Evaluation and management of pediatric refractory constipation: Recommendations from the NASPGHAN neurogastroenterology and motility committee
CME module may be found at https://learnonline.naspghan.org/jpgn2
Alexandra L. Kilgore and Mary K. Rogers Boruta contributed equally to the work as co-first authors.
Anil Darbari and Leonel Rodriguez contributed equally to the work as co-senior authors.
Disclaimer: The NASPGHAN clinical practice guidelines and position papers are evidence-based decision-making tools for managing health conditions. This document is not a disease management requirement or rule and should not be construed as establishing a legal standard of care, or as encouraging, advocating for, mandating or discouraging any particular diagnostic methodology or treatment. Our clinical practice guidelines and position papers should also not be used in support of medical complaints, legal proceedings, and/or litigation, as they were not designed for this purpose. The NASPGHAN clinical practice guidelines and position papers should also not be utilized by insurance companies or pharmacy benefit managers to deny treatment that is deemed medically necessary by a patient’s physician.
The health care team, patient, and family should make all decisions regarding the care of a patient, after consideration of individual specific medical circumstances. While NASPGHAN makes every effort to present accurate and reliable evidence-based information, these clinical practice guidelines and position papers are provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, nor consequential damages, incurred in connection with the clinical practice guidelines and/or position papers or reliance on the information presented.
Disclaimer: Although this paper is produced by the NASPGHAN Neurogastroenterology and Motility Committee it does not necessarily represent NASPGHAN policy and is not endorsed by NASPGHAN.
Abstract
Refractory constipation (RC) in pediatric patients should be recognized as a distinct condition with long-term impacts on patient and family quality of life. RC requires a more targeted diagnostic evaluation and complex management strategy that may involve management by pediatric neurogastroenterology and motility specialists and multidisciplinary teams including surgeons. Currently, there is a lack of a clear definition, evaluation, and management strategies for RC. This is the first North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition position paper to address pediatric RC regarding its definition, evaluation, and management.
Highlights
What is Known
-
Pediatric gastroenterologists frequently treat patients with refractory constipation (RC).
-
Currently, there is no clear definition, evaluation, and management strategies for RC.
What is New
-
Proposed definition for RC in pediatric patients.
-
Pathway for the evaluation and management of pediatric patients with RC.
1 INTRODUCTION
While most pediatric patients struggling with constipation have functional constipation (FC) that is well managed with dietary changes, behavioral modifications, and an osmotic laxative, pediatric gastroenterologists also commonly treat children with severe constipation that is refractory to these interventions. Refractory constipation (RC) requires a targeted diagnostic evaluation and complex management strategy which may involve management by pediatric neurogastroenterology and motility (NGM) specialists and multidisciplinary teams including surgeons. Although many studies have identified pediatric patients who are refractory to traditional constipation management, the definition of RC is nebulous without consensus1, 2 and its prevalence is unknown. Rheumatology has defined refractory disease as the “resistance to multiple drugs with different mechanisms of action by persistence of physical symptoms and high disease activity” and agrees that a unifying definition is needed for appropriate evidence-based identification and treatment of these patients.3 For instance, approximately one third of patients referred to a tertiary care center for “refractory functional constipation” were not on a laxative at their first pediatric gastroenterology clinic visit.4 The tertiary care center referral may have been avoided if the patient had been initially treated with a stimulant laxative. Furthermore, pediatric patients with long term FC scored significantly lower on health-related quality of life (HRQoL) scores when compared to healthy populations5 and about a quarter of pediatric patients struggle with constipation into adulthood.6 Current recommendations for the evaluation and management of pediatric constipation need to be enhanced to improve these outcomes.
RC has a significant impact on the lives of pediatric patients, making evaluation and management strategies a vital topic for pediatric gastrointestinal (GI). Patients with RC are often treated with osmotic laxatives alone when referred to NGM or colorectal surgery clinics, suggesting that some may be able to avoid referral to these specialized providers with daily use of a stimulant laxative. The following recommendations represent the first North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) position paper to provide guidance around the definition, evaluation, and management of pediatric RC. They follow evidence-based recommendations published in 2014 by the NASPGHAN and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) for the evaluation and treatment of pediatric constipation that includes the use of nonpharmacologic interventions such as water and fiber intake.7 The current manuscript should be used by pediatric gastroenterologists as an adjunct to the best practices outlined in the NASPGHAN/ESPGHAN guidelines for treating FC in children, as it identifies the utilization of optimal FC treatments as a first step for defining patients with RC. In addition, this position paper serves as encouragement to pediatric gastroenterologists to optimize therapy in FC (including stimulant laxatives) and to provide an evaluation and management pathway to improve outcomes and hopefully change the natural history of FC transitioning to RC as we currently know it.
2 METHODS
This position paper was initially conceived in the Spring of 2021 due to the lack of a clear definition, evaluation, and management strategies for RC. The authors are NASPGHAN NGM committee members and select chosen leaders in the NGM field. They include a diverse selection of authors regarding gender, ethnicity, geographic location, and practice size. The eighth author is a colorectal surgeon. The authors were approved by the NASPGHAN Clinical Care and Quality Committee and the NASPGHAN Council.
PubMed and Cochrane databases were utilized for literature searches through January 31, 2023 to assess the specific topics relating to the evaluation and management of “pediatric functional constipation,” “pediatric refractory constipation,” and “pediatric intractable constipation.” When pediatric (≤18 years of age) data were limited, adult studies were used and disclosed in their respective sections. Literature written in English was included. Sections were written using a combination of literature review and expert opinion. Authors anonymously voted on position statements using an online survey to indicate “agree,” “disagree,” or “want to reword the statement before voting.” A statement vote for “want to reword the statement before voting” prompted further discussion and modification in a virtual meeting. The edited statement was subsequently voted on again in an online survey with the statement's final wording determined by the highest number of votes. In the case of a tie-in statement agreement, the latest statement was published. No statements were voted on more than three times. “Recommended” statements were defined by at least seven out of eight authors in agreement.
Due to the lack of consensus for the definition of pediatric RC within literature, all data and literature reviewed and referenced in this position paper are based on FC studies whose patient population corresponded with our definition of RC. Table 1 is an overview of the 11 clinical questions addressed in this position paper. The authors agreed that these recommendations should be considered for revision in 5 years from the date of publication.
| Question 1: What is the definition of pediatric refractory constipation? |
| Question 2: What assessment is needed for pediatric patients with refractory constipation? |
| 2.1 History and physical exam |
| 2.2 Laboratory studies |
| 2.3 Imaging studies |
| 2.4 Manometry studies |
| 2.5 Rectal biopsy |
| Question 3: What is the effectiveness of nonpharmacologic treatments for pediatric patients with refractory constipation? |
| 3.1 Pelvic floor physical therapy with or without biofeedback |
| 3.2 Behavioral therapy |
| Question 4: What are the pharmacologic treatment options for maintenance therapy in pediatric patients with refractory constipation? |
| 4.1 Oral high-dose stimulant laxatives |
| 4.2 Secretagogues |
| 4.3 Serotonin agonists |
| Question 5: What are the maintenance options for retrograde therapy in pediatric patients with refractory constipation? |
| Question 6: What is the effectiveness of surgical interventions in pediatric patients with refractory constipation? |
| 6.1 Anal dilation, internal anal sphincter myectomy, and anal botulinum toxin injection |
| 6.2 ACEs |
| 6.3 Sacral nerve stimulation |
| 6.4 Colonic diversion and colectomy |
| Question 7: What are the recommended pharmaceutical options for ACE flushes? |
| 7.1 Weaning and discontinuing the ACE |
| Question 8: What is the prognosis for children with refractory constipation? |
| Question 9: How can refractory constipation be prevented? |
| Question 10: What is the recommended treatment pathway for pediatric refractory constipation? |
| Question 11: What research is needed for the care of children with refractory constipation? |
- Abbreviation: ACE, antegrade continence enema.
Question 1: What is the definition of pediatric refractory constipation?
The authors recommend that pediatric RC be defined by the presence of 4 specific clinical criteria listed in Table 2. Explicitly, RC is defined by the presence of ongoing constipation symptoms in children who meet Rome IV criteria for pediatric FC,8 and who have had failure to improve after a minimum of 3 months,7 usage of age- and developmentally appropriate conventional constipation therapies, and in whom there is impaired QoL. Ongoing symptoms are defined by ≤2 voluntary defecations per week and/or ≥1 episode of fecal incontinence per week. Conventional constipation therapies should include the use of daily stimulant laxatives at appropriate dosages in addition to behavioral and biomechanical interventions (Agreement 8/8). Appropriate daily stimulant dosage is defined in Table 3 and is considered to be the initial weight-based dosage listed up to the maximum defined dose. This is inclusive of pediatric patients who are unable to achieve or participate in conventional interventions due to physical impairments, developmental differences, and/or geographic limitations. Historically, 3 months of treatment has been considered the appropriate length of time to reassess a patient's symptoms and determine if it is refractory to treatment.7, 9 The authors agreed upon a 3-month duration of ongoing symptoms per the defined parameters to deter the delay in treatment, worsening QoL, and long-term outcomes.
| 1. Must meet ROME IV criteria for functional constipation |
| 2. Failure of age and developmentally appropriate conventional therapies to improve symptoms after a minimum of 3 months on the following therapies |
| a. Daily use of a stimulant laxative at appropriate dosage (see Table 3 for dosing recommendations) regardless of osmotic laxative use |
| b. Behavioral interventions |
| c. Biomechanical interventions (i.e., correct positioning on the toilet) |
| 3. Ongoing symptoms of constipation |
| a. ≤2 voluntary defecations per week |
| and/or |
| b. ≥1 episode of fecal incontinence per week |
| 4. Impaired QoL for the patient or family due to constipation symptoms |
- Abbreviation: QoL, quality of life.
| Medication | Formulation | Dosing |
|---|---|---|
| Stimulant laxatives | ||
| Sennosidesa | Chew tablet | 1–2 mg/kg qHS, max 120 mg |
| Gummy | ||
| Liquid | ||
| Tablet | ||
| Bisacodyl | Tablet | 0.2 mg/kg qHS, max 20 mg |
| Compounded | ||
| Enema/suppository | 5–10 mg (0.5–1 enema or suppository) qday or BID | |
| Secretagogues | ||
| Linaclotideb | Tablet | 72, 145, or 290 µg qday |
| Take on an empty stomach, 30 min before the first meal of the day | ||
| Plecanatidec, d | Tablet | 3 mg qday |
| Lubiprostoned | Capsule | 8, 16, or 24 g BID |
| Take with food | ||
| Serotonin agonists | ||
| Prucaloprided | Liquid | 0.02–0.04 mg/kg, max 2 mg qday |
| Tablet | ||
- Abbreviations: BID, twice a day; FDA, Food and Drug Administration.
- a Sennoside and senna leaf extract are not interchangeable.
- b Linaclotide is contraindicated for patients less than 2 years of age and is FDA approved for patients greater than 6 years of age.
- c Plecanatide is contraindicated for patients less than 6 years of age.
- d This medication is not FDA approved for children less than 18 years of age.
The NASPGHAN/ESPGHAN guideline on the evaluation and treatment of pediatric FC from 2014 suggests that polyethylene glycol (PEG) 3350 is the most effective pharmacologic treatment for maintenance therapy.7 Since publication, bisacodyl4, 10 and senna11-13 have been shown to be safe and effective in pediatric patients. Per the expert author's opinion, some pediatric patients referred to a pediatric neurogastroenterologist can avoid invasive testing with a maintenance stimulant laxative. Our authors support the use of a scheduled stimulant laxative over PEG 3350 or other osmotic laxatives alone before classifying a patient refractory to the treatment of FC. In addition, postprandial toilet sits should be implemented in developmentally appropriate patients at a minimum14 before labeling a pediatric patient with RC. Modifications to toilet sit biomechanics should also be performed to allow for relaxed posturing14 and a squatting position to straighten the anorectal canal.15
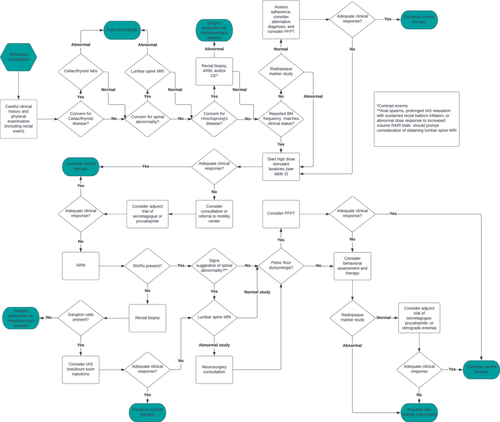
Question 2: What assessment is needed for pediatric patients with refractory constipation?
Most diagnostic tests used in the evaluation of RC do not have normative data or a unified consensus on their performance, indications, and clinical utility.
2.1 History and physical exam
A thorough clinical history and physical examination, including a digital rectal examination (DRE), is invaluable in the evaluation of children with RC to identify red flags such as delayed passage of meconium and the presence of sacral abnormalities.
2.2 Laboratory studies
There is no evidence to recommend the routine use of laboratory studies in the evaluation of RC. Studies evaluating the association of celiac disease with FC have yielded controversial results. Some reported a similar constipation prevalence in patients with celiac disease compared to healthy controls,16, 17 while others found constipation to be the most common GI symptom.18-20
Recent studies have discovered that 3.25%–7.6% of children with RC have celiac disease and up to 76% have resolution of constipation symptoms after initiating a gluten-free diet.21, 22 No study has evaluated the prevalence of thyroid disease in RC, but hypothyroidism infrequently presents as constipation.23
-
Pediatric patients with RC should undergo serologic screening for celiac disease before performing an invasive test or surgical intervention. (Agreement: 8/8).
-
Pediatric patients with RC should be screened for thyroid disease if there is a red flag present for thyroid disease before performing an invasive test or surgical intervention. (Agreement: 8/8).
2.3 Imaging studies
2.3.1 Abdominal x-ray
The use of an abdominal x-ray (AXR) in the evaluation of constipation is controversial. Multiple radiologic scores have been developed in the attempt to standardize stool burden interpretation on an AXR, but their use has not been widespread given the lack of reproducibility, reliability, sensitivity, and specificity when tested in clinical practice. Two systematic reviews did not find evidence to support the routine use of AXR in the evaluation of FC,24, 25 and no study has reported its utility in pediatric RC.
-
The use of an AXR in RC should be reserved for those patients unable to provide a reliable medical history and/or unable to allow for a physical exam (including a DRE), or to evaluate for mechanical obstruction or colonic distention when considering surgical interventions. (Agreement: 8/8).
2.3.2 Colonic transit studies
Colonic transit studies (CTTs) can be performed using radiopaque markers (ROMs) or nuclear medicine.
2.3.2.1 Radiopaque markers
CTT with ROM has been used to assess for slow transit constipation.26 Two protocols involving a capsule containing 24 ROM are widely accepted and validated in adults: simplified and segmental. The simplified method consists of ingesting a single capsule and obtaining an AXR 5 days later.27 The segmental method involves ingesting a capsule daily for 3 consecutive days followed by an AXR on Days 4 and 7 after ingestion.28 No ROM protocol is validated in children, but studies have advocated for the use of ROM to predict colonic manometry (CM) results in children with RC. When considering ordering this study, it is important to recognize that the patient will need to swallow the markers (within the capsule or mixed into a spoonful of food). Two studies assessing children 3–18 years of age (median 11.5–12 years old) have demonstrated that a normal CTT correlated to a normal CM, while 47%–1% of children with an abnormal CTT (defined per the simplified method with >6 ROM present within the colon, proximal to the rectum on the Day 5 AXR) had an abnormal CM.29, 30 If the reported symptoms of RC cannot be validated on patient evaluation by an expert, ROM can be helpful to determine if the patient truly has RC before proceeding with invasive testing.
-
CTT via ROM should be completed for patients with RC with equivocal medical history and to screen for the need to perform CM. (Agreement: 7/8).
2.3.2.2 Colonic scintigraphy
Colonic scintigraphy (CsC) involves the ingestion of a nonabsorbable form of a radioisotope and requires a series of measurements for up to 72 h. Normative data and clinical utility have been defined in adults but not yet in children.31 A study classifying CTT in children with RC using CsC values from healthy children reported values similar to adult studies.32 In pediatric RC, CsC has been shown to be reproducible33 and to have good correlation between CsC and CM.34 CsC may also be useful in differentiating between slow transit constipation and fecal retention.35, 36 Limitations of CsC in children include low availability, high cost, and lack of normative data due to no standardized protocol.
-
CsC should not be performed routinely, but can be considered as an alternative to the ROM. (Agreement: 8/8).
2.3.3 Lumbar spine magnetic resonance imaging
A lumbar spine MRI (LSMRI) is not routinely recommended in the evaluation of patients with FC unless there is concern for a spinal anomaly that presented as constipation before development of neurological symptoms,37, 38 especially in patients with concomitant urodynamic abnormalities39 or suspected Currarino triad.40 This holds true for patients with pediatric RC as well. In addition, anal spasms and prolonged anal relaxation with small volumes of rectal balloon inflation during anorectal manometry (ARM) may prompt further evaluation with LSMRI as the findings have been correlated with spinal cord abnormalities.41, 42
-
An LSMRI should be performed in pediatric patients with RC associated with physical or neurological signs of spinal anomalies, signs of neurogenic bladder on urodynamics, or when the ARM is abnormal suggesting spinal cord abnormalities (see ARM section below). (Agreement: 8/8).
2.3.4 Contrast enema
The contrast enema (CE) may be used in pediatric RC to screen for Hirschsprung's disease (HD) although its positive predictive value is 65.1% and rectal biopsy is the gold standard.43 Findings may include proximal dilation of the rectosigmoid with increased rectosigmoid ratio (normal is 1:1) and retained contrast following defecation. In addition, children with FC have a larger sigmoid colon diameter than the average population44 and larger sigmoid diameter is correlated with the premature termination of high amplitude propagating contractions (HAPCs) during CM.45 The CE may be most helpful in understanding the caliber of the colon in a pediatric patient with RC.
-
A CE can be used to screen for HD or to assess colorectal anatomy. (Agreement: 8/8).
-
In pediatric patients with significant abdominal distension, a CE can be used to assess the colonic caliber before surgical intervention. (Agreement: 8/8).
2.3.5 Defecography
Defecography has been well defined and validated in adults in the evaluation of defecation dynamics. The study can be performed with fluoroscopy, MRI, or scintigraphy. A recent study using fluoroscopic defecography in children with abnormal CTT reported abnormalities in 53% of patients including pelvic floor dyssynergia and/or structural abnormalities.46 Fluoroscopic defecography may also aid in successful management changes.47 No study has reported the use of MRI defecography in children.
-
Despite its widespread use and extensive data available in adults, there is no evidence to recommend its routine use in children. Defecography can be considered in pediatric patients with abnormal bear-down maneuvers on ARM (see Section 2.4.1) refractory to conventional therapies and/or concerns of anatomic problems of the pelvic floor. (Agreement: 8/8).
2.3.6 Transabdominal ultrasonography
In pediatric patients, transabdominal ultrasonography (TAU) has been primarily used to evaluate the transverse diameter of the rectum to diagnose constipation or fecal impaction.48-50 There are concerns about its lack of reproducibility due to variations in the rectal distention according to defecation51 and unsatisfactory correlation with clinical diagnosis of constipation.52 A systematic review of TAU yielded insufficient evidence for a diagnostic association between clinical constipation symptoms and rectal diameter in children,24 but it has good agreement with detecting fecal impaction.53
-
TAU has a good agreement with DRE to evaluate for fecal impaction but should not be performed in place of DRE. (Agreement: 8/8).
2.4 Manometry studies
2.4.1 Anorectal manometry
ARM is the most performed motility study in children and is used to evaluate the length, resting, and squeeze pressures of the anal canal, rectal sensation, presence and quality of recto-anal inhibitory reflex (RAIR), and recto-anal coordination during simulated defecation. Common indications for ARM include screening for HD, assessing for pelvic floor dyssynergia, and evaluating for postsurgical defecation disorders in children with anorectal malformations. ARM performance and interpretation guidelines including normal values were revised and published by the American Neurogastroenterology and Motility Society in conjunction with NASPGHAN54 and the British Society of Paediatric Gastroenterology, Hepatology, and Nutrition.55
A randomized prospective trial comparing conventional therapy against conventional therapy combined with ARM results in children with severe constipation showed no added clinical value of the ARM on outcome.56 The importance of ARM in RC is likely best defined according to the indication and potential findings.
Anal sphincter resting pressure (ARP) has not been systematically evaluated in RC, but most studies have reported it as within normal range. Pediatric patients with fecal incontinence (FI) have a lower ARP on ARM than patients without FI, although its presence does not predict clinical outcomes.57, 58
Rectal sensation (first sensation, urge to defecate, and pain) is delayed in children actively or previously afflicted with constipation compared to healthy controls.59 Rectal compliance per barostat in children with RC does not correlate with response to treatment,60 suggesting a large rectum and potentially delayed rectal sensation may not be associated with outcomes.
RAIR is the reflexive relaxation of the internal anal sphincter (IAS) elicited by the inflation of a balloon within the rectum. RAIR is defined as a ≥15% reduction in IAS pressure compared to the ARP and subsequent return to the ARP.61
Absent RAIR is observed in patients with HD and anal achalasia61 and can be evaluated with rectal biopsy. Its absence may be seen in patients with megarectum,54 although a recent study showed no correlation between the volume required to elicit the RAIR and transverse rectal diameter on TAU.62
Anal spasms when obtaining a RAIR and prolonged sphincter relaxation with sustained rectal balloon inflation have been seen in higher frequency in children with spinal anomalies.41, 42, 63
Bear-down maneuver (BDM) includes simulated defecation and balloon expulsion test (BET). Recent studies using 3D high-resolution ARM reported a lower percent of anal relaxation in the pediatric FC group compared to historical controls during simulated defecation64 and dyssynergic defecation in 69%–81% of patients aged 5–17 years old (median 8–10 years old) using adult criteria.64, 65 Simulated defecation should be interpreted with caution in pediatric patients due to potential patient lack of understanding, cooperation, or the use of sedation. BET and ARM in children have good correlation66 with abnormal BET in up to 42% of adolescents with pelvic floor dysfunction assessed by ARM.67 No information concerning clinical outcomes with BDM is available.
Squeeze effort in children has conflicting utility. One study demonstrated that children with FI had lower maximum squeeze pressures compared to those without FI,57 while another reported no difference in squeeze pressures.58
-
ARM should be used to screen for the presence of a RAIR. (Agreement: 8/8).
-
If anal spasms and prolonged sphincter relaxation are detected during ARM, an assessment for spinal abnormalities can be considered. (Agreement: 8/8).
-
The BDM performed during ARM can be used as a surrogate for the BET in age-appropriate patients. (Agreement: 8/8).
2.4.2 Colonic manometry
CM is performed with a water-perfused or solid-state catheter lined with multiple pressure sensors. The catheter is placed endoscopically via fluoroscopic-assisted colonoscopy or with fluoroscopy alone.68 The standard protocol takes at least 6 h and can be completed the day of placement; however, if the study is abnormal, the study should be repeated the following day in case of potential anesthesia effect.69 The study includes recording of a fasting period, a meal challenge and post-prandial recovery, and medication challenge with intracolonic bisacodyl.54 CM is considered normal when both a normal gastrocolic response to a meal and normally propagated HAPCs (from cecum to rectosigmoid junction) are present.54 A normal CM has been associated with functional GI disorders.70
CM has been shown to have utility in guiding surgical interventions but not medical therapy in pediatric RC.71 This suggests that CM should be performed after all medical interventions have been exhausted and surgery is being considered. The presence of HAPCs was the most important variable associated with outcome.72
2.4.2.1 Colonic manometry—Antegrade continence enemas
CM has been instrumental in understanding the potential mechanisms of action with antegrade continence enema (ACE) use aside from the mechanical washout of the colon. One RC study with a median age of 9.4 years old (±5.8 years) showed that a 10–20 mL/kg saline infusion into the cecum via the catheter lumen resulted in increased colonic motility compared to baseline, but significantly less than seen with bisacodyl.73 Balloon distention of the proximal colon can elicit HAPCs in some children with normal CM with similar characteristics as the bisacodyl-induced HAPCs.74 A normal response to intracolonic bisacodyl on CM was predictive of a greater likelihood to respond to treatment with ACE than patients with absent HAPCs.75 Colonic motility has also been shown to improve with prolonged use of ACE in patients with RC, with 33%–83% of patients having normalization of colonic motility on follow-up CM.76, 77 Normalization of colonic function was associated with successful discontinuation of ACE.77
2.4.2.2 Colonic manometry—Diverting ostomy
CM is particularly useful in planning the takedown of a diverting ileostomy in patients with RC78, 79 and can potentially guide the timing and the type of surgery. The presence of pancolonic HAPCs and gastrocolonic response may predict improved clinical outcomes of ileostomy takedown without partial colectomy.78
-
CM does not have predictive value to guide medical therapy. (Agreement: 8/8).
-
CM should be performed only after medical therapy has been exhausted and surgical therapy is being considered. (Agreement: 7/8).
-
CM should be used to guide the timing and type of surgery to address RC. (Agreement: 7/8).
-
CM should be used to guide when to perform an ostomy takedown. (Agreement: 8/8).
2.4.3 Wireless motility capsule
The wireless motility capsule allows for the simultaneous evaluation of transit and contractility. It has been extensively evaluated in adult patients and has a strong correlation with CTT by ROM. The study is well tolerated in children and showed a strong agreement with ROM.80 This device is no longer available from the manufacturer.
-
There is insufficient data to recommend the use of the wireless motility capsule as a routine test for RC. (Agreement: 8/8).
2.5 Rectal biopsy
Rectal biopsies are not routinely performed in patients with RC and are indicated exclusively in patients with a suspected diagnosis of HD including patients with an equivocal or no RAIR on ARM.
-
Rectal biopsies should not be used routinely in patients with RC and are indicated exclusively in patients with a suspected diagnosis of HD. (Agreement: 7/8).
Q3: What is the effectiveness of nonpharmacologic treatments for children with refractory constipation?
Patients and their families frequently seek out nonpharmacologic treatment options due to preference and perceived lack of response to prescribed pharmacologic therapy. Common nonpharmacologic options include botanicals, dietary changes and supplements, pelvic floor physical therapy (PFPT), behavioral therapy, and occupational therapy.81
2.6 Pelvic floor physical therapy with or without biofeedback
The overall goal of PFPT with or without biofeedback is to improve detection of rectal distention from stool and to improve control of the pelvic floor muscles and external anal sphincter (EAS); thereby, decreasing FI and enhancing voluntary defecation. One randomized controlled trial (RCT) in children with FC demonstrated pelvic physiotherapy combined with conventional medical therapy was more efficacious at 6 months when compared to conventional therapy alone (92.3% vs. 63.0%).82 Other RCTs have not demonstrated long-term improvement with PFPT with or without biofeedback in children with FC.83, 84 Studies for the efficacy of PFPT in children who meet criteria for RC are lacking, which could be in part due to variations in clinical practices.
-
PFPT can be used in pediatric patients with demonstrated dyssynergic defecation on ARM. (Agreement: 8/8).
-
PFPT can be used for patients with RC. (Agreement: 7/8).
2.7 Behavioral therapy
Children can develop anxiety, fear, and pain with defecation which may contribute to the development of FC.81 Children with FC can be afraid of toileting, resulting in lower self-efficacy. One study illustrated improved self-efficacy scores in children who responded to constipation management,85 suggesting a possible target for behavioral therapy. Another RCT demonstrated no significant clinical improvement in FC with age-appropriate behavioral therapy (parental education and behavioral play therapy) and conventional therapy (PEG with rectal therapy as needed) compared to conventional therapy alone.86
-
Behavioral therapy has no clearly defined role in the management of children with RC in RCTs. (Agreement: 8/8).
Question 4: What are the pharmacologic treatment options for maintenance therapy in pediatric patients with refractory constipation?
There are limited studies on medication use for pediatric RC. The following literature review primarily originates from adult FC studies. The discussed medications have not been approved by the Food and Drug Administration (FDA) for use in pediatric patients except for linaclotide. Please reference Table 3 for dosing recommendations on the medications discussed below. The typical practice of dosing for the stimulant laxatives is to start low and increase to the maximum dosing to achieve improved stooling frequency and resolution of fecal incontinence if present.
2.8 Oral high-dose stimulant laxatives
2.8.1 Senna/sennosides
Sennosides, a plant derivative anthraquinone laxative, has long been used to treat constipation because of its low toxicity, cost-effectiveness, and high accessibility. There are no pediatric RCTs to demonstrate its effectiveness or long-term side effects, and most observations are derived from comparative or open-label studies. No dosing guidelines exist; however, common practice is delineated in Table 3. Sennosides should be administered 6–12 h before the goal time for the bowel movement (BM) for optimal success.81 Stomach cramping and diarrhea are the most common side effects, although contact dermatitis can occur as well. Sennosides-induced dermatitis occurs primarily in patients on high-dose sennosides and with prolonged stool-to-skin exposure (i.e., diapered) leading to perianal blisters.12 An analysis on the use of sennosides did not find overwhelming evidence of tolerance development and considered it a safe treatment alternative for FC.12 While pediatric RCTs on the use of sennosides in RC are lacking, it is the author's expert opinion that the use of a stimulant laxative such as sennosides is beneficial in RC treatment.
-
High-dose sennoside is a mainstay of management of RC and should be optimized for the individual patient before considering further management options. (Agreement: 7/8).
2.8.2 Bisacodyl
Bisacodyl is a stimulant diphenylmethane laxative that acts directly on the intestinal myenteric plexus to increase intestinal motility and decrease fluid absorption to promote BMs. There is no large pediatric randomized double-blind placebo-controlled (RDBPC) trial on the effectiveness of bisacodyl. There is a large multicenter adult RDBPC trial that showed a higher number of complete spontaneous bowel movements (SBMs) weekly with bisacodyl compared to placebo.87 In patients aged 0.9–21 years old (median age 9.45 years) with RC, the median number of BMs doubled after the initiation of bisacodyl. After following up long term, 55% of patients were successfully weaned off bisacodyl with a median time of 18 months. GI upset was reported in 9% of patients, and there were no complications with long-term bisacodyl use in the pediatric population.10 RCTs are needed to further evaluate the safety and efficacy of bisacodyl, yet it is the author's expert opinion that the use of stimulant laxatives such as bisacodyl is beneficial in the treatment of pediatric RC.
-
High-dose bisacodyl is a mainstay of management of RC and should be optimized for the individual patient before considering further management options. (Agreement: 7/8).
2.9 Secretagogues
Over the last few years, more data has been released on the safety and efficacy of secretagogues in the treatment of constipation in the pediatric population. Current evidence (further discussed below) demonstrates modest improvement in symptoms with secretagogue use alone. Per the author's expert opinion, secretagogues are beneficial in conjunction with stimulant use in the treatment of pediatric RC.
2.9.1 Linaclotide
Linaclotide is a guanylate cyclase-C receptor agonist that increases intestinal fluid secretion, accelerates intestinal transit, and decreases visceral pain. Data on the efficacy and tolerability of linaclotide in pediatric patients is limited.88 A retrospective study of 60 children with a median age of 13.9 years old with FC showed that 45% had a positive clinical response from the initiation of linaclotide at the first follow-up (median 2.5 months). Reported constipation decreased from 83% to 64% and median BM frequency increased from 4 to 7 per week. However, 18% of patients stopped using linaclotide due to adverse events.89 The safety profile of long-term use of linaclotide has yet to be determined in pediatric patients. Linaclotide has received FDA approval for use in ages 6–17 years with FC. Linaclotide can cause excessive diarrhea which may lead to its discontinuation. This medication is contraindicated in patients with concern for intestinal obstruction and in children younger than 2 years of age.90
2.9.2 Plecanatide
Plecanatide is the newest secretagogue compound that has been approved by the FDA for the treatment of adults with chronic idiopathic constipation. It is a guanylate cyclase agonist that is a structural analog of human uroguanylin, a cGMP activator with a similar mechanism to linaclotide to increase intestinal fluid secretion, accelerate intestinal transit, and decrease visceral pain.91, 92 Several RCTs of 12 weeks duration in adults with chronic idiopathic constipation showed improved efficacy of plecanatide over placebo.93, 94 There are no pediatric RCTs. Pediatric studies are needed to better understand the adverse effects, dosing regimens, and long-term safety profile of plecanatide. This medication is contraindicated in patients with known or suspected mechanical intestinal obstruction and in children younger than 6 years of age due to risk of severe dehydration. Plecanatide is not FDA approved for children less than 18 years of age.95
2.9.3 Lubiprostone
Lubiprostone is a prostone, a bicyclic fatty acid metabolite of prostaglandin E1. It activates a chloride channel (ClC-2) in the GI tract to enhance intestinal fluid secretion passively without stimulating intestinal smooth muscles.96 In adults, lubiprostone significantly improves constipation symptoms in Phase 3 RCTs.97 Conversely, a pediatric RDBPC trial found no statistically significant difference in SBM frequency comparing lubiprostone to placebo. The 12 and 24 μg twice daily doses were well tolerated by subjects in the double-blind and extension phases, with a similar safety profile seen in adult studies.98 Pediatric studies are needed to better understand adverse effects, dosing regimens, and long-term safety profile of lubiprostone. This medication is contraindicated in patients with known or suspected mechanical intestinal obstruction. Lubiprostone is not FDA approved for children less than 18 years of age.99
-
A secretagogue should be considered as an adjunct to a high-dose stimulant laxative when treating RC with poor response to optimized high-dose stimulant laxatives or when high-dose stimulant laxatives are not tolerated. (Agreement: 8/8).
2.10 Serotonin agonists
2.10.1 Prucalopride
Prucalopride is a dihydro-benzofuran-carboxamide derivative and novel serotonin 5-HT4 agonist that promotes GI motility. Given its highly selective affinity to only 5-HT4, the risk of target-unrelated side effects like cardiac toxicity is theoretically minimized when compared to cisapride and tegaserod.100, 101 Prucalopride has been shown to be well tolerated by toilet-trained pediatric patients with FC, but it is no better than placebo in improving weekly SBMs or minimizing fecal incontinence.102 Long-term data evaluating the safety and efficacy of prucalopride in the treatment of pediatric FC are still warranted. Prucalopride is not FDA approved for children less than 18 years of age. This medication is contraindicated in patients with prucalopride hypersensitivity reactions, intestinal perforation or obstruction, obstructive ileus, inflammatory bowel disease, and toxic megacolon. Patients starting prucalopride should be monitored for suicidal thoughts or behavior and new onset or worsening depression.103
-
Prucalopride should be considered as an adjunct to a high-dose stimulant laxative when treating RC with poor response to optimized high-dose stimulant laxatives. (Agreement: 7/8).
2.10.2 Tegaserod
Tegaserod is a serotonin 5-HT4 agonist that promotes GI motility in patients with constipation that was withdrawn from the market in the United States due to serious adverse events.
-
We do not recommend the use of tegaserod in pediatric patients. (Agreement: 8/8).
Question 5: What are the maintenance options for retrograde therapy in pediatric patients with refractory constipation?
Retrograde therapy options include suppositories, small-volume enemas, and large-volume retrograde irrigation. Commercially available suppositories are often glycerin or bisacodyl, while small-volume retrograde enemas are typically sold with sodium phosphate (saline enema), mineral oil, or bisacodyl as the active ingredient. There is no published data on their effectiveness in pediatric RC management, but bisacodyl suppositories and enemas (Table 3) are commonly used. Phosphate-containing solutions should not be used chronically due to the risk of rectal retention resulting in electrolyte derangements (hyperphosphatemia, hypocalcemia, and hypokalemia),104, 105 colitis, and spastic left-colon syndrome.106, 107
Large-volume retrograde enemas can be administered via catheters with or without a balloon. Also, there are several retrograde irrigation devices for the administration of high-volume enemas to facilitate colonic emptying.108 In children with normal anorectal anatomy, the catheter is inserted through the anus into the rectum and, if present, the balloon is inflated to hold the catheter in place. The irrigation solution is administered via gravity, manual pump, or electric pump depending on the type of retrograde irrigation device. Once the solution is administered, the catheter is removed, and the colonic contents are expelled.
No RCTs evaluate the effectiveness of retrograde irrigations. A 2017 consensus review reported best-practice recommendations regarding the indications, patient selection, treatment regiments, troubleshooting, and practical aspects of retrograde irrigations.109 Commonly used retrograde irrigation solutions are tap water and normal saline (NS). Some centers prefer NS enemas over tap water for patients with mega-rectosigmoid and/or colonic dysmotility due to the potential for iatrogenic hyponatremia with rectal retention of the solution. It is important to note that some commercially available retrograde irrigation devices are approved for tap water use only. As with ACEs, additives, including glycerin, castile soap, and bisacodyl, may be mixed with the irrigation solution to facilitate colonic emptying. Additives are considered “off-label” due to limited data and variable practice among centers.109
Common retrograde irrigation complications include pain with catheter insertion, catheter expulsion, balloon breakage, solution leakage, and abdominal pain. Bowel perforation following rectal irrigation is estimated at <0.002%.110, 111 Based on expert opinion, if retrograde enemas are used for maintenance therapy, provider-directed large-volume enemas or bisacodyl enemas are recommended over commercially purchased sodium phosphate enemas.
-
We do not recommend the use of sodium phosphate enemas for the maintenance treatment of pediatric RC (Agreement: 8/8).
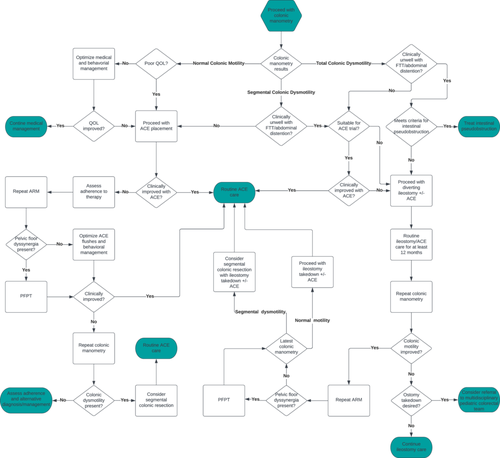
Question 6: What is the effectiveness of surgical interventions in pediatric patients with refractory constipation?
Surgical management for children with RC has been an area of debate and controversy primarily due to the lack of a universal definition of RC in surgical literature and the constant advancement of surgical procedures. Despite these challenges, there are some prior studies to help guide management in this population.
2.11 Anal dilation, IAS myectomy, and anal botulinum toxin injection
IAS myectomy lacks evidence to guide its use, but there is some data on the role of anal dilation and anal botulinum toxin injection (ABTI).
2.11.1 Anal dilation
While sedated anal dilation is a historically common treatment option for pediatric RC, there has been no clinical advantage demonstrated for anal dilation with medical management when compared to medical management alone.112 Possible anesthesia complications, risk for loss of anal sphincter tone, FI, and lack of efficacy in the literature have all contributed to a decreased use of anal dilation.
2.11.2 IAS myectomy
IAS myectomy was another common historical surgical option for treatment of pediatric RC, but it is irreversible and can result in permanent FI. The procedure has been shown to reduce symptom severity in RC but is less efficacious than ABTI.113 Due to the risk for permanent complications when compared to ABTI, routine use of IAS myectomy has declined.
2.11.3 Anal botulinum toxin injections
Botulinum toxin can be injected into the IAS and EAS, decreasing muscle tone by impairing nerve signaling. This neurotoxin has been used with RC, IAS achalasia, and HD. Common practice is to use Botox® 6 units/kg (maximum 100 units) diluted in 2–5 mL of normal saline and divided evenly into four injection sites. Results from several studies assessing the response in patients with RC to ABTI have been varied, with some demonstrating clinical improvement and others showing no superiority when compared to medical therapy alone.113-115 There is more consistent data supporting the use of ABTIs in anal achalasia.61, 116 Complications from ABTI are rare.117 ABTI may be more relevant in patients with RC with withholding behavior who are unable to engage in other therapies, like psychological interventions, PFPT, and biofeedback.
-
The use of anal dilation and anal sphincter myectomy is not recommended for use in patients with RC. (Agreement: 8/8).
-
ABTI into the IAS is beneficial in patients diagnosed with IAS achalasia. (Agreement: 8/8).
-
There is no clear role of ABTI in the treatment of patients with RC without a diagnosis of IAS achalasia. (Agreement: 7/8).
2.12 Antegrade continence enemas
Retrograde enemas are less invasive than surgery in the treatment of RC, yet some children struggle to tolerate them. ACE is a surgical alternative that has been shown to improve fecal impaction incidence, frequency of stooling, and FI. These benefits may be aided by an intensive bowel management program to establish an enema with the correct volume and stimulant content if required for effective emptying.106, 118, 119 Concerns have existed regarding the effectiveness of ACE in sub-groups of patients. Behavioral concerns and pelvic floor dyssynergia have been used as relative contraindications for ACE; however, two recent publications have shown that ACE is highly effective in these groups.120, 121 ACE may also provide increased independence for pediatric patients over retrograde enemas. ACE has been shown to improve colonic dysmotility, likely due to improved colonic emptying.76 For patients with partial or segmental dysmotility, ACE combined with PFPT and biofeedback have been shown to be very effective.122 The two most common surgical options for the placement of an ACE device are appendicostomy and cecostomy. In patients with an unavailable appendix, a cecal flap neo-appendicostomy can be fashioned to create a channel that can be catheterized. Both procedures can be done with minimally invasive techniques with good outcomes,123, 124 but there are risks and benefits to each ACE procedure that must be considered and discussed with patients and caregivers.
-
Indications for consideration of surgical placement of ACE include improving autonomy in patients regularly using retrograde enemas, the failure to adequately treat RC after maximizing pharmaceutical options, and the inability to use oral or rectal therapies in the treatment of RC. (Agreement: 8/8).
2.13 Sacral nerve stimulation
Most studies reviewing the benefits of sacral nerve stimulation (SNS) include patients with organic causes of constipation as well as FC. The exact mechanism of improvement with SNS has not yet been demonstrated. A recent pediatric study compared outcomes with ACE and SNS, which showed FI was improved with SNS when compared to ACE. Stool frequency and ability to wean laxatives were superior in patients with ACE.125 Medical providers must consider the high rate of complications with SNS placement. One study showed that 40% of patients experienced pain, neurologic symptoms, wound infections, or rash following the procedure, with the same proportion of patients requiring reoperation.126
-
SNS is a treatment option in select children with RC and may be considered as an adjunct treatment. (Agreement: 8/8).
2.14 Colonic diversion and colectomy
2.14.1 Segmental colonic dysmotility
There is significant variation in the treatment of patients with colonic dysmotility due to concomitant dyssynergic defecation.127 A recent multicenter study by the Pediatric Colorectal and Pelvic Learning Consortium reviewed the treatment of pediatric patients (median age of 9.6 years) with segmental colonic dysmotility with RC and found that 92% of patients avoided colonic resection after treatment with ACE. The majority of patients (7/8) who failed treatment with ACE underwent a subsequent colonic resection, and the need of such intervention was higher in those without pelvic floor dyssynergia.128, 27 Per expert opinion, medical management with possible PFPT should be considered before colonic resection in patients with segmental colonic dysmotility and pelvic floor dyssynergia. As previously discussed (see Section 2.4.2), colonic motility improves following prolonged use of ACE.77 Regardless, per expert opinion, the placement of a diverting ileostomy can be considered in patients with segmental dysmotility who are intolerant of ACE. Distinctly, per expert author opinion, patients who have known segmental colonic and concomitant symptoms of failure-to-thrive and/or symptomatic abdominal distension may benefit from a diverting ileostomy.
-
For segmental colonic dysfunction, an ACE trial should be performed before colonic resection. Patients may have recovery of segmental dysmotility with appropriate ACE use. (Agreement: 8/8).
-
Patients who have ongoing difficulties with the management of their RC with ACE should be further evaluated and treated for other conditions (including pelvic floor dysfunction) before the consideration of a colonic resection. (Agreement: 7/8).
-
Patients diagnosed with segmental colonic dysmotility who do not tolerate ACE may benefit from a diverting ileostomy. (Agreement: 7/8).
-
Patients diagnosed with segmental colonic dysmotility and failure-to-thrive and/or abdominal distention may benefit from a diverting ileostomy. (Agreement: 7/8).
2.14.2 Total colonic dysmotility
Surgical options for pediatric patients with total colonic dysmotility include ACE placement or primary diverting ileostomy. Patients with malnutrition or significant colonic distention may benefit from a primary diverting ileostomy over ACE and may also require additional testing to screen for chronic intestinal pseudo-obstruction.
Timing for eventual ileostomy takedown needs to be individualized. If dyssynergic defecation is present, it should be addressed. If unable to improve dyssynergic defecation, consider maintaining the ileostomy until it is treated. Improved colonic motility during CM can be detected 6–12 months following diverting ileostomy. The etiology for the recovery in function has not been elucidated.76 Placement of ACE at time of ileostomy takedown should be considered to aid with independent stooling. If there is ongoing significant partial colonic dysmotility on follow-up CM, options that should be considered include segmental colonic resection, Deloyers-type extended colectomy, or sub-total colectomy with ileo-rectal anastomosis. These decisions and surgical risks should be balanced with possible successful bowel management with antegrade or rectal enemas.129
-
Patients diagnosed with total colonic dysmotility and failure-to-thrive and/or abdominal distention may benefit from a diverting ileostomy. (Agreement: 8/8).
-
The placement of ACE should be considered to be performed at the same time as a diverting ileostomy takedown. (Agreement: 7/8).
-
A CM should be repeated at least 12 months after the placement of a diverting ileostomy to help guide management before ileostomy takedown. (Agreement: 8/8)
-
An ARM study should be considered before ileostomy takedown to assess for pelvic outlet obstruction. (Agreement: 7/8).
Question 7: What are the recommended pharmaceutical options for antegrade continence enema flushes?
No RCTs demonstrate the optimal administration of ACE regarding duration, frequency, or solution recipes.130 Commonly used irrigation base solutions for daily ACE use in pediatrics are NS and PEG with electrolytes (Table 4).131-137 Patients should avoid softened tap water due to increased sodium content.131 NS can be obtained from a pharmacy or prepared at home by mixing 1.5 teaspoons of noniodized salt with 1000 mL of tap water. Parents should be educated on the correct measurement technique to prevent hyper- or hyponatremia.106 PEG with electrolytes can be used as the initial irrigation solution or alternatively upon failing NS irrigations.132, 133, 135, 136
| Solution | |
| NS | 10–30 mL/kg |
| Polyethylene glycol | 17 g per 240 mL flush solution |
| Additives | |
| Glycerin | Start with 5% concentration and can increase to maximum of 15% (i.e., 20 mL glycerin/400 mL of NS to 60 mL glycerin/400 mL NS) |
| Castile soap | Start with 5% concentration and can increase to maximum of 18–20 mL |
| Bisacodyl | 0.2 mg/kg/dose up to 20 mg
|
- Abbreviations: ACE, antegrade continence enema; NS, normal saline.
Commonly used flush additives include glycerin, castile soap, and bisacodyl (Table 4), although there are no established guidelines regarding dosing or administration.135-137 Additives may be administered before or after the flush, or be mixed with the base solution to facilitate colonic emptying. The use of additives has been shown to improve overall success with ACE.134, 136, 137 Optimal additive concentrations in the base solution vary in the literature and among centers. Caution should be taken when increasing the concentration of castile soap and glycerin given their association with chemical colitis.138 Again, phosphate-containing solutions should be avoided due to the possible development of electrolyte derangements,104, 105 colitis, and spastic left-colon syndrome.106, 107
2.15 Weaning and discontinuing the antegrade continence enema
To date, there are no studies that assess how to successfully wean and discontinue the use of ACE in pediatric RC. Short- and long-term data on ACE outcomes are limited due to the heterogeneity of patients. In patients with FC refractory to medical therapy on successful ACE treatment, 10%–42% discontinued ACE use 1.2–8.8 years after placement.121, 122, 132, 133, 135, 136, 139, 140
Colonic motility has been shown to improve after ACE. In a heterogeneous group of seven patients who underwent ACE placement, the CM normalized in five patients at a median time of 30 months. Two patients discontinued the use of ACE and three underwent progressive wean at time of publication.76 One study used CM before and after ACE placement to demonstrate factors associated with the successful decrease and discontinuation of the device. The study reported a significant improvement in multiple CM parameters with normalization of motility in 13 out of 40 patients at a median time of 19 months. Normalization of HAPCs and older age predicted a decrease in the use of ACE. Older age was the only predictive factor in the discontinuation of the ACE.77 The timing of colonic motility recovery is not known.
-
Weaning of ACE should be considered in patients who are clinically stable on antegrade flushes. (Agreement: 8/8).
-
Repeat CM should be considered in patients who are not able to wean or discontinue the use of ACE. (Agreement: 8/8).
Question 8: What is the prognosis for children with refractory constipation?
No longitudinal studies were identified that evaluate the long-term prognosis of children with RC. Looking at pediatric FC data, a 2010 systematic review reported 44% of patients remained symptomatic after 5–10 years of laxative use141 with another study finding 25% of children having symptoms into adulthood.6 After a mean follow-up of 2.8 years, a retrospective study including 79 pediatric patients with RC reported 48.1% on three or more treatment modalities, 5.1% requiring high volume retrograde enemas, and 3.8% undergoing ACE placement4 demonstrating the escalation of therapy needed over time in RC.
From a HRQoL standpoint, symptoms of poorly controlled constipation into adulthood have been shown to negatively affect HRQoL such as general health, social contact, and intimacy in adults.142 To date, there are no longitudinal studies evaluating the HRQoL in pediatric RC. A 2010 systematic review and meta-analysis of 20 pediatric studies reported that children with FC have a significantly lower HRQoL (self-report and parent proxy-report) when compared to their healthy controls. While HRQoL scores were comparable to those with IBS, they were lower than children with IBD and GERD.5 A multicenter study on children with FC and FI had significantly decreased QoL when compared to children with FC alone.143 QoL in children with RC and FI have been shown to improve following treatment with ACE144, 145 and retrograde irrigation system.146
Question 9: How can refractory constipation be prevented?
No studies investigate how to prevent RC, but some studies have explored risks for ongoing constipation despite typical therapeutic interventions. Potential prognostic symptoms for poor response to therapy include the presence of constipation before 1 year of life, duration of symptoms greater than 3 months before presentation, infrequent stooling, FI, nocturnal enuresis, large diameter stools, and fecal mass present in the abdomen and/or rectum.147 The presence of a megarectum is often found in imaging in patients with RC.148 In addition, some patients with RC have been found to have dyssynergic defecation (21%–31%) on ARM, colonic inertia (13%–50%) on colonic transit studies, partial or complete absence of HAPCs on CM, or absent gastrocolic reflex on CM.148, 149 Additional studies are warranted on the prevention of pediatric RC.
-
Early intervention with daily stimulant laxatives in the treatment of FC is encouraged to try to prevent the disease progression from FC to RC. (Agreement: 8/8).
-
Delaying proper treatment by persistently searching for an underlying etiology of patient's constipation, minimizing symptoms, or avoidance of stimulant laxatives due to concern for dependence may result in long-term complications from RC. (Agreement: 8/8).
Question 10: What is the recommended treatment pathway for pediatric refractory constipation?
Question 11: What research is needed for the care of children with refractory constipation?
Over the last decades, our knowledge of colonic and anorectal function has improved; however, management and patient outcomes have had limited progress. Pediatric RC is often multifactorial making its evaluation and treatment more complicated.
Advancement is needed in the evaluation of RC. Current testing options are limited by requiring the cooperation of pediatric patients for uncomfortable studies such as unsedated ARM, BET, and CM. Studies are needed to investigate the role of less invasive tests like CTT beyond guiding the timing of more advanced, invasive testing like CM. The role of ARM in understanding defecation dynamics in children needs to be better elucidated. It is paramount to define the utility of diagnostic tests to avoid unnecessary interventions.
In the treatment of pediatric patients with RC, RCTs are needed to guide when to use specific constipation medications over others based on history and exam, assess the safety and efficacy of long-term laxative use, and investigate the safety, efficacy, and outcomes of secretagogues or serotonin agonists alone or in combination with stimulant laxatives. Further assessment of the benefit of transcutaneous tibial nerve stimulation, protocolization of PFPT with and without feedback, and employment of occupational therapists would also help advance the field. From a surgical standpoint, we need to develop biomarkers associated with colonic distention and dysfunction to better understand the indications of surgical interventions in RC, refine the identification and long-term management of diverting ileostomies, and protocolization of tapering off and discontinuing ACEs.
While this paper presumes there is a progression to pediatric RC from prolonged, inadequate treatment of FC, in practice we know that this is not always the case. Pediatric patients with FC would greatly benefit from longitudinal studies to determine the natural history, risk factors, and prognosis for RC. Longitudinal studies would also allow pediatric gastroenterologists to further categorize pediatric RC by those who would benefit from medical intervention alone and those who need earlier surgical management.
3 LIMITATIONS
There are many limitations to this paper. The major limitation is in our search methods due to the lack of a clear definition for pediatric RC. In addition, much of the current literature referenced is retrospective and involves sample size requiring the inclusion of author expertise and experience. Due to the lack of consensus for the definition of pediatric RC in current literature, an organized framework like Grading of Recommendations, Assessment, Development, and Evaluations approach could not be utilized for this position paper. Additionally, the absence of a common definition of RC has limited the creation of predictive models through longitudinal studies that could better elucidate the etiologies, optimal treatment strategies, and outcomes of RC.
4 CONCLUSION
Constipation is one of the most common reasons for referral to pediatric gastroenterology. Patients who do not respond to treatment are frequently diagnosed with RC, but their management has been challenging due to lack of consensus about the definition. As a result, there is variability in the evaluation and management of RC among pediatric gastroenterologists. This article is an attempt to provide a standardized definition of pediatric RC and establish a common management pathway for this complex patient population. Timely identification of pediatric RC (Table 2) combined with consistent use of stimulant laxatives (Table 3) and use of common management strategies (Figures 1 and 2) may improve QoL for pediatric patients. Finally, a standardized pediatric RC definition will benefit the advancement of evaluation and management through future targeted studies.
CONFLICT OF INTEREST STATEMENT
Dr. Anil Darbari is on the speaker's bureau for Abbott Nutritionals, which is unrelated to this topic. Dr. Leonel Rodriguez is a reviewer and writer for UpToDate and on the advisory board for Neuraxis, which is unrelated to this work. The remaining authors declare no conflict of interest.




