Operational Soil Warming by Underground Transmission Lines Impacts on Soil Microorganisms and Related Metabolic Activities
Academic Editor: Stefan Scheu
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
During operation, underground transmission lines (UTLs) emit heat leading to soil warming, especially of the subsoil within the cable trench. This fundamentally changes the natural vertical temperature gradient in soil and the environment of microorganisms and may contribute to the variations in microbe community composition, microbial biomass, and microbial, and enzyme activities. Along with this, N-transformation could result in environmental and groundwater pollution by nitrate-N.
Aims
The aim of the study was to decode the impact of operational (sub)soil warming by UTL on soil microorganisms and their metabolic activities specifically in the subsoil.
Methods
At four study sites along an existing 320 kV UTL near Aachen, Germany, soils were sampled from topsoil to subsoil at 120 cm depths from UTL and control sites. A supplemental laboratory experiment was established to investigate soil samples from the entire soil at specific temperature and moisture conditions.
Results
UTL operation resulted in low (0.6K) to moderate (1.7K) soil warming in topsoil and subsoil, respectively, which partly increased soil DNA content and microbial biomass, abundance of soil bacteria, and metabolic and enzyme activities, especially in subsoil samples. For example, in the topsoil soil, microbial biomass was 13% higher in UTL relative to control and increased extraordinarily by 35%–37% in the subsoil. The abundance of soil bacteria was as well enhanced, but no effect was found for amoA copy numbers. Total Nmin contents were lower in UTL compared to control sites indicating that probably N uptake by vegetation was as well increased.
Conclusions
In prospect of the imminent grid expansion of extra-high voltage transmission lines, there was substantial evidence that the operation of underground cables will not have any critical impact on soil microorganisms and their metabolic activities.
1 Introduction
Soil warming induced by climate change has been the focus of research for 2.5 decades (Grime et al. 2000; Khan et al. 2024; Lükewille and Wright 1997; Rustad et al. 2001; Weedon et al. 2023). The Intergovernmental Panel on Climate Change assessment reports that global average temperatures will rise by 2.1°C–3.5°C (Tollefson 2021). The extent to which global warming influences the soil heat balance is not yet well documented. Anyhow, it is hypothesized that the expected increase in ambient temperatures due to climate change is likely to have less impact on metabolic processes in the soil, especially in the living topsoil, due to the enormous diurnal and annual temperature fluctuations in the top centimeters of soil. The indirect effects on the water balance are probably more substantial. Warming of the subsoil, on the other hand, might have a stronger impact on soil microorganisms and their metabolic processes.
The current transformation of the entire energy system within the EU will lead to a large-scale expansion of extra-high-voltage underground transmission lines (UTLs). Thus, in the near future, the operation of extra-high-voltage UTL will additionally contribute to locally increased soil warming. In Germany, for example, it is expected that approx. 10,000 km of electricity grids will be installed until the year 2030 (FMEC 2023), the majority as UTL.
During operation, UTL emits heat, and this will lead to a warming of the soil, especially the subsoil, whereby temperature increases in a range of 1.7–5.3 K at 120 cm depth for base load operation are documented (Ahl et al. 2023; Emmerling et al. 2024). Increased heat emissions in the subsoil in the vicinity of the cable trench thus may reverse the natural temperature profile in soil in the way that soil temperature will increase from the topsoil to the subsoil which requires the soil organisms to adapt to this anomaly. This phenomenon has already been observed in anthropogenic soils, namely, in cover soils above waste and sewage sludge landfills, where the decomposition of organic compounds in the landfill body by microorganisms can result in a constant supply of heat (+2°C to 4°C) to these soils (Blume 2021). Knowledge of the impact of UTL on soil temperature and soil moisture dynamics and related metabolic transformations and microbial adaptation is fundamental for environmental evaluation. Earlier work by Trüby (2018), for example, has shown that the operation of extra-high-voltage UTL promotes microbial respiration activity in soils.
The vertical profile of soil microbial distribution and activity in relation to subsoil warming has not yet been studied in detail. It is therefore imperative to understand how microorganisms can adapt to the increasing warming of the soil with depth and which consequences occur for metabolic activity. Microorganisms exist throughout the soil profile, and those microorganisms living in deeper soil horizons likely play key roles in regulating biogeochemical processes (Dickie et al. 2002). According to Eilers et al. (2012), microbial biomass and bacterial diversity are usually lower in the subsoil than in the topsoil (Spohn et al. 2016). Results from a meta-analysis revealed that among examined edaphic factors, the depth variation in soil pH exhibited significant negative associations with the depth change in microbial biomass and diversity, whereas soil total organic carbon and total nitrogen exhibited significant positive associations (He et al. 2023).
Rustad et al. (2001) could show that the response of artificial soil warming by underground cables in a range of 0.3°C–6°C significantly increased soil respiration, net N mineralization and nitrification. Changes in N-cycling are likely to potentially result in substantial nitrate-leaching and production of gaseous nitrous oxide (Daebeler et al. 2017; Hui et al. 2024).
Weedon et al. (2023) investigated the shape of the response of soil microbial communities to soil warming and their associated community temperature adaptation along a geothermal gradient on Iceland. The mechanisms behind temperature adaptations of soil microorganisms could be physiological adaptations of single species (Malcolm et al. 2008) or species shifts within the microbial community (Schindlbacher et al. 2011). According to Hall et al. (2010), soil bacteria may adjust their physiology to temperature by altering cell membrane permeability. Focusing on the microbial community composition, Radujković et al. (2018) found that bacterial and fungal communities changed only at warming levels exceeding +6°C to 8°C above ambient. It is still not clear, whether this change was a direct effect of temperature or indirect effects due to, for example, effects on vegetation growth and phenology (Leblans et al. 2017), reduced soil organic matter concentration or differences in soil texture (Verbrigghe et al. 2022). However, Weedon et al. (2023) provided much evidence that this community shift may be driven by direct responses to temperature.
The aim of the study was to decode the impact of operational (sub)soil warming by UTL on soil microorganisms and their metabolic activities. We thus hypothesized that operational soil warming will enhance both, specifically in the subsoil. We investigated the amounts of soil microbial biomass, soil respiration, enzyme (dehydrogenase) activity as well as N-transformation (the amounts of Nmin) up to 120 cm depth during 2 years in a field study at four different study sites in West Germany. A supplementary laboratory experiment was conducted in order to test the combined impact of specific temperatures and moisture conditions at highest cable load on soil DNA and the relative abundance of soil bacteria assessed by 16S rRNA and amoA genes encoding for microbial nitrification.
2 Materials and Methods
2.1 Field Study
The field study on soil microbial properties was part of a monitoring program on the impact of operational soil warming caused by extra-high voltage UTL on soil behavior (Emmerling et al. 2024). Soil monitoring was carried out at four different study sites in West Germany along an existing 320 kV UTL, acronym ALEGrO (Aachen—Liège Electrical Grid Overlay). ALEGrO was planned and implemented by the Amprion Ltd, one of the four transmission system operators in Germany, in cooperation with the Belgian transmission system operator “Elia.” The electrical transmission line acts as a power link between both countries from Oberzier (near Aachen, Germany) to Lixhe (near Liège, Belgium) over a distance of approx. 90 km. It was constructed between 2018 and 2020 and is in operation since November 2020.
Soil was investigated in the area of the cable trench at four different study sites—Weisweiler, Dürwiss, Haaren, and Brand—near the city of Aachen, Germany. Soil in a distance of 4.5–5.5 m without UTL impact was used as a control at each study site, respectively. At each study site, soil samples were taken at four soil depths, at 20, 60, 90, and 120 cm depth, representing the plough horizon, the main rooting zone, as well as the subsoil and the deeper subsoil. At each UTL treatment, sampling was thus in close proximity to the cables laying within a bedding material in 180 cm depth. Sampling strategy for Nmin analysis was different and represented three depths, 0–30, 30–60, and 60–90 cm depth according to Wehrmann and Scharpf (1979). All soil samples were kept cool at approx. 4°C–6°C until they were brought to the laboratory and immediately analyzed.
At each study site and for each soil depth, composite samples were obtained from two replicates each in the UTL and control variants. Soil samples were taken in 2 years, each in spring (May 2022, April 2023) and autumn (October 2022 and October 2023) with a split-tube auger (Eijkelkamp Inc.), respectively. Due to the limited study area and in order to minimize the sampling impact, only two replicate samples were taken at each treatment per date and depth and combined to form a composite sample.
At three out of four study sites, soils were under arable land use prior to the start of the investigations, and one soil was managed as grassland. The parent material of each soil derived from air-blown silt (loess), and thus soils showed low variation in soil physical properties with the exception that the soil water regime of the grassland soil at location Brand differed markedly. Arable soils at the study sites Weisweiler, Dürwiss, and Haaren were characterized as Luvisols (partly with stagnic properties), whereas the soil type at grassland study site Brand was a Gleysol in slope position. All in all, soil textures, soil pore distribution, and bulk density showed only small differences between the four soils and between UTL cable lines and the control sites, respectively. Soil pH values of the arable soils ranged from 6.7 to 7.7 at Weisweiler and Dürwiss study sites, whereas the pH was lower in a range of 6.3–4.6 at Haaren study site. At the grassland site Brand, pH values varied especially vertically in soil from 6.4 to 7.3 (Emmerling et al. 2024).
2.2 Laboratory Experiment
The 1-year monitoring program of soil temperature and moisture dynamics at the ALEGrO-UTL revealed that on average, soil temperature was increased in a range of 0.6 K in the topsoil, approx. 1 K in the rooting zone, and 1.3–1.7 K in the subsoil from 90 to 120 cm depth (Table 1). Soil warming was restricted mainly to the immediate vicinity of the cable route (Emmerling et al. 2024).
| (a) | UTL | Control | ||||
|---|---|---|---|---|---|---|
| Depth (cm) | Mean (°C) | SD | Mean (°C) | SD | K | p value |
| 20 | 12.89 | 6.26 | 12.28 | 6.11 | 0.61 | *** |
| 60 | 13.20 | 5.21 | 12.23 | 5.05 | 0.97 | *** |
| 90 | 13.46 | 4.52 | 12.17 | 4.46 | 1.29 | *** |
| 120 | 13.82 | 3.93 | 12.13 | 3.94 | 1.69 | *** |
| (b) | UTL | Control | ||||
|---|---|---|---|---|---|---|
| Depth (cm) | Mean (wt%) | SD | Mean (wt%) | SD | Change (wt%) | p value |
| 20 | 19.04 | 6.04 | 20.03 | 7.36 | −1.00 | *** |
| 60 | 19.10 | 4.55 | 20.13 | 4.83 | −1.02 | *** |
| 90 | n.m. | n.m. | n.m. | n.m. | n.m. | |
| 120 | 20.83 | 6.39 | 23.28 | 3.07 | −2.45 | *** |
- Note: Soil water content at 90 cm depth was not recorded as part of the monitoring (data acc. to ALEGrO-monitoring; Emmerling et al., 2024).
The average cable load of the ALEGrO-UTL during the study period was 65% (Emmerling et al. 2024). In order to test the impact of higher cable loads above average, we additionally calculated a 100% permissible cable capacity of the ALEGrO cables by using the factor 2.4 as the cable load is squared in the heat loss of the cables and correlates with the current intensity. It must be emphasized that in practice, a 100% utilization is an exceptional case that only occurs, if at all, for a limited period of time. As a result, the calculated soil warming increased on average up to 1.5 K (20 cm depth), 2.3 K (60 cm depth), 3.1 K (90 cm depth), and 4.1 K (120 cm depth). Within the ALEGrO monitoring, it was found that the differences in soil moisture content between UTL and control were on average in a range of −1.00 wt% in 0–60 cm depth and −2.45 wt% in the deeper subsoil in 120 cm depth (for further details see Emmerling et al. 2024; Table 1).
For the laboratory experiment, only the soil at the representative Weisweiler study site was sampled at the appropriate soil depths as described above. The samples were immediately cooled in thermo boxes in the field and brought to the laboratory on the same day, then sieved to <2 mm, and the water content was determined gravimetrically. The samples were stored in the refrigerator for 2 days before the experiment. For the laboratory experiment, moist soil with various soil moisture contents was incubated at different temperatures in a dry-bath thermo-block incubator (BIOER, USA) for at least 72 h. For each soil sample, five Eppendorf pipettes of 2.0 mL volume were filled with approximately 1.0 g of soil. For this purpose, the monitored soil temperature and soil moisture at the various soil depths of UTL and control sites of the ALEGrO-soil monitoring for the Weisweiler study site were used (Table 2). The experimentally set conditions for soil temperature and soil moisture corresponded to the spring mean values from March to May 2023 at this site.
| Soil depth (cm) | Control | UTL | UTL+ | |
|---|---|---|---|---|
| Temperature (°C) | 20 | 13.0 | 13.5 | 14.5 |
| Water content (wt%) | 18.0 | 17.0 | 16.0 | |
| Temperature (°C) | 60 | 13.0 | 14.0 | 15.5 |
| Water content (wt%) | 20.0 | 19.0 | 18.0 | |
| Temperature (°C) | 90 | 13.0 | 14.5 | 16.0 |
| Water content (wt%) | 22.0 | 20.0 | 18.0 | |
| Temperature (°C) | 120 | 14.0 | 15.5 | 18.0 |
| Water content (wt%) | 24.0 | 21.0 | 18.0 |
- Note: Data are based on sensor records from the ALEGrO-monitoring program for springtime (period of March 15–May 15, 2023). UTL = Underground transmission line at standard operation with a mean cable load of 65%; UTL+ = calculated cable load of 100%.
2.3 Analytical Methods
Soil pH was determined using air-dried soil in a 0.01 M CaCl2 solution. Samples were extracted in an end-to-end shaker for 2 h. Measurement was done using a pH Cond 340i glass electrode (WTW Ltd, Germany). Amounts of total soil organic carbon (TOC) and total nitrogen (Nt) were quantified using an elemental analyzer vario EL cube (Elementar Ltd, Germany).
Soil microbial carbon was determined on moist soil (adjusted to approx. 50% of maximum water-holding capacity) according to the chloroform fumigation extraction method (Vance et al. 1987). Extraction was performed using a 0.01 M CaCl2 solution. Extracts were analyzed for organic carbon with a TOC-TN Analyzer (Shimadzu TOC-V + TNN, Kyoto, Japan). A kEC coefficient of 0.45 was used to calculate microbial biomass carbon (MBC) from extract-C values. Soil microbial respiration (Rb) was determined according to Heinemeyer et al. (1989). Moist (40%–60% of WHCmax) soil samples were continuously flushed with air, and released CO2 was determined automatically by an infrared gas analyzer (ADC Model 225-MK3, Hoddesdon, England). Difference between CO2 released from the samples and ambient air was considered as soil respiration. Measurements were done for 24 h at constant ambient air of 22°C. Dehydrogenase activity (DHA) was determined on the basis of Thalmann (1968) and Alef and Nannipieri (1995). Moist soil samples were incubated with a triphenyl tetrazolium chloride (TTC) solution dissolved in 0.1 M Tris buffer for 24 h at 27°C. After 2 h of reaction time with shaking at regular intervals, the colored sample was filtrated, and the liquid phase was measured at 546 nm against blank values on a spectrometer (Shimadzu UV-1650 PC; Shimadzu Europe GmbH, Duisburg, Germany).
For Nmin analyses, 12 g moist soil samples were immediately extracted with 2 M KCl, centrifuged at 3.000 UPM for 10 min, and subsequently analyzed with a continuous flow analyzer (SEAL analytical AA3, Norderstedt, Germany). Soil water content was determined separately.
Amounts of DNA were investigated as an indicator for the estimation of soil microbial biomass (Joergensen and Emmerling 2006; Gong et al. 2021). DNA extraction and purification were performed using the Genomic DNA from Macherey-Nagel NucleoSpin Soil kit (Düren, Germany). DNA yield was measured in a microplate at 260 nm (Töwe et al. 2010) using a Victor3 MultiLabel Reader (Perkin Elmer, Germany).
The qPCR assay was prepared to 20 µL volume consisting of InnuMix SYBR-Green qPCR Master-Mix (Analytik Jena), and the reaction was carried out in a thermal cycler with an optical module (Analytik Jena, Jena, Germany). 16S rRNA genes were used as a proxy to quantify the abundance of bacteria in soil as described by Bach et al. (2002). To analyze the effect of temperature and moisture content on ammonia-oxidizing bacteria (AOB), the abundance of bacteria harboring the amoA gene was used as a marker for bacterial N mineralization according to Rotthauwe et al. (1997). Amplification for 16S rRNA and amoA genes was carried out using primers FP 16S rDNA and RP 16S rDNA (Bach et al. 2002) as well as amoA 1F and amoA 2R (Rotthauwe et al. 1997), respectively. Reaction conditions for 16S rRNA gene consisted of initial denaturation at 95°C for 10 min, 95°C for 20 s, 62°C for 1 min, and 72°C for 30 s for 35 cycles. The amoA genes were amplified at 94°C for 1 min, 60°C for 1 min 30 s, 72°C for 1 min for 39 cycles, and 95°C for 15 s, 60°C for 30 s, and 95°C for 15 s. Standard curves were generated from cloned amplicons of Bacillus subtilis and Nitrosomonas sp. for 16S rRNA and amoA, respectively. The qPCR reaction efficiency of the standards was 102% and 92% for 16S rRNA and amoA, respectively.
2.4 Statistical Analyses
Statistical analyses were performed with the SPSS statistical package, version 29. For pairwise comparisons between the two treatments, UTL and control, of the field study, a nonparametric Wilcoxon test was used. The mean values of the study sites refer to a total of four samples taken during the 2-year study period. For a general comparison of UTL and control plots, the mean values were combined into an overall mean. A total of three variants (control, UTL, and UTL+) were compared in the laboratory experiment. Accordingly, a Kruskal–Wallis H-test was applied. A post hoc test with the Bonferroni correction was carried out to verify significant differences between the subgroups. Individual negative measured values for amoA, especially for samples from 60 and 90 cm depth, were set to zero.
3 Results
3.1 Field Study
At each study site, the measured soil microbiological properties, namely, microbial biomass, basal respiration, and DHA, showed a vertical gradient in the soils with decreasing contents with increasing soil depth (Table 3). Amounts of microbial biomass, for example, were in a range of 292 mg Cmic kg−1 dry matter (DM) in the topsoil to 18.7 mg Cmic kg−1 DM in 120 cm depth for the UTL treatment at the Weisweiler study site (Table 3). As well, microbial respiration activity ranged from 0.48 to 0.09 mg CO2-C kg−1 DM h−1 and enzyme activity (DHA) from 85.0 to 0.8 mg TPF kg−1 DM d−1, respectively.
| Study site | Soil depth (cm) | Soil organic C (mg TOC g−1 DM) | Microbial biomass (mg C kg−1 DM) | Soil respiration (mg CO2-C kg−1 DM h−1) | Dehydrogenase activity (mg TPF kg−1 DM d−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| UTL | Control | UTL | Control | UTL | Control | UTL | Control | ||
| Weisweiler | 20 | 23.31 | 23.63 | 292.8 ± 31 | 215.0 ± 28 | 0.48 ± 0.03 | 0.36 ± 0.04 | 85.0 ± 26.6 | 50.6 ± 13.7 |
| 60 | 5.50 | 2.20 | 62.6 ± 12 | 44.9 ± 9 | 0.14 ± 0.02 | 0.14 ± 0.01 | 16.0 ± 7.6 | 9.7 ± 3.3 | |
| 90 | 5.04 | 1.51 | 28.9 ± 5 | 20.0 ± 5 | 0.11 ± 0.01 | 0.08 ± 0.01 | 4.3 ± 1.4 | 2.7 ± 1.8 | |
| 120 | 4.92 | 1.53 | 18.7 ± 3 | 12.4 ± 3 | 0.09 ± 0.03 | 0.06 ± 0.02 | 0.8 ± 0.3 | 1.1 ± 1.0 | |
| Dürwiss | 20 | 18.33 | 13.94 | 273.8 ± 32 | 243.1 ± 22 | 0.41 ± 0.03 | 0.32 ± 0.02 | 59.1 ± 9.7 | 57.2 ± 17.0 |
| 60 | 5.31 | 9.11 | 69.0 ± 25 | 44.8 ± 16 | 0.15 ± 0.01 | 0.15 ± 0.02 | 9.9 ± 4.4 | 12.7 ± 4.9 | |
| 90 | 6.44 | 8.13 | 38.7 ±14 | 15.5 ± 2 | 0.12 ± 0.02 | 0.09 ± 0.02 | 2.9 ± 1.5 | 1.3 ± 0.7 | |
| 120 | 4.86 | 4.42 | 18.2 ± 2 | 14.9 ± 5 | 0.10 ± 0.01 | 0.07 ± 0.02 | 0.6 ± 0.4 | 0.4 ± 0.3 | |
| Haaren | 20 | 12.23 | 15.00 | 189.4 ± 24 | 175.0 ± 16 | 0.37 ± 0.04 | 0.30 ± 0.03 | 61.4 ± 22.5 | 54.9 ± 8.1 |
| 60 | 1.53 | 2.39 | 45.1 ± 23 | 37.7 ± 12 | 0.15 ± 0.04 | 0.12 ± 0.01 | 18.8 ± 8.4 | 16.3 ± 6.6 | |
| 90 | 1.68 | 1.58 | 28.8 ± 9 | 20.0 ± 6 | 0.09 ± 0.01 | 0.07 ± 0.01 | 10.4 ± 2.8 | 4.9 ± 2.1 | |
| 120 | 1.51 | 3.32 | 19.9 ± 5 | 14.1 ± 5 | 0.10 ± 0.01* | 0.04 ± 0.01 | 3.0 ± 1.0 | 1.1 ± 1.0 | |
| Brand | 20 | 24.22 | 26.81 | 436.4 ± 22 | 407.8 ± 26 | 0.55 ± 0.09 | 0.44 ± 0.03 | 77.3 ± 4.0 | 69.6 ± 4.2 |
| 60 | 7.13 | 6.69 | 116.8 ± 35 | 88.7 ± 13 | 0.25 ± 0.04 | 0.15 ± 0.04 | 14.3 ± 8.5 | 14.6 ± 10.5 | |
| 90 | 7.86 | 5.78 | 54.8 ±9 | 40.7 ± 4 | 0.18 ± 0.03 | 0.06 ± 0.02 | 7.2 ± 2.2 | 8.5 ± 6.8 | |
| 120 | 4.52 | 3.12 | 24.7 ± 7 | 18.2 ± 3 | 0.10 ± 0.01 | 0.08 ± 0.01 | 2.1 ± 1.1 | 1.7 ± 0.5 | |
| Average | 20 | 19.5 ± 5 | 19.9 ± 6 | 298.1 ± 26 | 260.2 ± 25 | 0.45 ± 0.03** | 0.33 ± 0.02 | 70.7 ± 8.6 | 58.1 ± 5.6 |
| 60 | 4.9 ± 2 | 5.0 ± 4 | 73.4 ± 13 | 54.0 ± 8 | 0.17 ± 0.02 | 0.14 ± 0.01 | 14.7 ± 3.4 | 13.3 ± 3.1 | |
| 90 | 5.0 ± 4 | 4.3 ± 3 | 37.8 ± 5* | 24.0 ± 3 | 0.13 ± 0.01** | 0.08 ± 0.01 | 6.2 ± 1.2* | 4.3 ± 1.8 | |
| 120 | 4.0 ± 2 | 3.1 ± 2 | 20.4 ± 2* | 12.9 ± 2 | 0.10 ± 0.01** | 0.06 ± 0.01 | 1.6 ± 0.4*** | 1.0 ± 0.4 | |
- Note: Significant differences between UTL versus control are marked; Wilcoxon test, n = 4 (each study site) or 16 (average).
- + p < 0.10.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.10.
From a soil depth of 90 to 120 cm, only very low amounts of microbial biomass and metabolic activities were detected. The values in the respective UTL variant were consistently higher than the controls at each soil depth and on average across all four study sites. On total average, amounts of microbial biomass, for example, accounted for 13% higher in UTL relative to control soils for the top horizon, 26% for 60 cm depth, 37% for 90 cm, and 35% for 120 cm depth, respectively (Table 3). It was noticeable that the differences were particularly significant in the subsoils at depths of 90 and 120 cm (Table 3). In the slightly warmed subsoils of the UTLs, the contents and activities were increased by 35%–37% (microbial biomass), 38%–40% (respiration), and 31%–38% (DHA), however, at an overall low measurement level.
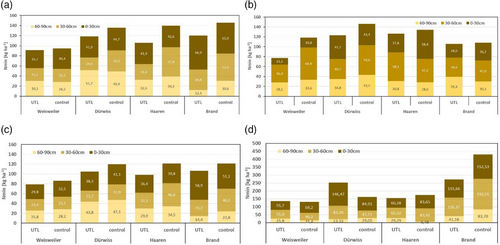
In general, mean Nmin levels in the arable sites ranged between 80 and 250 kg Nmin ha−1 at a depth of 0–90 cm, except at Brand grassland site in autumn 2023, where levels of up to 430 kg Nmin ha−1 were measured (Figure 1). The overall mean (±SD) Nmin amounts in the entire soil from 0 to 90 cm depth across all study sites and sampling dates was 136.8 kg ha−1 (±55.4) in UTL compared to 150.9 kg ha−1 (±78.3), respectively. The differences between UTL and control were on average not significant (p = 0.291).
3.2 Laboratory Experiment
The DNA content in both the control and the UTL decreased continuously with depth. In the topsoil, the DNA content in the UTL was slightly below that of the control. In contrast, the DNA content in the subsoil was reversed from a depth of 90 cm, showing significantly higher amounts in the UTL soil (Figure 2).
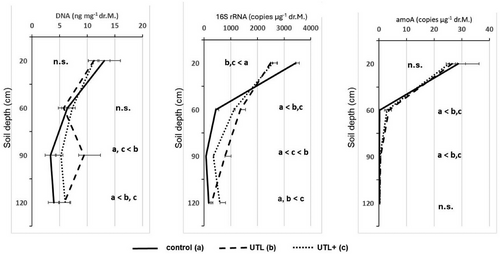
As part of the laboratory experiment, a UTL variant with 100% cable utilization was also tested (UTL+). The results of this variant did not match the UTL variant as the effects of increased temperature were less pronounced. Particularly, at 90 cm depth, DNA content of UTL was higher relative to UTL+ and the control.
Copy numbers of 16S rRNA genes encoding for soil bacteria decreased with depth as well (Figure 2). In the top horizon, copy numbers of 16S rRNA were significantly higher in UTL than in the control. A clear reaction to the increasing operational warming was found for the subsoil, in which 16S rRNA copies at a depth of 60 and 90 cm increased significantly compared to the control. However, the simulation of increased cable utilization apparently showed no operational effects on the abundance of soil bacteria (Figure 2).
No effects of the experimentally conducted soil warming by high-voltage UTL on the number of amoA genes copies encoding for the activity of AOB could be detected in the laboratory experiment (Figure 2c).
4 Discussion
Temperature is one of the most important environmental factors known to affect the growth of microorganisms and the biological activity of soils. Elevated temperatures per se may promote growth and biodiversity of most soil communities, especially in cold ecosystems (FAO 2020), and thus, this might be transferred to the temperature conditions in subsoils. However, little is still known about soil microorganisms and their activities in the subsoil, or about the effects of relatively uniform elevated temperatures on biological systems (Fierer et al. 2003; Yan et al. 2017). The population density of soil organisms is physiologically and morphologically closely linked to the abiotic soil properties, which typically change with increasing soil depth. Numerous studies have shown that the population density, biomass, and activities of soil organisms decrease with increasing soil depth (Eilers et al. 2012; He et al. 2023; Spohn et al. 2016). Operational heat emissions permanently change the natural vertical temperature and moisture amplitudes in soils, which might lead to physiological adaptation of soil organisms. In this context, both positive and negative feedback effects on microbial community might be conceivable.
The results received from the ALEGrO field survey emphasized that the impact of operational soil warming by UTL on soil water dynamics was very low accounting for −1 to −2.4 wt% (see Table 1), and this was in accordance with Brakelmann (1984). Soil microorganisms can be active in a wide temperature range between 1°C and 55°C if sufficient moisture is available (Ottow 2011). However, it has to be taken into account that different groups of microorganisms have different temperature preferences, so that a change in temperature might also lead to a shift in the microbial community structure. We hypothesize that an increase in soil temperature may cause a shift from cold-loving (psychrophilic) bacteria, whose optimum temperature is approx. 10°C, to mesophilic bacteria, whose optimum temperature is approx. 15°C, so that the absolute reaction rate does not change with an increase in temperature, especially in resource-limited habitats such as subsoils.
The value levels measured in the field study were typical for the analyzed soils and their soil chemical properties. The decline of the microbial properties with increasing soil depth was consistent with previously published work (He et al. 2023; Sun et al. 2021). The assumption that both microbial biomass and microbial metabolic activities will be stimulated by the operational warming of the soil by high-voltage underground cables was only partly confirmed by our field study, whereas the increase of soil respiration during artificial soil warming simulating heat emissions from underground cables was already documented by Trüby (2018). The microbial biomass in the soil, soil respiration, and DHA in our study corresponded to the vertical gradient of the soil organic carbon content (see also Table 3) but were further modified by the vertical temperature increase. The operation of the underground cables generally causes a temperature anomaly in the soil, in that the temperature did not decrease with increasing soil depth but rather increased (Emmerling et al. 2024). Operational heating of the soil by UTL is therefore more pronounced in the subsoil than in the topsoil. It is thus not surprising that a disproportionate promotion of microorganisms and their metabolic activities occurs in the subsoil at a depth of 90 and 120 cm, against the background of an overall low measured value level within the subsoil.
According to van't Hoff's rule, metabolic activities should have doubled with a temperature increase of 10°C in each case. The direct and indirect influence of temperature changes on microbial communities and their metabolic activities is undisputed; however, it applies above all to laboratory cultures that are not limited in terms of nutrients and the overall growth conditions. Our results revealed that on total average of the four study sites within the field investigation, microbial biomass and metabolic activities (respiration, DHA) were enhanced by 13%–27% in the top 60 cm of UTL soils relative to the respective controls. However, the influence of moderate soil warming due to the operation of UTL was evidently particularly pronounced in the subsoil, where the microbial biomass and its activities are in general low. Here, the promotion of soil microbial activities ranged from 31% to 40%. The Q10 value of microbial activity, for example, respiration rate, is usually between 2 and 2.4 within the mesophilic physiological temperature range (approx. between 0°C and 35°C) as the turnover rate increases exponentially with increasing temperature (Ottow 2011). Even more, under field conditions, values of 2–4 were determined (Janssens and Pilegaard 2003). It can be assumed that, among other factors, the diffusion rate of soluble substrates and O2 is increased at higher temperatures, which can lead to an increase in turnover rates (Davidson et al. 2006).
Our results revealed that metabolic activities were considerably enhanced, especially in the subsoil, taking into account that the temperature increase was only in a range of 0.6 K (topsoil)–1.7 K (subsoil). Copies of various microorganism groups usually decrease with depth in the soil, analogous to the SOM content, whereby the decrease in the biomass of fungi is greater than that of prokaryotes. This may lead to a relative enrichment of prokaryotes in the subsoil (Ottow 2011). As the operational heating of the subsoil by underground cables stimulates microbial growth but is not accompanied by an increased availability of organic substances, it can be assumed that the stimulation of the turnover rate of microbial biomass is lower.
Among the plant nutrients, special attention must be paid to nitrogen (N), as increased microbial N transformation due to soil warming could have undesirable environmental effects. The Nmin contents determined at the four study sites and at the various soil depth levels were within the usual range for arable and grassland sites. Averaged results of Nmin revealed no significant lower amounts in UTL-affected soils. This contrasted with results from Lükewille and Wright (1997) who found an N release in a boreal forest due to experimentally increased temperatures. Our results revealed that the operational soil warming caused by UTL had no significant effect on the release of ammonium-N and nitrate-N. It might be hypothesized that this is attributed to better N utilization and root uptake of N due to increased ambient temperatures in the vicinity of the cables. Another reason could be that an increase in temperature may cause increases in denitrification by 30%–100% (Alster et al. 2018).
The results of the laboratory experiment basically confirmed the field study. This experiment also tested the influence of higher soil warming as a result of an increased operational cable load of 100%. According to the DNA contents and 16S rRNA copies, soil microorganisms were further stimulated, whereas no clear effect was detectable for the copy number of amoA genes, thus for the nitrification activity.
It is still an open question whether operational (sub)soil warming has a stronger effect on soil bacteria or on soil fungi and whether this could explain the low impact on amoA genes encoding for bacterial N nitrification. According to Hall et al. (2010), soil warming promoted fungi, whereas according to Ottow (2011), this is the case for soil bacteria. Anyway, our results of amoA copy numbers indicated that operational soil warming by underground cables seemed not to impact N-nitrification in soil.
5 Conclusions
Factors affecting the vertical distribution of microorganisms, their community structure, and metabolic activities have not yet been fully understood, especially in the light of soil warming. Soil warming due to anthropogenic activities is quite unresearched as well. One example is the operation of UTLs which emit heat during operation leading to soil warming especially in the subsoil. This fundamentally changes the environment of microorganisms and may result in variations in microbe community composition, microbial biomass, and microbial and enzyme activities.
Overall, operational soil warming by high-voltage underground cables improved microbial properties of the entire soils, extraordinarily of the subsoils. Nmin contents were tendentially but not significantly lower in UTL compared to control sites.
In prospect of the imminent grid expansion of extra-high-voltage transmission lines, it can be assumed that the environmental impact of underground cables during operation within the entire soil profile up to 120 cm depth will be low in terms of soil microbial properties and Nmin contents and thus relates to the soil pedon, which is of particular importance for soil organisms, metabolic processes, and the growth of agricultural crops. Nevertheless, a comprehensive soil monitoring appears appropriate in order to achieve public acceptance of underground cables and to reduce public concerns.
Author Contributions
Christoph Emmerling: conceptualization, resources, writing–original draft. Maren Herzog: project administration, review and editing. Celine Hoffmann: formal analysis, validation. Benjamin Schieber: reviewing and editing.
Acknowledgments
Many thanks are dedicated to Elvira Sieberger who helped with the analyses in the laboratory. Alena Förster, Michelle Fonfara as well as student assistants of the soil science department of the University of Trier are kindly acknowledged for assistance during project realization and sampling campaigns.
Open access funding enabled and organized by Projekt DEAL.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




