Awareness, referral and age at Kasai surgery for biliary atresia in Europe: A survey of the Quality-of-Care Task Force of ESPGHAN
See related article, pages 1207-1209.
Disclaimer: Although this paper is produced by the ESPGHAN Quality of Care Task Force, it does not necessarily represent ESPGHAN policy and is not endorsed by ESPGHAN.
Abstract
Objectives
To identify infants with biliary atresia (BA), European Society of Paediatric Gastroenteroloy and Nutrition (ESPGHAN)/North American Society of Pediatric Gastroenteroloy and Nutrition (NASPGHAN) guidelines recommend measurement of conjugated/direct bilirubin in infants with prolonged jaundice and using a stool colour card (SCC). The ‘Quality of Care’ Task Force of ESPGHAN performed two surveys to assess current case finding for BA and age at Kasai portoenterostomy (KPE).
Methods
The first survey approached 26 European hepatology centres to report age at referral and age at KPE of all infants diagnosed with BA from 2015 to 2019. The second survey targeted paediatricians in France to assess awareness and compliance with the recently introduced SCC.
Results
Data from 785 patients with BA from 18 centres in 15 countries revealed a mean age at referral to tertiary centre of 55 days (median 53, IQR 48–60) (n = 636). The mean age at KPE was 61 days (median 60; IQR 54–67) (n = 772). For 6% of patients, cirrhosis was too advanced for surgery.
Of 392 paediatricians answering the second survey, 53% felt familiar with the target diseases, 80% correctly identified cholestasis and 59% always inquired about the infant's stool colour. If abnormal, 93% would order blood tests and 85% call for advice. The SCC screening was considered helpful for case finding and improving knowledge of cholestatic diseases by 62% and 45% paediatricians, respectively.
Conclusions
Referral of infants for KPE remains late, indicating low adherence to search for cholestasis in icteric infants by age 2−3 weeks. Knowledge and structures need improvement to allow earlier guideline conform case finding, diagnosis and therapy.
Highlights
What is Known
-
Timely diagnosis and Kasai hepatoportoenterostomy in infants with biliary atresia (BA) is vital to improve survival and to avoid complications and liver transplantation.
-
Prolonged jaundice (>2 weeks) in exclusively breastfed babies is common and mostly benign. The risk of missing cholestatic diseases without structured screening is high.
-
The European Society of Paediatric Gastroenteroloy and Nutrition (ESPGHAN)/North American Society of Pediatric Gastroenteroloy and Nutrition (NASPGHAN) guidelines recommend to measure total/conjugated bilirubin in all neonates with prolonged jaundice, if breastfed latest by 3 weeks of age.
What is New
-
Our survey covering >50% of infants diagnosed with BA from 2015 to 2019 in the European Union, UK and Switzerland disclosed a mean age at Kasai surgery of 60 days, similar to results obtained in Europe 10–30 years earlier.
-
Screening with a stool colour card improves awareness, but additional efforts are required to reduce the age at Kasai surgery below 30 days.
1 INTRODUCTION
Biliary atresia (BA) is an idiopathic congenital disease resulting in progressive fibrosclerosing obliteration of the large extrahepatic and then intrahepatic bile ducts, beginning prenatally with rapid progression after birth.1 Although rare, affecting 1/5000 new-borns in Taiwan to 1/15,000 to 1/20,000 in Europe, Canada and the USA, BA is the most common indication for liver transplantation in childhood, and the leading hepatic cause of liver failure and death.1 Early Kasai portoenterostomy (KPE) aims to reestablish the bile flow, and suspend or slow down the development of biliary cirrhosis. The earlier the surgery is performed, the better is the survival rate with the native liver without the need for transplantation.2-4
BA presents with prolonged jaundice, yellow as opposed to colourless urine (a common feature to all cholestatic disorders), and pale (acholic) stools in otherwise entirely well infants. Since the early 90s, public awareness has been raised about the importance of identifying new-borns with cholestasis by 2−3 weeks of age among those with prolonged jaundice, and referring them to a specialist centre for investigation and treatment, including vitamin K injection to avoid haemorrhage.5-7 In spite of several educational programmes, the average age at KPE did not significantly change over 20 years, and remained around 60 days of life, both in the USA8, 9 and Europe.2, 4, 10
The joint evidence-based guidelines of European Society of Paediatric Gastroenteroloy and Nutrition (ESPGHAN) and North American Society of Pediatric Gastroenteroloy and Nutrition (NASPGHAN) from 2017 on the evaluation of cholestatic jaundice in infants, recommend the measurement of total and conjugated (direct) bilirubin in non-breastfed neonates with visible jaundice at 2 weeks of age, and no later than 3 weeks of age if breastfed.11 An elevated serum conjugated bilirubin level (>1 mg/dL or >17 µmol/L) warrants timely referral to a paediatric liver specialist. The above cut off is highly sensitive to detect BA in infants between 3 and 60 days of age.12 In practice, this recommendation is not followed. In 2020, ESPGHAN council initiated a Quality of Care (QoC) task force initiative, with the main goal to survey the implementation of diagnostic and management ESPGHAN guidelines. If deficits are identified, targeted education should further promote the recommendation in clinical practice. The Hepatology Committee of ESPGHAN selected early case finding of infants with BA as the first topic. To date neonatal dried blood spot screening is not available for this condition, although a pilot study was promising measuring matrix metalloproteinase-7 (MMP-7) levels in BA and control patients from stored dried blood spots taken within the first 3 days of life.13, 14 We aimed to assess the age of referral for possible KPE in a representative sample of infants and providing the rationale for referring infants for possible BA as early as possible given the improved outcome if KPE is performed before 4 weeks of age.4
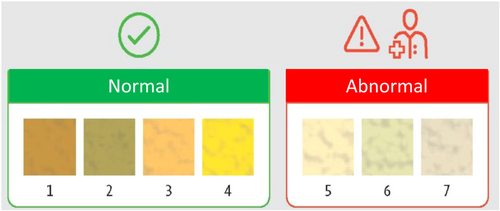
In contrast to jaundice, an acholic stool is quite specific for obstructive cholestasis. The stool colour card (SCC picturing normal and abnormally pale or depigmented stools) (Figure 1) is noninvasive, cost effective, simple and suitable for mass screening.15 A systematic review and meta-analysis of intervention trials concluded that the SCC reduces the age at KPE to around 60 days in countries with a pre-intervention age of more than 70 days at surgery.13 The SCC was included in the items systematically assessed during the ‘well baby visits’ of the first weeks of life, first in Taiwan in 2002, followed by Japan, China, Canada and recently in Switzerland, France and Germany.13, 16-23 In Taiwan, the SCC improved the prognosis of infants with BA due to timely surgery.21 Screening induced anxiety in parents was not demonstrated in a Swiss survey after SCC introduction.24
Here we report the results of two surveys as initial steps of the QoC Task Force activity on BA: first, to assess the age of referral and KPE in a representative sample of infants with BA in Europe; second, to survey the visibility and acceptance among French paediatricians of the recently initiated screening for BA with the SCC.
2 METHODS
2.1 First survey: Age at referral and Kasai hepatoportoenterostomy
Hepatologists of the QoC Task force developed the questionnaire which was applied by Surveymonkey®. The survey targeted paediatric hepatologists working in 26 tertiary centres for paediatric liver diseases in 22 different European countries. At least one paediatric hepatologist in each country received the survey. The survey was sent out twice (August and December 2020) to improve the response rate.
The following items were requested: number of infants with newly diagnosed BA, including the isolated form and BA with splenic malformation syndrome, during a 5-year period (2015–2019), either in their centre (partial) or if data were available all diagnosed infants in the country (total); the infants' mean age at referral to the hospital and at Kasai operation; the organization for Kasai surgery in their country (centralized or not), the number of KPE performed per year at their centre (<5 or 5 to <15 or ≥15), how many of newly diagnosed infants with BA per year did not undergo KPE because of advanced cirrhosis or too old age, and whether the centre performed paediatric liver transplantation. The survey also included general information about management of neonates: age (weeks) of the first recommended postnatal health check visit; title of the visiting person (neonatologist, general paediatrician, general practitioner, midwife or specialized nurse); if an infant is still jaundiced at discharge from maternity, whether only total or also conjugated bilirubin is measured at discharge, and whether a follow-up visit with bilirubin measurement is foreseen. Finally, it was asked whether in their country a protocol or guidelines exists for jaundiced babies.
2.2 Second survey: Perceived usefulness of the SCC by paediatricians
The second survey explored the acceptance und perceived usefulness of the SCC in paediatricians in France, where it had been implemented 2 years before. The SCC is included in the child's health booklet, handed out when the mother is discharged from the maternity unit. The anonymous survey was sent once via Surveymonkey® to French general paediatricians through the electronic mailing lists of their two associations [Société Française de Pédiatrie (SFP), Association Française de Pédiatrie Ambulatoire (AFPA), France]. The eight questions in the survey aimed to record perceptions of the SCC, and how the card affected their approach towards a jaundiced infant.
2.3 Statistical analysis
We presented frequencies (N) and percentages (%) for categorial variables, mean (standard deviation) and median (interquartile range, IQR) for continuous variables to summarize survey responses both on a centre-specific and country-specific basis. Due to different patient numbers reported by centres, we used a weighted mean that involves specific weights to the patient numbers of each centre for a more balanced analysis.
Statistical analysis was performed using SAS 9.4 (Statistical Analysis Software; SAS Institute Inc.) and GraphPad Prism 9.4.1 (GraphPad Software LLC).
3 RESULTS
3.1 First survey: Age at referral and Kasai hepatoportoenterostomy
The questionnaire was sent to 26 centres of paediatric Hepatology in 22 European countries, thereof 18 (69%) from 15 countries answered [Austria, Belgium, France, Germany, Greece, Hungary, Ireland, Italy (three hospitals), Poland, Serbia (two hospitals), Spain, Sweden, Switzerland, The Netherlands and United Kingdom]. Sixteen centres provided data on age of Kasai surgery, thereof 14 reported also the age of referral to their centre. Seven centres (France, Hungary, Ireland, Poland, Switzerland, The Netherlands and UK) could provide data on infants with BA for the whole country during the 5-year period, while 10 centres reported only their own data. Six centres represented the only hospital for Kasai operation in their country, and 11 were also transplant centres.
A total number of 785 patients with BA were reported from 17 centres during the 5-year period (2015−2019), ranging from 3 to 160 children per centre or country, respectively. The weighted mean age at referral to the tertiary centre was 55 days, with a median of 53 day (IQR 48–60 days) (n = 636) (Table 1). The weighted mean age at Kasai surgery was 61 days, the median age 60 days (IQR 54–67 days) (n = 772), resulting in a weighted mean duration of six hospital days in the centre prior surgery (median 7 days, IQR 3−10 days) (Table 1). During the 5 years, 37 children with newly diagnosed BA (6%) did not undergo Kasai operation due to advanced cirrhosis and/or too late referral, thereof four children died on the waiting list for a primary liver transplantation.
| N (%) | |
|---|---|
| Age (days) at referral to centre performing Kasai surgery for biliary atresia, N = 14 | |
| Range (minimum−maximum) | (35–67) |
| Weighted mean (sum) | 55 (total = 636) |
| Median (IQR) | 53 (48–60) |
| Age (days) at Kasai surgery in infants with biliary atresia, N = 16 | |
| Range (minimum−maximum) | (42–81) |
| Weighted mean (sum) | 61 (total = 772) |
| Median (IQR) | 60 (54–67) |
| Calculated hospital days spent in centre prior Kasai surgery, N = 14 | |
| Range (minimum−maximum) | (0–18) |
| Weighted mean (sum) | 6 (total = 636) |
| Median (IQR) | 7 (3–10) |
| Only one centre performs Kasai surgery in the country, N = 16 | |
| Yes | 5 (31%) |
| No | 11 (69%) |
| Number of Kasai operations performed in the centre per year, N = 16 | |
| <5 | 7 (44%) |
| 5−15 | 6 (38%) |
| >15 | 3 (19%) |
| Paediatric liver transplantation performed in the centre, N = 16 | |
| Yes | 11 (69%) |
| No | 5 (31%) |
| Number of infants with confirmed biliary atresia during the 5 years period who did not receive Kasai surgery because they were too old or had advanced cirrhosis at diagnosis? | |
| 0 | 6 (38%) |
| 1 | 2 (13%) |
| 2 | 1 (6%) |
| 3 | 3 (19%) |
| 5 | 3 (19%) |
| 9 | 1 (6%) |
- Abbreviation: IQR, interquartile range.
Of 16 centres providing data on age of surgery, 7 (44%) perform less than 5 operations per year, 6 (38%) between 5 and 15 and 3 (19%) centres more than 15. Five (31%) of the 15 centres reported to be the only hospital providing Kasai surgery in their country, and 11 (69%) centres perform paediatric liver transplantation (Table 1).
In 13 countries (87%) a ‘well baby visit’ is offered for free to infants after discharge from the maternity unit as part of the national regular health checks. These visits are performed by physicians, and scheduled at 2−3 weeks of age in six countries, at 4−5 weeks in five countries and at 6 weeks of age in two countries (Table 2).
| N (%) | |
|---|---|
| Are well-child visits planned after discharge from maternity in your country? N = 15 | |
| Yes | 13 (87%) |
| No | 2 (13%) |
| At what age is the first well-child visit is performed after discharge in your country? N = 13 | |
| 2−3 weeks | 6 (46%) |
| 4−5 weeks | 5 (38%) |
| 6 weeks or later | 2 (15%) |
| Who is performing the well-child visit after discharge in your country? N = 13 | |
| Physician | 13 (100%) |
| Do the parents in your country get information about jaundice when the neonate is discharged home (leaflets, notice in the health booklet, stool colour card, etc.)? N = 15 | |
| Yes | 4 (27%) |
| No | 11 (73%) |
| If an infant is jaundiced at discharge from maternity, is there control visit planned? N = 15 | |
| Yes | 9 (60%) |
| No | 6 (40%) |
| Who does perform a control visit in a jaundiced baby after discharge from maternity? N = 14 | |
| General paediatrician | 7 (50%) |
| General paediatrician or midwife | 1 (7%) |
| General practitioner | 2 (14%) |
| Neonatologist | 2 (14%) |
| Unclear | 2 (14%) |
| In your country, is direct bilirubin routinely measured in jaundiced neonates at discharge? N = 15 | |
| Yes | 2 (13%) |
| No | 13 (87%) |
| Is there any protocol/guideline how to approach jaundiced neonates in your country? N = 15 | |
| Yes | 9 (60%) |
| No | 6 (40%) |
Information about jaundice is enclosed in the baby's health booklet in only four countries (Table 2). Two centres reported that total and conjugated bilirubin are measured in jaundiced infants before discharge from maternity unit. In infants with visible jaundice at discharge, a control visit is routinely scheduled in nine countries. A protocol of standard care for the jaundiced infants is available in nine countries. A national registry on incident cases of BA is established in six of the 14 countries, and was launched after the survey in another one in 2022 (Table 2).
3.2 Second survey: Perceived usefulness of the SCC by paediatricians
The SFP and the AFPA have about 750 and 1500 members, respectively. In total, 392 paediatricians (16%) completed the survey. Most respondents (96%) had seen the SCC in the booklet.
Half of the participants (53%) felt familiar with the target diseases and their symptoms, but 40% admitted to have little knowledge and 7% reported hardly any or no knowledge of the diseases screened with the SCC. When they were asked what diseases would be screened by the SCC, most paediatricians correctly chose cholestasis (78%) and BA (97%), while only a small fraction incorrectly chose jaundice (6%), or others (4%) (reported as free text: e.g., melena, rectal bleeding, hepatitis, cow's milk allergy, infections).
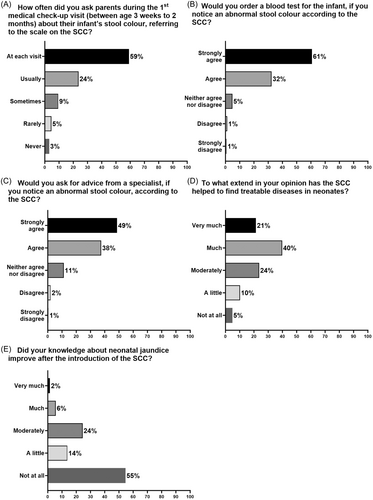
The subsequent questions explored the paediatricians' compliance with this new screening tool and how the implementation of the SCC had changed their practice (Figure 2A–E). When asked how often they inquired about the infant's stool colour, referring to the scale, during the first medical check-up visit (at age 2 weeks to 3 months), 59% ask always (at each visit), and a further 24% usually, while the remaining 17% ask the parents sometimes (9%), rarely (5%) or never (3%) (Figure 2A). In case of an abnormal stool colour, 61% would strongly agree to order a blood test, and 32% would agree, while 7% do not agree (Figure 2B). A high percentage (87%) would already ask a specialist for advice (Figure 2C). When the participants were asked whether the SCC has helped to diagnose treatable diseases in neonates, 62% considered it as very much or much helpful, while the remainder were more or less sceptical (Figure 2D). For the question of the impact of the SCC on their knowledge of neonatal jaundice, 45% reported that it improved (Figure 2E).
4 DISCUSSION
Improvement in QoC for children with neonatal cholestasis, in particular those with BA, is one of the main goals for paediatric hepatologists. The most comprehensive longitudinal data come from the French cohort covering 1428 infants diagnosed with BA between 1986 and 2015.4, 10 More recent data are only available from selected patients with isolated BA, excluding those with splenic malformation syndrome, comparing the incidence of BA prior and during the lockdown during the COVID pandemic.25 The first step of our QoC initiative was thus to get a broad picture of case finding and age of KPE of infants with BA in Europe. While two decades ago, the goal was to perform KPE before the age of 60 days, large cohort studies indicate a highly significant inverse relationship between age at surgery and the survival rates with native liver, with the highest rate when the KPE is performed within the first 30 days of life.2, 4, 10
Our European survey covered 772 infants diagnosed in the years from 2015 until 2019 and disclosed a median age at Kasai surgery of 60 days (IQR from 54 to 67 days). In addition, 6% had been referred too late to perform KPE due to advanced age and cirrhosis, which is similar to the 5.6% reported in the French cohort.4 Considering about 5.1 million infants born annually in the EU plus UK and Switzerland, and an incidence rate of BA in 1 of 18,000, about 1.416 infants with BA are expected during the 5-year period. Our cohort of 772 infants from all over Europe accounts for about 55% and therefore, seems quite representative for all infants with BA born in Europe during these years. Data from the French cohort 1986 and 2015 of 1340 infants disclosed median age at KPE of 59 days (range 6−199 days) with no significant improvement over 3 decades.4 The results of our survey confirmed the stagnation for age at surgery. Although many other factors are important (e.g., anatomic pattern, isolated vs. syndromic form, surgeon's experience, postoperative management), age at surgery is most significant for outcome and the need for liver transplantation.2, 26
The median age at referral to the KPE performing centre was 53 days (IQR 48−60), with a calculated median of 7 (IQR 3−10) hospital days in the tertiary centres before surgery. The delay in the centres can be decreased by optimizing the structure, resources and process of diagnostic work up and around surgery. In contrast, the time point to identify an infant with cholestasis, further diagnostic work-up outside and final referral to the tertiary centre for surgery depends on the healthcare system of the country for example, time points for first well baby visit after discharge from maternity unit and awareness of paediatricians, general practitioners, midwifes or other healthcare professionals to initiate timely blood testing for conjugated bilirubin and speed up further work-up and referral. Measurement of conjugated bilirubin of jaundiced babies should be targeted to ‘well child visit’ if performed at 2 and not later than 3 weeks of age. In countries with later or no regular check-up, different strategies need to be established.
Several interventional trials aimed to reduce the age at KPE applying different screening strategies.13 One study from Texas measured conjugated bilirubin as first step in all jaundiced neonates within 60 h after birth, with retesting until 2 weeks of age if values were >95th percentile. Infants with a conjugated bilirubin of >1 mg/day or >17 μmol/L were referred for diagnostic work up.27 This approach reduced the mean age at KPE from 56 days prior intervention to 36 days of age with a group difference of 19 days [95% CI: 7–32] (p = 0.004). Most intervention trials used the SCC for screening with blood sampling if stool colour is abnormal, which is less costly.13 A recent meta-analysis of eight such studies from Taiwan, Japan and China revealed a significant lower age at KPE in settings with a mean age at surgery of >70 days. However, in countries with a pre-screening age at KPE of <60 days, no significant reduction could be achieved. Multiple reasons for no further improvement may explain this: later development or fluctuation of abnormal stool in some infants, delayed detection and reporting by caregivers, and delays within the health care system to reach the diagnosis and referral to surgery, in part due to insufficient knowledge of healthcare professionals.
The second survey aimed to gather experience and awareness about the SCC from paediatricians practicing in France, where the card had been introduced about 2 years ago. The answers of almost 400 paediatricians to simple questions disclosed the rather low level of knowledge about cholestatic diseases in infancy, despite previous awareness campaigns in France. Reassuring was the high visibility of the SCC, its routine use, and the adequate resulting management: calling for advice. The survey has several limitations, particularly the low response rate of 16% which cannot exclude a selection bias. We also cannot exclude the results from self-assessment reflect real performance. An evaluation of the effectiveness to improve long-term outcome of infants with BA after introduction of the SCC—as paper version or digital application28 as currently tested—will hopefully be reported from more countries where it has been introduced.
Besides the urgency for surgery in BA, other complications call for an early diagnosis of neonatal cholestasis regardless of the underlying disease. Malabsorption of fat-soluble vitamins, including vitamin K, impairs coagulation in spite of postnatal oral vitamin K supplementation increasing the risk of cerebral bleeding, a dreadful but preventable complication.5
Physiological jaundice in neonates is common, and prolonged jaundice in exclusively breastfed babies is most often not due to cholestasis, therefore, the risk of missing cholestatic diseases is very high. The knowledge of the metabolism and routes of elimination of bilirubin is poor, as the survey confirmed, and the relationship with urine or stool colour is at best confused. In the dark skinned babies, jaundice is more easily missed. Many general practitioners, paediatricians and midwifes are not aware of the current guidelines on infantile jaundice.9 Therefore, the QoC task force has developed a case-based awareness campaign which will be delivered to all European countries in their respective languages for distribution to health care professionals to deliver the main messages of the ESPGHAN guidelines (https://www.espghan.org/our-organisation/Quality-of-Care-Initiative).
In conclusion, our survey covering about half of all infants with BA born in the European Union, UK and Switzerland during a 5 year period disclosed a mean age at Kasai surgery of 60 days. These figures are similar to the French data obtained over the last 30 years showing no improvement over that period. Better knowledge of current guidelines and implementation of structures in the different countries which allow case finding by latest 3 weeks of age and standards in diagnostic work up and timely referral pathways, would benefit not only infants with BA, but also neonates with other diseases causing cholestasis.
ACKNOWLEDGEMENTS
We thank the ESPGHAN Council for initiating the Quality-of-Care project and providing financial support, and members of the Hepatology Committee to review the questionnaires prior start of the project. We thank all colleagues at the participating hospitals for their valuable contributions and the French paediatricians who answered to the 2nd survey. We particularly acknowledge the following colleagues who contributed data to the 1st survey: Jörg Jahnel, Graz, Austria, Xavier Stephenne, Bruxelles, Belgium, Claus Petersen, Hannover, Germany, Aglaia Zellos, Athens, Greece, Florence Lacaille, Paris HNEM, France, Antal Dezsofi, Budapest, Hungary, Emer Fitzpatrick, Dublin, Ireland, Daniele Alberti, Brescia, Italy, Pietro Betalli, Bergamo, Italy, Marco Spada, OPBG Rome, Italy, Piotr Czubkowski, Warsaw, Poland, Jelena Antic, Novi Sad, Serbia, Natasa Dragutinovic, Belgrade, Serbia, Loreto Hierro, Madrid, Spain, Bjorn Fischler, Stockholm, Sweden, Valerie McLin, Geneva, Switzerland, Henkjan Verkade, Groningen, The Netherlands, Mark Davenport, London, UK. ESPGHAN provided financial support to set up the Quality-of-Care project. Although this paper is produced by the ESPGHAN Quality of Care Task Force' it does not necessarily represent ESPGHAN policy and is not endorsed by ESPGHAN. Open Access funding enabled and organized by Projekt DEAL.
THE ESPGHAN QUALITY-OF-CARE TASK FORCE
Piotr Czubkowski, Cristina Campos Goncalves, Paula Crespo Escobar, Nabil El-Lababidi, Konstantinos Gerasimidis, Katharina Ikrath, Angelika Kindermann, Sibylle Koletzko, Florence Lacaille, Thu Giang Le Thi, Anna Litwin, Emanuele Nicastro, Tena Niseteo, Rouzha Pancheva.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




