Formulation of spray-dried phenytoin loaded poly(ε-caprolactone) microcarrier intended for brain delivery to treat epilepsy
Abstract
This study evaluates the efficacy of the spray-drying technique in the bioengineering of phenytoin (PHT) containing poly(ε-caprolactone) (PCL) microcarrier intended for brain delivery for long-term treatment of epilepsy. Through orthogonally designed experiments, the optimal formulation and process variables for the preparation of PCL-microcarriers containing PHT were obtained. The produced microcarriers were characterized by coulter counter, scanning electron, scanning transmission electron microscopies, differential scanning calorimetry, powder X-ray diffraction, and in vitro release. The results showed that the produced microcarriers have a spherical structure, uniform size distribution, and a particle mean diameter of about 4.0 µm, which is suitable for brain delivery. The PHT was loaded as dispersed microcrystals within the PCL-microcarriers. From this system, PHT was released slowly into a buffer solution for approximately 14 days without any burst effect. These data suggested that PHT containing spray-dried PCL-microcarrier may be a promising drug delivery system for local brain delivery and long-term treatment of pharmacoresistant epilepsy. © 2007 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 96: 1018–1030, 2007
INTRODUCTION
At least 50 million people worldwide suffer from epilepsy. Despite the continued development of antiepileptic drugs (AEDs), about 30% of epileptic patients suffer from uncontrolled seizures or pharmacoresistant epilepsy. Only about 60–80% of the pharmacoresistant epilepsies respond to surgical removal of the seizure focus, a radical procedure, which can lead to language and memory problems. Moreover, some patients cannot be surgically treated because of unacceptable risks of loss of brain functions. Considering these facts, better and improved epilepsy treatments, which are effective in pharmacoresistant epilepsy and represent an alternative or an addition to surgery, are urgently needed. An AEDs containing microparticulate delivery system or microcarrier that could directly target the epileptic region in the brain would offer enormous advantages including: (i) controlled drug release for long-term therapeutic effect based on the versatile properties of the drug carrier polymer to be selected, (ii) by-passing the multidrug resistance drug transporters of brain endothelial cells which limit the therapeutic drug concentration in the brain, (iii) implantation after surgery to increase surgical success, (iv) production of higher AEDs concentrations compared with oral and systemic delivery without reaching “toxic” plasma levels leading to side effects, (v) minimized drug interactions, (e.g., related to liver metabolism and protein binding), (vi) relatively higher adaptability of the treatment to the surgical act (area covered by drug) compared with monolithic implants.
The intracerebral injection of microparticles is an emerging alternative for drug delivery into the brain, for example, for treatment of brain cancers.1-6 Furthermore, phenytoin (PHT) release from controlled release polymers surgically implanted into rat brains significantly reduced experimental seizures.7 However, the polymeric drug carriers were large in size (5 mm diameter and 4 mm high), which produces more damage when implanted in the brain compared to the injection of particle with diameter less than 5 µm. Moreover, polymers such as ethylene-vinyl acetate (EVAC), are not biodegradable and contains inflammatory contaminants such as butylhydroxytoluene that would need to be removed through a complex process before implantation into humans.7 The biodegradable and biocompatible polymers proposed in this study, namely poly(ε-caprolactone) (PCL), is far more preferable for drug delivery.8 Moreover, PCL has undergone clinical evaluation for sustained delivery of levonorgestrel worldwide.9
For this study, PHT was chosen as a model AED drug because it is an effective anticonvulsant used for monotherapy of partial seizures, PHT predominantly acts by reducing voltage-gated sodium and calcium channel currents.10, 11 In recent clinical trials, none of the newer anticonvulsants or AEDs was superior to PHT in their anticonvulsant action, although PHT when given orally produces more side effects12 and is teratogenic. PHT is a substrate for multidrug transporters, including P-glycoprotein,13, 14 which is upregulated in epileptic tissue.6 Therefore, it is likely that PHT resistance is largely caused by its restricted entry into the brain, specifically in epileptic tissue.14 For example, modulation of sodium currents by PHT did not significantly differ in acutely isolated hippocampal CA1 neurons from kindled rats that were PHT-resistant or that did respond to PHT.15 Moreover, the modulation of postsynaptic calcium channels by PHT was also found in human hippocampal granule cells from intractable TLE patients and appears to contribute to its anticonvulsant action.16 Therefore, it is conceivable that when present in locally therapeutic concentrations, PHT may be an efficient anticonvulsant in TLE patients that are pharmacoresistant to oral PHT. Finally, genetic differences were identified in the ABCB1 transporter gene (also known as MDR1 and P-glycoprotein 170), associated with resistance to AEDs.17, 18
We have previously reported that the PCL-microparticles could successfully be prepared by using spray-drying method for other biopharmaceuticals.19 In this article, process and formulation variables influencing the preparation of PHT loaded PCL microcarrier by spray-drying technique were studied through the orthogonal experimental design. To assure the robustness, the efficacy and safety of the novel delivery system, the produced PCL-microcarriers were characterized by coulter counter, scanning electron and scanning transmission electron microscopies, DSC and powder X-ray diffraction (XRD). The encapsulation efficiency and loading of PHT and drug release profile were investigated by HPLC analysis.
EXPERIMENTAL
Materials
Poly(ε-caprolactone) (PCL, Tone® Polymer P-757) was kindly provided by Union Carbide (Danbury, CT). This polymer was used without further purification and processing. The following chemicals were purchased from commercial suppliers: Phenytoin, span 40, sodium azide and phosphate-buffered saline (PBS, pH 7.4) from Sigma Chemical Co. (St. Louis, MO); dichloromethane (DCM), reagent grade from VWR International, Inc. (West Chester, PA); Slide-A-Lyzer Dialysis Cassettes, 7K MWCO, 3–12 mL Capacity from PIERCE Chemical Co. (Rockford, IL). All chemicals were reagent grade and used as purchased without further purification. Distilled water was prepared with a Mega-Pure™ system from Cole-Palmer (Vernon Hills, IL).
Preparation of PHT Containing Spray-Dried Microcarrier
The PCL microcarriers preparation was adapted from the work of Baras et al.20 Briefly, PCL and span 40 were dissolved in DCM; PHT was dissolved in water. The two solutions were mixed and then emulsified using an Ultraturrax model T25 (IKA Laboratory Technology, Staufen, Germany) at high speed (11000–16000 rpm for 5 min) and room temperature. Microcarriers were obtained by spraying this emulsion through the nozzle of a Buchi Mini Spray Dryer Model B-290 (Buchi Laboratoriums—Technik AG, Flawil, Switzerland). Typically, the process parameters were set as follows: inlet temperature (50°C); pump setting (25); aspirator (setting 80); spray flow (30 mmHg and 357 L/h). The solid microcarriers that had precipitated into the bottom collector (32–33°C outlet temperature) were collected and kept at room temperature.
The Orthogonal Experimental Design
In these studies, the effects of formulation variables on microcarriers characteristics such as particle size and PHT entrapment were investigated. These factors included: (a) the presence of an emulsion stabilizer (span 40) with variable concentration in the organic phase, (b) the concentration of the polymer solution, (c) the concentration of the PHT solution, and (d) the ratio of organic/aqueous phase volumes. In order to obtain the optimal synthesis conditions, experiments have been orthogonally designed for arranging the four factors above with three levels for each factor as shown in Table 1. Nine different sets of experiments have been conducted under conditions of different parameter combinations according to the standard L9(34) table as shown in Table 2. Each experiment was repeated twice.
| Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| Concentration of the polymer (%), (A) | 0.1% | 0.5% | 1.0% |
| Concentration of the phenytoin (%), (B) | 0.1% | 1.0% | 2.0% |
| Ratio of organic/aqueous phase (C) | 100/1 | 100/5 | 100/10 |
| Concentration of span 40 (%), (D) | 0.05% | 0.1% | 0.5% |
Characterization of PHT Containing Spray-Dried Microcarriers
Particle Size Analysis
The spray-dried microcarriers were redispersed in an Isoton® II Diluent solution and sized by a Z2™ Coulter Counter® Cell and Particle Counter (Beckman Coulter, Inc., Fullerton, CA) equipped with a 100 µm aperture size tube. Particle size was expressed as number mean diameter (µm). Scanning-transmission electron microscopy (STEM) was also used to study the size of the spray-dried microcarriers.
Morphological Analysis
The external morphology of the microcarriers was analyzed both by optical and scanning electron microscopy. The optical microscopy (Fisher Scientific, Houston, TX) was used at a magnification 1000×. For scanning electron microscopy (SEM), the spray-dried microcarriers were redispersed in water, air-dried, mounted on stubs and coated with gold-palladium under an argon atmosphere. Examination was carried out using SEM (Hitachi S-3400-II Scanning electron microscope, Hitachi High Technologies America, Inc., Schaumburg, IL) equipped with an image analyzer. Scanning-transmission electron microscope (STEM) examination of the fine powders was carried out with an Analytical Electron Microscope, JEOL 100 CX, which was operated at 100 kV and 100 µA. For this purpose fine powders of PCL, PHT, and their mixtures before and after processing were diluted in distilled deionized water with traces of tertiary butylamine. A drip of this dispersion was dried on a slim bar copper grid coated with a thin former film. The grids were subsequently coated with carbon and platinum.
Determination of Amount of PHT Encapsulated in PCL-Microcarriers
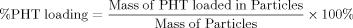 (1)
(1)Differential Scanning Calorimetry (DSC)
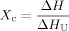 (2)
(2)Powder X-Ray Diffraction Analysis
XRD of the fine powders of the samples were carried out with a Panalytical (former Philips-Norelco) powder diffractometer with CuKα-radiation (λ = 0.154184 nm) generated at 40 kV and 20 µA and monochromotized with a graphite crystal. The fine powders were scanned from 4° 2Θ to 44° 2Θ which was the range showing the most important X-ray reflections for these samples.
In Vitro Release Study
The in vitro release behavior of PHT from PCL-microcarrier sample was investigated as follows: 60 mg sample of drug loaded spray-dried PCL microcarriers was suspended in 4 mL of 0.1 M PBS (pH 7.4) containing sodium azide 0.01%(w/v), and then was placed into the Slide-A-Lyzer Dialysis Cassettes that was immersed into 250 mL 0.1 M PBS (pH 7.4) containing sodium azide 0.01%(w/v). The system was shaken at 60 rpm and temperatures 37.4°C. At predetermined intervals, 300 µL PBS solution was taken out from the 250 mL immersion medium and replaced by the same volume of fresh medium. The PHT content of the release solution was measured by HPCL using a C18 column with UV detection at 220 nm.13 All release experiments were carried out in triplicate, and averages and standard errors of the mean are presented in Figure 7.
RESULTS AND DISCUSSION
Determination of the Optimal Parameters
The analysis of preparation variables (the carrier size) is shown from Tables 2–3, and for drug loading is shown from Table 4 and Figure 1. As it is noteworthy, the size of microcarriers was remarkably affected by the organic/aqueous phase ratio. It was also affected by the amount of stabilizer. However, the concentration of polymer solution and the concentration of the PHT had a much less significant influence on particle size, but their impacts on the PHT loading of microcarriers cannot be overlooked. For the intended efficient brain delivery of microcarriers, our goal was to obtain small size microcarriers with high drug loading. For parameter A (concentration of polymer solution), the size of A1 was the smallest and the amount of PHT loading was also higher than that of A2 and A3 (Fig. 1), therefore A1 was chosen as one of the best preparation conditions. For parameter B (conc. of the PHT), the particle size with B1 was the smallest, but the drug loading amount was low (about 0.5%), which is not suitable for the successful application for brain delivery. The particle size with B2 was smaller than that of B3, and the loading amount was not different. So B2 was chosen. For parameter C (ratio of organic/aqueous phase), the size with C1 was the smallest, but the drug loading amount is low (about 1.0%), which is not suitable for the intended application into the brain. Although the drug loading amount of C3 was higher than that of C2, the size of C2 was smaller than that of C3. So the best parameter was C2. The particle sizes with D2 and D3 were not different, and they are smaller than that of D1, and the loading amount of D1, D2, and D3 were not remarkably different, leading us to choose D2. To summarize, A1B2C2D2 (i.e., 0.1% PCL, 1.0% PHT, 100/5 phase ratio, and 0.5% stabilizer) was the best process parameters combination. In the confirmation experiments under conditions of the best parameters combination, the particle size of the sample was about 4.0 µm (as shown in Fig. 2), and the loading amount was about 17.5% w/w.
| No. | A | B | C | D | Particle Size (µm) | PHT Loading (% w/w) | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | I | II | (I + II)/10 | Mean Value | |
| 1 | 1 | 1 | 1 | 1 | 4.01 | 4.21 | 0.822 | 0.61 |
| 2 | 1 | 2 | 2 | 2 | 4.69 | 4.51 | 0.920 | 18.8 |
| 3 | 1 | 3 | 3 | 3 | 4.62 | 4.80 | 0.942 | 21.7 |
| 4 | 2 | 1 | 2 | 3 | 4.41 | 4.61 | 0.902 | 0.48 |
| 5 | 2 | 2 | 3 | 1 | 4.85 | 5.11 | 0.996 | 14.1 |
| 6 | 2 | 3 | 1 | 2 | 3.90 | 4.12 | 0.802 | 3.11 |
| 7 | 3 | 1 | 3 | 2 | 4.90 | 4.80 | 0.970 | 0.81 |
| 8 | 3 | 2 | 1 | 3 | 3.99 | 4.11 | 0.810 | 0.40 |
| 9 | 3 | 3 | 2 | 1 | 5.01 | 5.21 | 1.022 | 8.55 |
| K1 | 2.684 | 2.694 | 2.434 | 2.840 | ||||
| K2 | 2.700 | 2.726 | 2.844 | 2.692 | ||||
| K3 | 2.802 | 2.766 | 2.908 | 2.654 | ||||
R |
22.3451 | 22.3395 | 22.4692 | 22.3562 | ||||
R /3m /3m |
3.7242 | 3.7233 | 3.7449 | 3.7260 | ||||
| Sj | 0.0014 | 0.0004 | 0.0220 | 0.0032 | ||||
| Source | DF | SS | MS | F | |
|---|---|---|---|---|---|
| Repeat | 1 | 0.0004 | 0.0004 | 2.0 | |
| A | 2 | 0.0014 | 0.0007 | 3.5 | |
| B | 2 | 0.0004 | 0.0002 | 1.0 | |
| C | 2 | 0.0220 | 0.0110 | 55.0 | ** |
| D | 2 | 0.0032 | 0.0016 | 8.0 | * |
| Error | 8 | 0.0016 | 0.0002 | ||
| Total | 17 |
- * F0.05 (2.8) = 4.46, F0.01 (2.8) = 8.65 (significant effect).
- ** F0.05 (1.8) = 5.32, F0.01 (1.8) = 11.26 (highly significant effect).
| Factor Level | K/3 | Margin |
|---|---|---|
| A1 | 14.07 | 11.15 × 8.17 |
| A2 | 5.90 | 2.98 |
| A3 | 2.92 | |
| B1 | 0.63 | 10.52 × 10.47 |
| B2 | 11.10 | 0.05 |
| B3 | 11.15 | |
| C1 | 1.37 | 10.87 × 7.91 |
| C2 | 9.28 | 2.96 |
| C3 | 12.24 | |
| D1 | 7.75 | 0.19 × 0.18 |
| D2 | 7.57 | 0.01 |
| D3 | 7.56 |
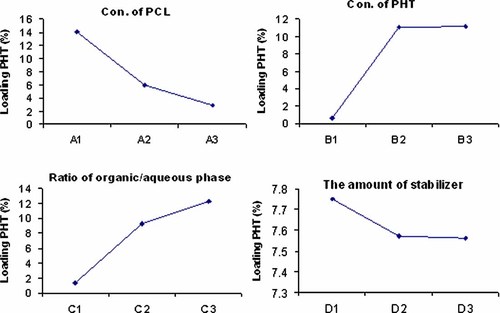
The relation of factor and index.
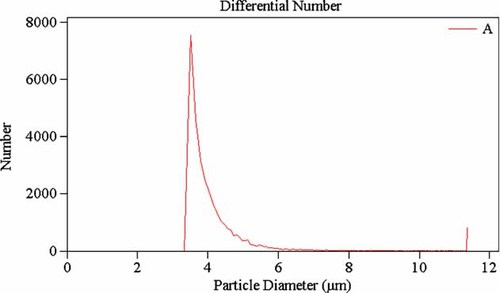
Size distribution of the phenytoin loaded PCL microcarrier. The formulation was made from 0.1% PCL, 1.0% PHT, 100/5 phase ratio, and 0.5% span 40.
Effects of Each Factor on the PCL Microcarrier Formulation
Concentration of the PCL Solution in the External Organic Phase
An increase in the initial polymer concentration (from 0.1% to 1.0%, w/v) resulted in a significant decrease in PHT loading (Fig. 1) and was associated with an increase of the particle size (Tab. 2). These results could be related to the increased viscosity and density of the emulsion or fluid, which increased significantly with the polymer concentration.23 These factors would also decrease the air/fluid mass ratio (Eqs. 3 and 4) or decrease of the energy of atomization resulting in coarser spray-droplets and leading overall to an increase in the mean size of solid microcarriers.23 Moreover, a considerable loss of materials occurred during spray-drying. This loss depended on the technical features of the apparatus: much of the powder adhering to the cyclone walls was lost during spray-drying and was related to the polymer concentration as well.
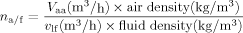 (3)
(3)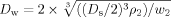 (4)
(4)Concentration of the PHT Solution in the Internal Aqueous Phase
There was a significant relationship between the initial concentration of PHT (0.1–2.0%, w/v) and both the particle size (Tab. 3) and the PHT loading (Fig. 1). It was postulated that, the PHT loading in PCL-microcarriers increases with the rise of the concentration of PHT in the internal aqueous solution. However, when the PHT/PCL ratio is high, the quantity of PCL present could be insufficient to cover the PHT completely. On the other hand, when the concentration of PHT was below 1.0%, the dispersion of PHT solution in the organic phase may be better than that at high concentration, leading to the formation of finer microdroplets during the spray-drying process.
The Organic/Aqueous Phase Ratio
As shown in Tables 2–4, increasing the volume of the aqueous phase (from ratio of 100/1 to 100/10) while maintaining a constant volume of the organic phase resulted in a significant increase of both the particle size and PHT loading (Fig. 1). The rise of the aqueous phase volume may increase the volume of aqueous phase microdroplets. Therefore, the produced PCL microcarriers had relatively larger size and a high PHT entrapment. However, the aqueous/organic phase ratio cannot be too high; otherwise, the polymer quantity dissolved in the organic phase may not be sufficient to encapsulate the PHT completely.
The Concentration of Emulsion Stabilizer in the External Organic Phase
The addition of insoluble PHT in organic solvents (DCM) could give rise to a nonuniform distribution of large PHT islands in the PCL matrix, presumably close to the microcarrier surface.25 Therefore, the emulsion stabilizer (span 40) is very important for the stability of the water in oil emulsion, which combines aqueous and organic phases that appeared suitable for this encapsulation process. Our preliminary studies with tween series, span 20, span 40 and span 80 showed that span 40 was the best Sorbitan to be used because its solubility in DCM was higher and it contributed to the production of particles with desirable features. The stability of this emulsion is a critical requirement, especially when the organic solvent is evaporated rapidly from the system by spray-drying to create well-defined microcarriers.
Even if it was possible to produce microcarriers in the absence of stabilizer, the obtained microcarriers would show a low drug loading because of the low stabilization of the microdroplets formed during the emulsification process in absence of stabilizer.20 The stabilization of the emulsion by the addition of span 40 is a prerequisite for the loading of a large amount of PHT within the microcarriers. Moreover, the stabilizer acts as a protective layer by adsorption at the organic/aqueous phase interface of the droplets and therefore prevents the coalescence of the microcarriers. When the amount of stabilizer is low (0.05%), the emulsion exhibited unstable microdroplets, which allowed to produce aggregated microdroplets during the spray-drying process, leading to relatively larger particles (Tab. 2).
The Morphology and Size of PHT Containing PCL Microcarriers
Particles prepared by spray-drying normally have a good spherical structure and uniform size distribution. A STEM of the PHT containing PCL microcarriers is shown in Figure 3 for the confirmation experiment under conditions of the best parameters combination (i.e., 0.1% PCL, 1.0% PHT, 100/5 phase ratio, and 0.5% stabilizer) and shows that the PCL-microcarriers have good spherical structure. SEM of the PHT containing PCL microparticles also shows spherical morphology and mainly uniform size (Fig. 4). The particle sizes are shown in Figure 2 and indicate that 100% of the PHT containing PCL microcarriers were in the range of 3.5–4.4 µm, with very narrow size distribution. The mean diameter of these particles is 4.3 ± 0.1 µm (n = 3), 90% of these particles had a size d90 = 3.5 ± 0.1 µm (n = 3), 50% of these particles had a size d50 = 3.9 ± 0.2 µm (n = 3).
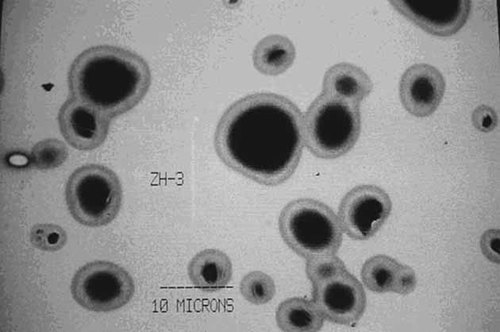
Scanning transmission electron micrograph (STEM) of the phenytoin-microcarrier. The formulation was made from 0.1% PCL, 1.0% phenytoin, 100/5 phase ratio, and 0.5% span 40.

Scanning electron micrograph (SEM) of the phenytoin-microcarrier. The formulation was made from 0.1% PCL, 1.0% phenytoin, 100/5 phase ratio, and 0.5% span 40.
DSC Analysis
- (1)
PHT decomposes shortly after melting in the same temperature range of PCL decomposition. Consequently, the heat of fusion of PHT and the percent of crystalline PHT cannot be determined.
- (2)
The degree of crystallinity in the PCL matrix is higher in the microcarrier than in the native PCL sample. The sample in which the components were unmixed also shows similar crystallinity to the native PCL indicating that the enhanced crystallinity in microcarrier may be due to templating of PCL on dispersed PHT crystals. This also suggests that most PHT crystals are dispersed within the PCL matrix, and not on the surface of PCL-microcarriers.
- (3)
The melting peak and the decomposition peak of PHT in the PCL microcarrier appear to shift to considerably lower temperatures. In the control sample of PCL containing 12 wt% PHT, the melting peak of PHT was depressed approximately 40°C. In the microcarrier, the depression was approximately 100°C. The melting point depression is suggested to arise from two sources: (1) the presence of PCL, which depresses the melting point due to the fact that PHT crystals are in equilibrium at their melting point with surrounding PHT/PCL liquid rather than with only liquid PHT,26 and (2) the size of the PHT crystals in the microcarrier is within the nanometer scale, which will depress the melting point according to the Gibbs–Thompson equation:27, 28
| Samples | Composition (%) | DSC Data | Tm (°C) of PHT | ||||
|---|---|---|---|---|---|---|---|
| PHT | PCL | Span 40 | Tm (°C) | ΔHm (J/gPCL) | Xc (%) | ||
| (A) Native PHT | 100.0 | 0.0 | 0.0 | 368.3 | |||
| (B) Blank PCL microcarrier | 0.0 | 84.0 | 16.0 | 53.6 | 98.0 | 68.6 | |
| (C) Physical mixture PHT/PCL | 12.0 | 88.0 | 0.0 | 54.9 | 85.4 | 59.7 | 320.8 |
| (D) PHT/PCL microcarrier | 14.3 | 71.4 | 14.3 | 56.6 | 98.9 | 69.2 | 277.0 |
| (E) Native PCL | 0.0 | 100.0 | 0.0 | 56.5 | 85.7 | 60.0 | |
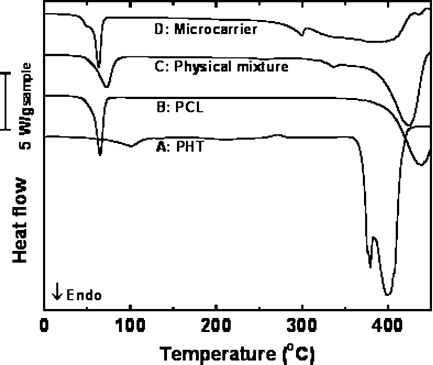
DSC thermogram of native phenytoin powder (A), blank PCL + span 40 microcarrier (B), physical mixture of phenytoin + PCL + Span 40 (C); PCL microcarrier containing 14.3% (w/w) of phenytoin (D).
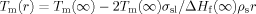 (5)
(5)PXRD Analysis
Powder X-ray patterns of PHT, PCL, their mixture, before the processing, and one of the processed samples (compositions are given in Tab. 6) are displayed in Table 7 and Figure 6. In terms of 2-theta angles, the X-ray diffractogram of PHT powder (Fig. 6), sharp peaks at diffraction angle 2θ, 2.78°, 3.29°, 5.58°, 7.57°, 10.99°, 11.24°, 11.92°, 13.02°, 14.34°. The highest peak and characteristic peak of PHT was the one at 2θ, 10.99°. These observations were similar to the previously reported diffractogram of PHT.29 These suggested that the drug was present as crystalline material. In the X-ray diffractogram of blank PCL powder, a combination of large diffraction peaks with sharp peaks at diffraction angle 2θ, 10.70°, 10.95° and 11.85° indicated that the polymer is semicrystalline material. The highest peak and characteristic peak of PCL was the one at 2θ, 10.74°. These observations were similar to the previously reported diffractogram of PCL.30 In the PHT containing microcarrier, the characteristic peak of PHT at 2θ, 10.99 slightly overlap with the PCL peak at 2θ, 10.70° suggesting that the drug was encapsulated in the crystalline state within the PCL matrix.
| Samples | Composition (%) | ||
|---|---|---|---|
| PHT | PCL | Span 40 | |
| ZH1 (processed mixture) | 4.8 | 79.4 | 15.8 |
| ZH2 (processed mixture) | 0.42 | 83.0 | 16.58 |
| ZH3 (processed mixture) | 14.3 | 71.4 | 14.3 |
| ZH4 (processed mixture) | 12.0 | 88.0 | 0.0 |
| ZH5: phenytoin | 100 | 0.0 | 0.0 |
| ZH6: blank PCL microcarrier | 0.0 | 84.0 | 16.0 |
| ZH7: physical mixture | 18.0 | 70.0 | 12.0 |
- Processed mixtures referred to spray-dried phenytoin loaded PCL microcarriers with different drug loadings.
| Sample d-value (nm) | Relative Intensity |
|---|---|
| ZH1 (processed) | |
| 0.561 | 3 |
| 0.413 | 100 |
| 0.403* | 14 |
| 0.374 | 47 |
| 0.299 | 5 |
| 0.248 | 4 |
| 0.234 | 1 |
| 0.223 | 5 |
| 0.219 | 2 |
| 0.207 | + |
| ZH2 (processed) | |
| 0.566 | 3 |
| 0.414 | 100 |
| 0.404* | 22 |
| 0.375 | 51 |
| 0.310 | 1 |
| 0.301 | + |
| 0.299 | 1 |
| 0.242 | 1 |
| 0.236 | + |
| 0.233 | 1 |
| 0.224 | 1 |
| 0.222 | 1 |
| 0.208 | 3 |
| ZH3 (processed) | |
| 0.575 | 2 |
| 0.415 | 100 |
| 0.405* | 27 |
| 0.375 | 48 |
| 0.309 | 1 |
| 0.300 | 1 |
| 0.234 | 2 |
| 0.224 | 3 |
| 0.211 | + |
| 0.208 | + |
| 0.207 | 1 |
| ZH4 (processed) | |
| 0.557 | 3 |
| 0.415 | 100 |
| 0.405* | 25 |
| 0.374 | 49 |
| 0.300 | 4 |
| 0.223 | 6 |
| 0.208 | 2 |
| ZH5 (phenytoin) | |
| 1.590 | 29 |
| 1.341 | 27 |
| 1.162 | 2 |
| 0.923 | 14 |
| 0.793 | 20 |
| 0.746 | 6 |
| 0.672 | 13 |
| 0.621 | 7 |
| 0.585 | 32 |
| 0.546 | 5 |
| 0.533 | 6 |
| 0.514 | + |
| 0.491 | 4 |
| 0.456 | 15 |
| 0.445 | 3 |
| 0.404* | 100 |
| 0.395 | 20 |
| 0.385 | 7 |
| 0.379 | 3 |
| 0.373 | 20 |
| 0.357 | 5 |
| 0.342 | 21 |
| 0.331 | 8 |
| 0.327 | 4 |
| 0.311 | 23 |
| 0.298 | 2 |
| 0.289 | 3 |
| 0.284 | 1 |
| 0.272 | 1 |
| 0.266 | 8 |
| 0.252 | 4 |
| 0.247 | 1 |
| 0.243 | 4 |
| 0.230 | 4 |
| 0.227 | 4 |
| 0.222 | 6 |
| ZH6 (PCL blank) | |
| 0.555 | 1 |
| 0.414 | 100 |
| 0.375 | 41 |
| 0.298 | 3 |
| 0.241 | 1 |
| 0.234 | + |
| 0.224 | 4 |
| 0.208 | 2 |
| ZH7 (mixture) | |
| 1.572 | 2 |
| 1.325 | 3 |
| 1.027 | + |
| 0.913 | 1 |
| 0.786 | 3 |
| 0.742 | + |
| 0.664 | + |
| 0.584 | 7 |
| 0.526 | 1 |
| 0.416 | 100 |
| 0.403* | 29 |
| 0.375 | 44 |
| 0.340 | 5 |
| 0.311 | 2 |
| 0.299 | 1 |
| 0.265 | 3 |
| 0.250 | 1 |
| 0.228 | 1 |
| 0.224 | + |
- The composition of sample ZH1 to ZH7 is available in Table 6.
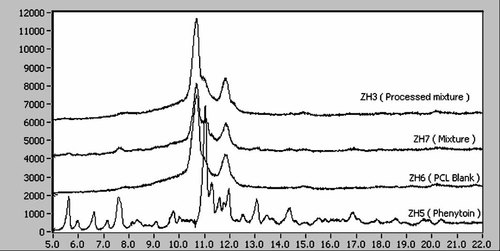
Powder X-ray diffractogram of from top to down: processed or spray-dried 14.3% w/w phenytoin loaded PCL microcarrier (ZH3), physical mixture of phenytoin and PCL (ZH7), blank PCL + span 40 microcarrier (ZH6), and native phenytoin powder (ZH5).
As angles vary with the type of radiation, it is also important to discuss these data in term of d-value. The strong reflections of PHT at 0.404 and 0.395 nm were useful for examining the effects of the mixing with PCL (sample ZH7) and after the processing the mixtures (ZH1, ZH2, ZH3, and ZH4). The polymer PCL blank has a PXRD pattern with only two broad but well-defined reflections at 0.414 and 0.375 nm (Tab. 7). The patterns in Figure 6 clearly shows that PCL has a crystallinity much lower than that of PHT. However the strong 0.404 nm reflections (marked with an asterisk in Tab. 7) can serve as a good indicator about the state of PHT after processing. The XRD pattern of the mixture (ZH7) before processing and that of the processed mixture (ZH3) are shown in Figure 6 and they are indistinguishable. This clearly shows that the PHT has not changed during the process. This is also true for all the other three XRD patterns for the processed mixtures ZH1, ZH2, and ZH4 as the data in Table 1 indicate. Thus, PHT seems to maintain its crystallinity during these processes. This XRD observation was consistent with the DSC data analysis.
The crystal structures of PHT (diphenylhydantoin) was reported as sodium salt and crystallized as the free acid from aqueous solution at pH 11. The crystals are orthorhombic and the defining characteristics of the respective unit cells are: a = 6.230, b = 13.531, c = 15.523 Angstrom; space group Pn21a with Z = 4 molecules per unit cell.31 Moreover, the stereochemical similarities and three-dimensional conformations of AEDs have been suggested to be having implication in their bioactivities.32 Therefore, the conservation of the crystalline structure of drug inside the PCL system suggested a preservation of the PHT bioactivity that remains to be demonstrated in biological systems in future studies.
In Vitro Release Profile of PHT Containing Spray-Dried PCL Microcarriers
PHT release from PCL-microcarriers was investigated in PBS (pH 7.4) at temperature 37.4°C. PHT release from the PCL-microcarrier samples generally showed multi-stage release behavior, as shown in Figure 7. During the first 4 days, release was linear with about 1.5 mg PHT released per day. The release occurred in the first stage may be due to the dissolution of the PHT adsorbed at the external surface of PCL-microcarriers. These results are consistent with Dong's report.33 During the following 8 days, the release rate decreased to about 0.4 mg/day and then it approached complete (80%) of the PHT release from the microcarriers. The release rate in this stage became much slower than that in the first stage, and we hypothesize that release at this point was a consequence of diffusion through pores generated in the dosage form, which was formed by the coalescence of some PHT crystals, since they might stick to each other when the PCL suspensions containing PHT was spray-dried. At the last stage, the PHT release almost not observable, which might have been PHT strongly isolated within the delivery matrix, and the release rate at this stage may be mainly controlled by the decomposition kinetics of PCL, leading to very slow and sustained drug release.
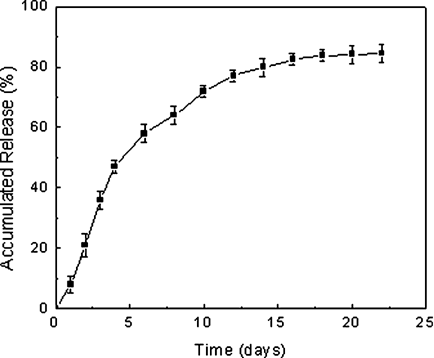
In vitro release profiles of drug from the phenytoin loaded PCL microcarrier in PBS pH 7.4 medium at 37.4°C. The formulation was made from 0.1% PCL, 1.0% phenytoin, 100/5 phase ratio, and 0.5% span 40.
CONCLUSIONS
PHT containing PCL-microcarriers were efficiently prepared by the spray-drying method. The effects of various technological parameters (concentration of the polymer and PHT solutions, organic/aqueous phase ratio, and the amount of stabilizer) on microcarrier size and PHT entrapment were studied. Based on the preparation method used, we can reasonably speculate that the produced microcarriers are microspheres. However, further analysis such as freeze fracture cryoelectron microscopy will be needed to confirm this speculation. DSC and PXRD analysis suggested that PHT was in crystalline state within the microcarrier. Previous studies34, 35 suggested that PHT exhibits different crystal habits depending on different factors such as cooling rate, nature of solvent, etc… Therefore, further studies would be required to elucidate the nature of the PHTs crystal habit inside the microcarrier. The optimal synthesis conditions were obtained through orthogonally designed experiments. Under our optimized conditions (i.e., 0.1% PCL, 1.0% PHT, 100/5 phase ratio, and 0.5% stabilizer), we obtained particles with 4.0 µm diameter and 17.5% (wt/wt) PHT loading, which released PHT slowly for about 14 days in vitro. The drug release exhibited a typical sustained release behavior without any burst effect. The focus of this manuscript was on the formulation aspect. The stability aspect which is also very important for the production of a robust and effective drug loaded microcarrier for brain injection, are still under current investigation. However, these preliminary features of the PHT-containing PCL microcarriers are suitable for the intended application for intermediate term management of epilepsy, such as after brain surgery.
Acknowledgements
This project was supported by the Epilepsy Research Foundation (New Therapy Grant). We are grateful to Mary Haestert (TTUHSC Electron Microscopy Center, Lubbock, TX) for her assistance for the scanning electron microscopy.




