The nature of the bonding between metalloporphyrins [Fe(II), Co(II), Mn(II)] and oxygen species (·O ·, H2+O·/HO·, HOO·, ·O·)
·, H2+O·/HO·, HOO·, ·O·)
Abstract
The interaction of reduced transition-metal porphyrins [e.g. PMn(II), PFe(II), PCo(II)] with oxygen species (·O ·, H2+O·/HO·, HOO·, ·O·) involves radical/radical coupling to form covalent bonds. Coordinately unsaturated iron(II) porphyrins form two sigma bonds with ·O
·, H2+O·/HO·, HOO·, ·O·) involves radical/radical coupling to form covalent bonds. Coordinately unsaturated iron(II) porphyrins form two sigma bonds with ·O ·(−ΔGBF = 1–6 kcal mol−1) via two of the four unpaired d-electrons of the iron center. The hydroxyl radical
·(−ΔGBF = 1–6 kcal mol−1) via two of the four unpaired d-electrons of the iron center. The hydroxyl radical 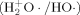 couples with an unpaired electron of the PM(II) porphyrins to form a single covalent bond
couples with an unpaired electron of the PM(II) porphyrins to form a single covalent bond 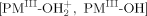 . The bond energies (−ΔGBF) for
. The bond energies (−ΔGBF) for 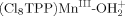 and
and 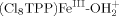 are 65 ± 4 and 58 kcal mol−1, respectively. Under neutral conditions the uncharged species [(Cl8TPP)MnIII–OH, (Cl8TPP)FeIII–OH, (Cl8TPP)CoIII–OH] have bond-formation energies (−ΔGBF) of 70 ± 4, 63, and 61 kcal mol−1, respectively. In acetonitrile solutions atomic oxygen (·O·; derived from ozone, O3) couples with (Cl8TPP)MnIII[ClO4], (Cl8TPP)MnII, (Cl8TPP)FeIII[ClO4], and (Cl8TPP)FeII to form (Cl8TPP+)MnVO (−ΔGBF = 59 ± 4 kcal mol−1), (Cl8TPP)MnIVO (85 kcal mol−1), (Cl8TPP+·)FeIVO (68 kcal mol−1; model for compound I of horseradish peroxidase), and (Cl8TPP)FeIVO (78 kcal mol−1; model for compound II of horseradish peroxidase). The weakly bonded species [(Cl8TPP+)MnVO and (Cl8TPP+·)FeIVO] epoxidize olefins with near-stoichiometric efficiency; the other two species are essentially unreactive with olefins. Copyright © 2001 John Wiley & Sons, Ltd.
are 65 ± 4 and 58 kcal mol−1, respectively. Under neutral conditions the uncharged species [(Cl8TPP)MnIII–OH, (Cl8TPP)FeIII–OH, (Cl8TPP)CoIII–OH] have bond-formation energies (−ΔGBF) of 70 ± 4, 63, and 61 kcal mol−1, respectively. In acetonitrile solutions atomic oxygen (·O·; derived from ozone, O3) couples with (Cl8TPP)MnIII[ClO4], (Cl8TPP)MnII, (Cl8TPP)FeIII[ClO4], and (Cl8TPP)FeII to form (Cl8TPP+)MnVO (−ΔGBF = 59 ± 4 kcal mol−1), (Cl8TPP)MnIVO (85 kcal mol−1), (Cl8TPP+·)FeIVO (68 kcal mol−1; model for compound I of horseradish peroxidase), and (Cl8TPP)FeIVO (78 kcal mol−1; model for compound II of horseradish peroxidase). The weakly bonded species [(Cl8TPP+)MnVO and (Cl8TPP+·)FeIVO] epoxidize olefins with near-stoichiometric efficiency; the other two species are essentially unreactive with olefins. Copyright © 2001 John Wiley & Sons, Ltd.




