pi,FSM of 3.5 cm s−1 because of the differences in the effective absorptive surface areas utilized. The VM-deduced PT values are compared with experimentally determined values, and are presented in Figure 7. Model-deduced PT values are found to be in good agreement with experimental PT values for capric acid over the range of HPB concentrations studied with the model-deduced values falling within experimental error for all conditions. While the VM and the FSM both offer good predictability of PT for capric acid at pH 7.4 over the range of HPB concentrations studied, the following discussion presents some important insights that are revealed from the VM analysis that would not be discernable from the FSM. The surface area based on the VM parameters (see the section on “Flat Surface versus Villus Surfaces”) is 13.3 times greater than the inside surface area of a smooth, hollow cylinder (the FSM). The extent to which capric acid may utilize this surface area in the presence and absence of HPB is illustrated in the concentration-distance profiles in Figure 8. It has been previously demonstrated14, 15 and is widely understood that highly permeable compounds are absorbed primarily at the villus tips. As can be seen in Figure 8, the VM demonstrates that capric acid alone behaves like other rapidly absorbed, highly lipophilic compounds. For this case the concentration gradient of capric acid decays rapidly across the ABL, with only about 5% of the original lumen concentration remaining at the tips of the villi; as the capric acid reaches the villi, it is rapidly absorbed at the villi tips, and the concentration decays rapidly to zero in the initial portion of the intervillus space. Because of the rapid absorption at the tips, capric acid is not able to diffuse more than approximately 10% into the intervillus space, leaving the majority of the crypt cells un-utilized for absorption. With increasing HPB concentrations, however, the concentration gradient across the ABL is decreased. Additionally, more capric acid is able to penetrate further into the villus crypts. For the capric acid plus 50 mM HPB formulation, the total capric acid concentration at the tips is approximately 40% of that in the bulk, and the concentration at the bottom of the crypts is approximately one-half of the concentration at the tips of the villi.
| HPB (mM) | PT × 105 (cm s−1) |  × 105 (cm s−1) × 105 (cm s−1) |
Pi,VM (cm s−1) | ABL Controlled from Experimental PT (%) | Accessibilitya | Accessibility/Accessibility at 0 mM HPB |
|---|---|---|---|---|---|---|
| 0 | 5.76 | 5.64 | – | 102.1 | 0.126 | 1.00 |
| 0.5 | 3.69 | 3.97 | – | 92.9 | 0.147 | 1.17 |
| 3 | 2.89 | 2.93 | – | 98.6 | 0.227 | 1.81 |
| 10 | 2.14 | 2.66 | 0.39 (±0.41) | 79.6 | 0.328 | 2.61 |
| 30 | 2.02 | 2.85 | 0.89 (±0.79) | 72.2 | 0.508 | 4.04 |
| 50 | 1.56 | 2.56 | 0.48 (±0.38) | 60.1 | 0.563 | 4.48 |
| Best fit | 0.48 (±0.38) |
- a Accessibility is defined as the extent to which a permeant utilizes the surface area available and is calculated by dividing the actual flux (Jactual) of the permeant by the flux, JSC,constant, that would occur if the permeant concentration was constant for all regions of the villi (i.e., tip, lateral surface, and crypt) and equal to that of the villus tip (i.e.,
 ). See Eq. 14.
). See Eq. 14.
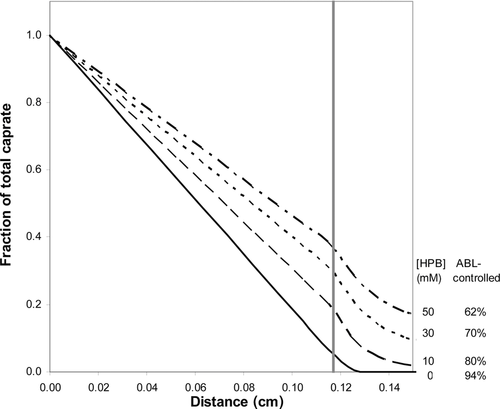
A comparison of the capric acid concentration distributions for capric acid alone and capric acid plus 50 mM HPB using iso-concentration profiles (representing total caprate, with a constant incremental concentration change between successive curves within the villus unit) provides further insight into the absorption characteristics of capric acid in the rat ileum (see Figs. 9 and 10). The comparison of the two figures is striking with respect to the differences in the slopes of the iso-concentration lines at and around the villus tips. For the case of the capric acid alone conditions, one can see that the caprate iso-concentration lines for the 0 mM HPB case are much more horizontally parallel than those of the 50 mM HPB case just above the villus tip, demonstrating much more vertical diffusion, with a significant fraction of the caprate being absorbed by the villus tip; this is due to the higher Pm in the absence of HPB. For the 50 mM HPB case (Fig. 10), the lines are more curved (more vertically inclined) just above the villus tip, demonstrating that the flux is directed more laterally (toward the intervillus space), resulting in a greater fraction of the caprate entering and diffusing down the villus crypt, this due to the lower Pm for the 50 mM HPB case. The result is more caprate in the villus crypt, allowing the HPB conditions to take fuller advantage of the available absorptive surface area at the lateral surface of the villus. Also of note is the flux and concentration gradient within the intervillus space. For the case of the 0 mM HPB (Fig. 9), the flux decays rapidly as demonstrated by the rapidly increasing space between successive iso-concentration lines. This is consistent with the rapidly decaying caprate concentration seen in Figure 8 for the 0 mM case. Additionally, in the 0 mM HPB case there is an observable lateral concentration gradient within the intervillus space of about 5% from the middle of the intervillus space (far right side of the figure) to the lateral surface of the villus, this due to the absorptive surface of the villus. For the 50 mM HPB case, however, the flux decay in the intervillus space and the lateral concentration gradients are much less pronounced. This result was expected due to the carrier effect of the HPB present in the intervillus space, this providing the means for greater access of caprate to the lateral surfaces of the villus. Taken together with the VM, Figures 9 and 10 demonstrate that HPB acts as a carrier molecule for caprate not only across the ABL, but also into the intervillus space, allowing capric acid to more effectively access and utilize the surface area available for absorption.
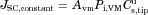 (15)
(15) is the free capric acid species concentration at the villus tip, and Pi,VM has been already defined. Jactual is the VM calculated flux. Accessibility was calculated for all the capric acid/HPB solutions using the VM (see Table 5, column 6). A comparison of the accessibilities for the different capric acid formulations quantitatively demonstrates what is already qualitatively understood from Figures 8-10: that HPB improves the access of capric acid to the surface area in the villus crypts. For the case of the 50 mM HPB formulation, capric acid is able to access 4.5-fold more surface area than the capric acid alone formulation. It should be noted that accessibility increases with increasing concentration of HPB, and that 50 mM HPB allowed access of capric acid to slightly more than one-half of the total surface area available for absorption.
is the free capric acid species concentration at the villus tip, and Pi,VM has been already defined. Jactual is the VM calculated flux. Accessibility was calculated for all the capric acid/HPB solutions using the VM (see Table 5, column 6). A comparison of the accessibilities for the different capric acid formulations quantitatively demonstrates what is already qualitatively understood from Figures 8-10: that HPB improves the access of capric acid to the surface area in the villus crypts. For the case of the 50 mM HPB formulation, capric acid is able to access 4.5-fold more surface area than the capric acid alone formulation. It should be noted that accessibility increases with increasing concentration of HPB, and that 50 mM HPB allowed access of capric acid to slightly more than one-half of the total surface area available for absorption.Additional insight is available from the accessibility value when we compare the VM with the FSM. The VM has 13.3 times more surface area than the FSM. Therefore, if we multiply the accessibility value determined from the VM by its surface area amplification factor (13.3), we can see how much surface area is effectively utilized for absorption as compared with the flat model (see Table 5). Column 7 in Table 5 shows that the more membrane-controlled conditions utilize much more surface area than would be predicted from the flat model. At this point it should be noted that the contribution from the microvilli present on the villi toward the effective surface area of the intestine is not included in the VM analysis. This contribution is actually “absorbed” into the Pi,VM and is considered to be an appropriate procedure in light of the fact that the dimensions of the microvilli are one to two orders of magnitude smaller than the smaller dimension of the villus in the present model and therefore ignoring the microvilli does not significantly influence the local concentration gradients in the intervillus space.
Several investigators22-24 have pointed out that the effective ABL thickness in anesthetized animals may be much thicker than in unanesthetized animals due to the influence of the anesthesia upon the intestinal motility. Yuasa et al.25 reported a twofold to threefold thicker ABL thickness in anesthetized rats compared with unanesthetized rats in studies using a single-pass perfusion technique with a flow rate of 0.16 mL/min in the rat jejunum. In order to examine how this may impact upon the principal conclusions of the present study, caprate transport was also modeled with the VM under the condition where the hABL value was reduced 10-fold from 0.117 to 0.0117 cm (10-fold is much greater than that indicated in the study by Yuasa et al.25). The VM simulation of capric acid concentration as a function of distance with varying concentrations of HPB demonstrated that, as expected, each condition was more membrane controlled than its counterpart with the thicker hABL (see Supplementary Material). It is quite noteworthy that even for a very thin hABL condition the intervillus concentration of caprate and the accessibility of caprate to the membrane surface sites also increased significantly with increasing concentration of HPB. This exercise of comparing the thicker hABL results encountered in in situ experiments (Fig. 8) to a thinner hABL expected in vivo demonstrates that even for in vivo conditions the hABL is likely a significant transport barrier for highly permeable molecules such as capric acid. Additionally, this exercise shows that a carrier molecule such as HPB can markedly improve the membrane accessibility of rapidly absorbed compounds under such in vivo conditions. This increased accessibility may become particularly useful when the molecule of interest is a membrane permeability enhancer.21
A Comparison of the VM with the FSM
With respect to the goodness of fit of experimental data to model-predicted PT values, the VM and the FSM models both predict PT equally well. In light of the above discussion, however, the VM clearly can provide additional valuable information concerning the intrinsic permeability coefficient values, effective surface area utilized for absorption, and extent of permeant penetration into the villus crypts. Although it is well understood that the small intestine contains villi which significantly increase the effective surface area available for absorption,26 the common approach for predicting permeability across the membranes of the small intestines simplifies the gut as a hollow, smooth cylinder. The VM, however, utilizes a better first approximation to the morphology of the absorptive surface of the small intestine than does the FSM. In addition to the visually apparent differences between a smooth cylinder and the small intestine, there is also experimental evidence that the villus structure can play an important role in the presentation of chemical absorption enhancers to the absorptive surfaces of the small intestine. Utilizing capric acid as an enhancer for mannitol absorption in the rat ileum, it was found that, in the presence of 30 mM HPB, a threefold to fourfold higher capric acid flux is observed before any indication of mannitol permeability enhancement is seen as compared with capric acid alone.21 This experimental evidence suggests that a significantly greater surface area is effectively utilized for capric acid absorption in the presence of 30 mM HPB; this is consistent with the VM, but not with the FSM. For the above stated reasons, the VM appears to be a more useful model for quantitatively investigating the effects of carrier molecules on the effectiveness of chemical permeation enhancers, such as capric acid, on absorption rates of polar molecules in the small intestine.
CONCLUSIONS
The contribution of the present study has been the mechanistic investigation of the influence of an aqueous phase carrier (HPB) upon the intestinal absorption of a highly lipophilic compound (capric acid) employing the physical model approach and experimental results from the in situ rat intestinal absorption experiments. The mechanistic analysis has shown how HPB is able to reduce the concentration gradient of capric acid in the ABL and also to increase the penetration of capric acid into the intervillus space to enhance its absorption. The model approach generally demonstrates and details the complex interplay of the key parameters of the system and their relative contributions to the overall capric acid flux. Basically, the analysis entails the following features. First, and importantly, the analysis is based upon assuming equal thermodynamic activity of capric acid in the intestinal lumen; therefore in the presence of HPB in the intestinal lumen, the total capric acid/caprate concentration in the intestinal lumen would be higher than in its absence. Secondly, HPB acts as a carrier for capric acid/caprate both in the ABL and in the intervillus space (but HPB is not a carrier across the biomembrane). Thus the transport resistances in the ABL and in the intervillus space are reduced by the presence of HPB; this then results in (a) the increased capric acid/caprate thermodynamic activity at the membrane interface and (b) in the intervillus space.
An obvious practical application of this study would be the use of this approach in predicting the optimum composition of cyclodextrin-containing formulations for the oral administration of highly lipophilic molecules.
GLOSSARY
-
- Notation
-
- Definition
-
- ABL
-
- aqueous boundary layer
-
- AFSM
-
- area of a smooth hollow cylinder for an ileal segment
-
- AVM
-
- surface area of the villi (13.3 × the area of a smooth cylinder)
-
- Atip
-
- area of the villus tip
-
- ALS
-
- area of the villus lateral surface
-
- Acrypt
-
- area of the villus crypt
-
- A−
-
- free caprate ion
-

-
- logarithmic mean concentration in the ileal segment
-
- C(0)
-
- inflow permeant concentration
-
- C(l)
-
- outflow permeant concentration
-
- Ci
-
- concentration of the ith species
-
- CT
-
- total caprate concentration
-

-
- total free caprate concentration at the villus tip
-
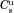
-
- the villus surface site concentration of free capric acid
-
- Di
-
- diffusion coefficient of the ith species
-
- Df
-
- diffusion coefficient of free capric acid or of caprate ion
-
- D*
-
- diffusion coefficient of capric acid·HPB complex or of the caprate ion·HPB complex
-
- Deff
-
- effective diffusion coefficient
-
- Fu
-
- fraction of the free capric acid species
-
- Ff
-
- sum of the fractions of the capric acid and the caprate ion species
-
- F*
-
- sum of the fractions of the HA·HPB and the A−·HPB complexes
-
- FSM
-
- flat surface model
-
- HA
-
- capric acid
-
- HPB
-
- 2-hydroxypropyl-β-cyclodextrin
-
- HPBf
-
- free 2-hydroxypropyl-β-cyclodextrin
-
- HA·HPB
-
- capric acid·2-hydroxypropyl-β-cyclodextrin complex
-
- A−·HPB
-
- caprate ion·2-hydroxypropyl-β-cyclodextrin complex
-
- hABL
-
- aqueous boundary layer thickness
-
- hV
-
- villus height
-
- J
-
- flux
-
- j
-
- local flux
-
- Jb
-
- flux into blood
-
- Jactual
-
- actual flux of the permeant
-
- JSC,constant
-
- flux when surface concentration of permeant at the tip, lateral surface, and crypt of the villi is constant
-
- k1
-
- A− + HPB association rate constant
-
- k2
-
- A−·HPB dissociation rate constant
-
- k3
-
- HA + HPB association rate constant
-
- k4
-
- HA·HPB dissociation rate constant
-
- Ka
-
- capric acid dissociation constant
-
- K−
-
- A−·HPB binding constant
-
- Ku
-
- HA·HPB binding constant
-
- l
-
- length of the ileal segment
-
- n
-
- some fraction greater than zero
-
- PT
-
- total permeability coefficient
-
- Pi
-
- intrinsic membrane permeability coefficient
-
- Pi,FSM
-
- Pi as determined from the FSM
-
- Pi,VM
-
- Pi as determined from the VM
-
- PABL
-
- the ABL permeability coefficient in the absence of a carrier
-

-
- ABL permeability coefficient in the presence of a carrier
-
- Pm
-
- effective membrane permeability coefficient
-
- r
-
- inside radius of the intestinal segment
-
- r1
-
- villus radius
-
- r2
-
- radius of the villus and associated intervillus space
-
- R
-
- rate of formation of caprate or the disappearance rate of the caprate·HPB complex
-
- RVM/FSM
-
- surface area ratio of the VM to the FSM
-
- VM
-
- villus model
-
- x
-
- position in the lumen (0 −l)
-
- ΔpH
-
- pH gradient
-
- ∇
-
- the standard del (nabla) operator for cylindrical coordinates
-
- β
-
- buffer capacity




